Disease Activity Is Associated with Obesity in Newly Diagnosed Pediatric Patients with Ulcerative Colitis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Assessment of Nutritional Status
2.3. Disease Activity of CD and UC at Diagnosis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Prevalence of Nutritional Status Deviations among Pediatric Patients with IBD in Hungary
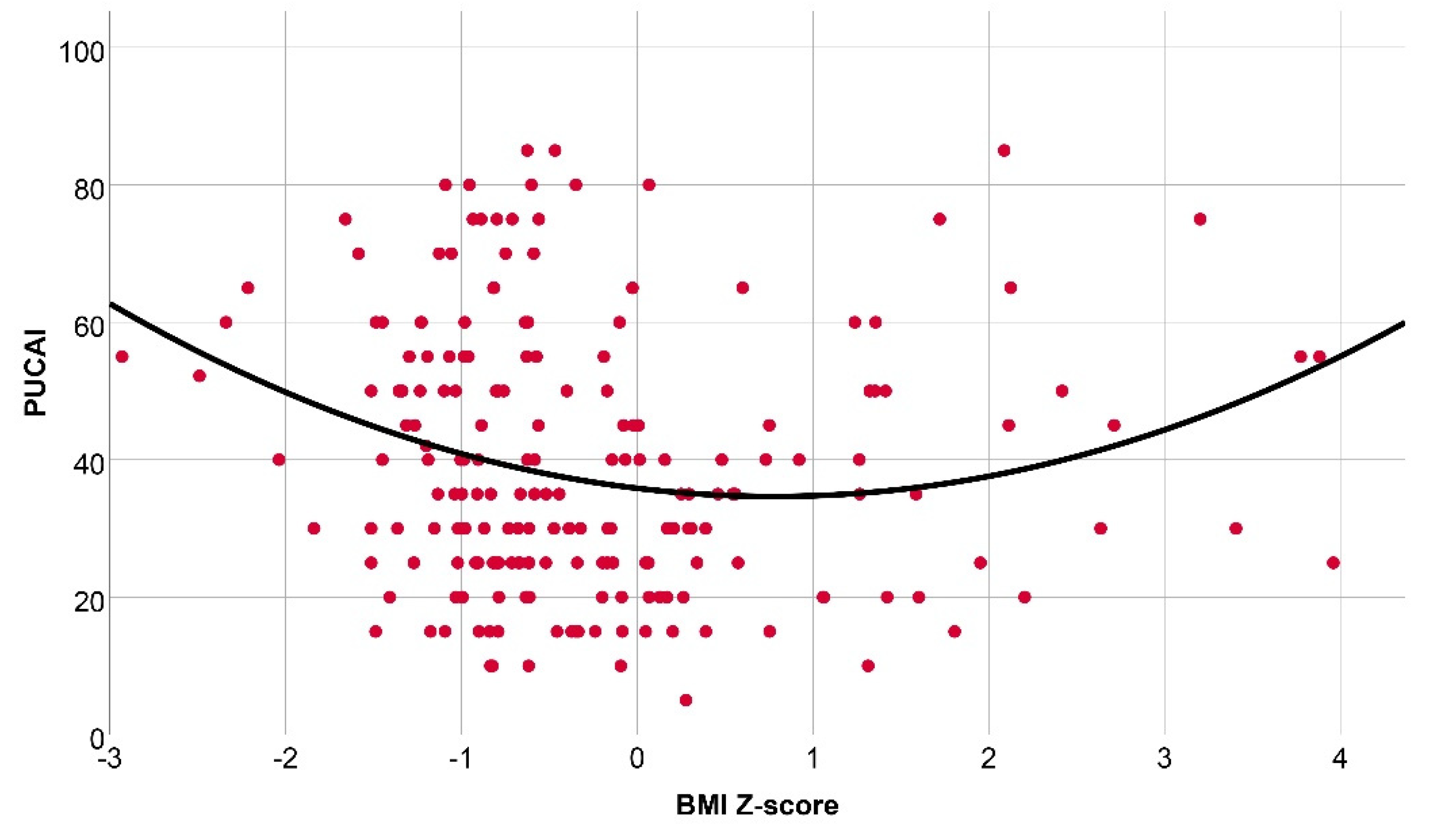
3.3. Assosiation between Nutritional Status Deviations and Disease Activity among Pediatric Patients with IBD in Hungary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.; Chan, F.K.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Sawczenko, A.; Sandhu, B.; Logan, R.; Jenkins, H.; Taylor, C.; Mian, S.; Lynn, R.M. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet 2001, 357, 1093–1094. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.; Buoncristiano, M.; Nardone, P.; Starc, G.; Hejgaard, T.; Júlíusson, P.B.; Fismen, A.S.; Weghuber, D.; Musić Milanović, S.; García-Solano, M.; et al. Thinness, overweight, and obesity in 6- to 9-year-old children from 36 countries: The World Health Organization European Childhood Obesity Surveillance Initiative—COSI 2015–2017. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22 (Suppl. 6), e13214. [Google Scholar] [CrossRef] [PubMed]
- Kádár, M.; Szőllősi, G.; Molnár, S.; Szabó, L. The incidence of malnutrition between 1 and 5 years of age on the basis of the preventive primary care data. Dev. Health Sci. DHS 2019, 2, 9–14. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity among Adults and Youth: United States, 2015–2016; NCHS Data Brief; National Library of Medicine: Bethesda, MD, USA, 2017; pp. 1–8. [Google Scholar]
- Ahrens, W.; Pigeot, I.; Pohlabeln, H.; De Henauw, S.; Lissner, L.; Molnár, D.; Moreno, L.A.; Tornaritis, M.; Veidebaum, T.; Siani, A. Prevalence of overweight and obesity in European children below the age of 10. Int. J. Obes. 2014, 38 (Suppl. 2), S99–S107. [Google Scholar] [CrossRef]
- OEU. Overweight and obesity among children and adolescents. In Health at a Glance: Europe 2020: State of Health in the EU Cycle; OECD: Paris, France, 2020. [Google Scholar]
- Jakab, A.E.; Hidvégi, E.V.; Illyés, M.; Cziráki, A.; Bereczki, C. Prevalence of Overweight and Obesity in Hungarian Children and Adolescents. Ann. Nutr. Metab. 2018, 72, 259–264. [Google Scholar] [CrossRef]
- Bammann, K.; Gwozdz, W.; Lanfer, A.; Barba, G.; De Henauw, S.; Eiben, G.; Fernandez-Alvira, J.M.; Kovács, E.; Lissner, L.; Moreno, L.A.; et al. Socioeconomic factors and childhood overweight in Europe: Results from the multi-centre IDEFICS study. Pediatr. Obes. 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Khalili, H.; Ananthakrishnan, A.N.; Konijeti, G.G.; Higuchi, L.M.; Fuchs, C.S.; Richter, J.M.; Chan, A.T. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2015, 21, 361–368. [Google Scholar] [CrossRef]
- Chan, S.S.M.; Luben, R.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Teucher, B.; Lindgren, S.; Grip, O.; Key, T.; Crowe, F.; et al. Body mass index and the risk for Crohn’s disease and ulcerative colitis: Data from a European Prospective Cohort Study (The IBD in EPIC Study). Am. J. Gastroenterol. 2013, 108, 575–582. [Google Scholar] [CrossRef]
- Kugathasan, S.; Nebel, J.; Skelton, J.A.; Markowitz, J.; Keljo, D.; Rosh, J.; Leleiko, N.; Mack, D.; Griffiths, A.; Bousvaros, A.; et al. Body mass index in children with newly diagnosed inflammatory bowel disease: Observations from two multicenter North American inception cohorts. J. Pediatr. 2007, 151, 523–527. [Google Scholar] [CrossRef]
- Pituch-Zdanowska, A.; Banaszkiewicz, A.; Dziekiewicz, M.; Łazowska-Przeorek, I.; Gawrońska, A.; Kowalska-Duplaga, K.; Iwańczak, B.; Klincewicz, B.; Grzybowska-Chlebowczyk, U.; Walkowiak, J.; et al. Overweight and obesity in children with newly diagnosed inflammatory bowel disease. Adv. Med. Sci. 2016, 61, 28–31. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Ben-Tov, A.; Weintraub, Y.; Amir, A.; Galai, T.; Moran-Lev, H.; Cohen, S. High and low body mass index may predict severe disease course in children with inflammatory bowel disease. Scand. J. Gastroenterol. 2018, 53, 708–713. [Google Scholar] [CrossRef]
- Long, M.D.; Crandall, W.V.; Leibowitz, I.H.; Duffy, L.; Del Rosario, F.; Kim, S.C.; Integlia, M.J.; Berman, J.; Grunow, J.; Colletti, R.B.; et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 2162–2168. [Google Scholar] [CrossRef]
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708. [Google Scholar] [CrossRef]
- Medina Carbonell, F.R.; Choyudhry Chandan, O. Body Mass Index at Presentation of Inflammatory Bowel Disease in Children. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 439–446. [Google Scholar] [CrossRef]
- Kadenczki, O.; Nagy, A.C.; Kiss, C. Prevalence of Undernutrition and Effect of Body Weight Loss on Survival among Pediatric Cancer Patients in Northeastern Hungary. Int. J. Environ. Res. Public Health 2021, 18, 1478. [Google Scholar] [CrossRef]
- Sullivan, J.S.; Mascarenhas, M.R. Nutrition: Prevention and management of nutritional failure in Cystic Fibrosis. J. Cyst. Fibros. 2017, 16 (Suppl. 2), S87–S93. [Google Scholar] [CrossRef]
- Yang, C.H.; Perumpail, B.J.; Yoo, E.R.; Ahmed, A.; Kerner, J.A., Jr. Nutritional Needs and Support for Children with Chronic Liver Disease. Nutrients 2017, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- RRoss, E.L.; Heizer, J.; Mixon, M.A.; Jorgensen, J.; Valdez, C.A.; Czaja, A.S.; Reiter, P.D. Development of recommendations for dosing of commonly prescribed medications in critically ill obese children. Am. J. Health Syst. Pharm. 2015, 72, 542–556. [Google Scholar] [CrossRef]
- Müller, K.E.; Lakatos, P.L.; Arató, A.; Kovács, J.B.; Várkonyi, A.; Szűcs, D.; Szakos, E.; Sólyom, E.; Kovács, M.; Polgár, M.; et al. Incidence, Paris classification, and follow-up in a nationwide incident cohort of pediatric patients with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Veres, G. A gyermekkori gyulladásos bélbetegségek hazai regiszterének (HUPIR) eredményei és az 5 éves nyomonkövetés hatása a diagnosztikai gyakorlatra. Magy. Belorv. Archivum. 2013, 66, 215–222. [Google Scholar]
- IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition. Inflammatory bowel disease in children and adolescents: Recommendations for diagnosis—The Porto criteria. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 1–7. [Google Scholar] [CrossRef]
- Joubert, K.G. The Hungarian Longitudinal Growth Study: From Birth to the Age of 18 Years; Working Papers on Population, Family and Welfare No. 23; Hungarian Demographic Research Institute: Budapest, Hungary, 2016. [Google Scholar]
- World Obesity Federation. Available online: https://www.worldobesity.org (accessed on 9 October 2022).
- Borowitz, D.; Baker, R.D.; Stallings, V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 246–259. [Google Scholar] [CrossRef]
- Turner, D.; Otley, A.R.; Mack, D.; Hyams, J.; De Bruijne, J.; Uusoue, K.; Walters, T.D.; Zachos, M.; Mamula, P.; Beaton, D.E.; et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology 2007, 133, 423–432. [Google Scholar] [CrossRef]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 439–447. [Google Scholar] [CrossRef]
- Sýkora, J.; Pomahačová, R.; Kreslová, M.; Cvalínová, D.; Štych, P.; Schwarz, J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 2741–2763. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 110–121. [Google Scholar] [CrossRef]
- Sila, S.; Trivić, I.; Pavić, A.M.; Niseteo, T.; Kolaček, S.; Hojsak, I. Nutritional status and food intake in pediatric patients with inflammatory bowel disease at diagnosis significantly differs from healthy controls. Eur. J. Pediatr. 2019, 178, 1519–1527. [Google Scholar] [CrossRef]
- Chandrakumar, A.; Wang, A.; Grover, K.; El-Matary, W. Obesity Is More Common in Children Newly Diagnosed with Ulcerative Colitis as Compared to Those with Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 593–597. [Google Scholar] [CrossRef]
- Karaskova, E.; Velganova-Veghova, M.; Geryk, M.; Foltenova, H.; Kucerova, V.; Karasek, D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 4226. [Google Scholar] [CrossRef]
- Dickson, I. Creeping fat in Crohn’s disease explained. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 713. [Google Scholar] [CrossRef]
- Schäffler, A.; Herfarth, H. Creeping fat in Crohn’s disease: Travelling in a creeper lane of research? Gut 2005, 54, 742–744. [Google Scholar] [CrossRef]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef]
- Kredel, L.I.; Siegmund, B. Adipose-tissue and intestinal inflammation—Visceral obesity and creeping fat. Front. Immunol. 2014, 5, 462. [Google Scholar] [CrossRef]
- Krebs, N.F.; Himes, J.H.; Jacobson, D.; Nicklas, T.A.; Guilday, P.; Styne, D. Assessment of child and adolescent overweight and obesity. Pediatrics 2007, 120 (Suppl. 4), S193–S228. [Google Scholar] [CrossRef]
- Yerushalmy-Feler, A.; Galai, T.; Moran-Lev, H.; Ben-Tov, A.; Dali-Levy, M.; Weintraub, Y.; Amir, A.; Cohen, S. BMI in the lower and upper quartiles at diagnosis and at 1-year follow-up is significantly associated with higher risk of disease exacerbation in pediatric inflammatory bowel disease. Eur. J. Pediatr. 2021, 180, 21–29. [Google Scholar] [CrossRef]
- Selbuz, S.; Kansu, A.; Berberoğlu, M.; Şıklar, Z.; Kuloğlu, Z. Nutritional status and body composition in children with inflammatory bowel disease: A prospective, controlled, and longitudinal study. Eur. J. Clin. Nutr. 2020, 74, 1173–1180. [Google Scholar] [CrossRef]
- Von Graffenried, T.; Schoepfer, A.M.; Rossel, J.B.; Greuter, T.; Safroneeva, E.; Godat, S.; Henchoz, S.; Vavricka, S.R.; Sokollik, C.; Spalinger, J.; et al. Impact of Overweight and Obesity on Disease Outcome in the Pediatric Swiss Inflammatory Bowel Disease Cohort. JPGN Rep. 2022, 3, e193. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The Therapeutic Role of Short-Chain Fatty Acids Mediated Very Low-Calorie Ketogenic Diet-Gut Microbiota Relationships in Paediatric Inflammatory Bowel Diseases. Nutrients 2022, 14, 4113. [Google Scholar] [CrossRef]

| CD (N = 699) 66.06% | UC (N = 328) 31.94% | |
|---|---|---|
| Age, mean (±SD) (years) | 13.7 (±3.19) | 12.6 (±3.73) |
| Age, median (range) (years) | 14.4 (1.1–18.0) | 13.4 (1.6–18.0) |
| Sex | ||
| Male, n (%) | 407 (58.22) | 170 (51.82) |
| Female, n (%) | 292 (41.77) | 158 (48.17) |
| PCDAI/PUCAI | (N = 677) | (N = 313) |
| Inactive disease | 30 (4.44) | 0 |
| Mild n (%) | 343 (50.66) | 143 (45.68) |
| Moderate n (%) | 155 (22.90) | 140 (44.72) |
| Severe n (%) | 149 (22.00) | 30 (9.60) |
| Anthropometric Parameter | CD N (%) | UC N (%) |
|---|---|---|
| Body weight | 699 (100) | 328 (100) |
| Undernourished (Z-score < −2) | 36 (5.16) | 2 (0.60) |
| Normal (−2 < Z-score < 2) | 644 (92.13) | 305 (92.98) |
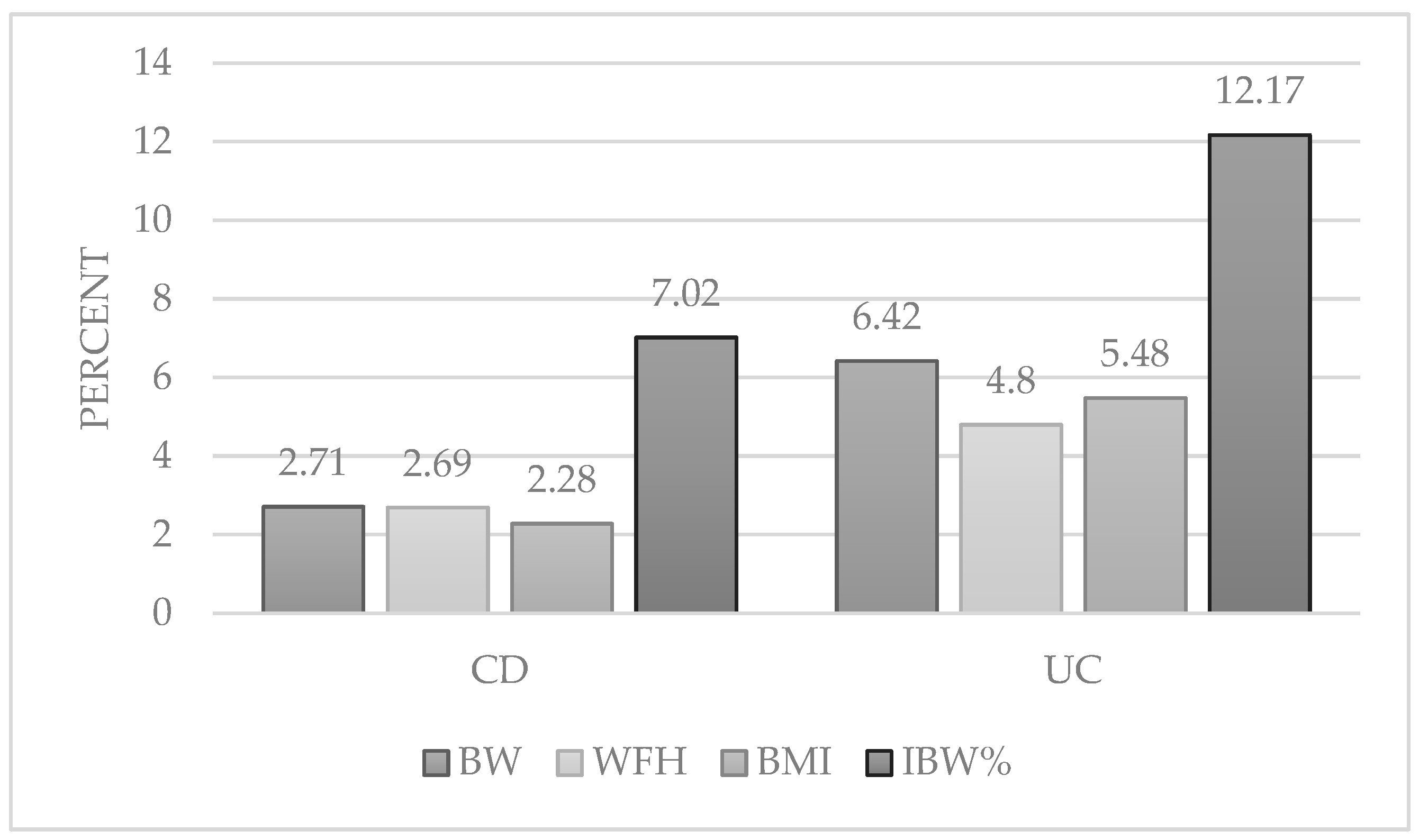
| Obese (Z-score > 2) | 19 (2.71) | 21 (6.42) |
| Body mass index | 699 (100%) | 328 (100%) |
| Undernourished (Z-score < −2) | 19 (2.70) | 6 (1.83) |
| Normal (−2< Z-score < 2) | 664 (94.99) | 304 (92.67) |
| Obese (Z-score > 2) | 16 (2.28) | 18 (5.48) |
| Weight-for-height | 669 (100%) | 312 (100%) |
| Undernourished (Z-score < −2) | 29 (4.34) | 7 (2.25) |
| Normal (−2 < Z-score < 2) | 622 (92.97) | 290 (92.95) |
| Obese (Z-score > 2) | 18 (2.69) | 15 (4.80) |
| Ideal body weight percent | 669 (100%) | 312 (100%) |
| <70 | 22 (3.28) | 2 (0.64) |
| 70–80 | 127 (18.90) | 24 (7.70) |
| 80–90 | 188 (28.30) | 79 (25.33) |
| 90–110 | 236 (35.20) | 141 (45.19) |
| 110–120 | 49 (7.32) | 28 (8.97) |
| >120 | 47 (7.02) | 38 (12.17) |
| Anthropometric Parameter | AI of Undernourished vs. Normal | p | AI of Normal vs. Obese | p | AI of Undernourished vs. Obese | p | |
|---|---|---|---|---|---|---|---|
| BW | CD | 38.63 vs. 30.5 | 0.06 | 30.50 vs. 18.67 | <0.01 | 38.63 vs. 18.67 | <0.01 |
| WFH | 45.00 vs. 31.13 | <0.01 | 31.13 vs. 18.92 | 0.022 | 45.00 vs. 18.92 | <0.01 | |
| BMI | 42.15 vs. 30.48 | 0.002 | 30.48 vs. 22.94 | 0.019 | 42.15 vs. 22.94 | <.0.01 | |
| IBW% | 43.29 vs. 30.60 | <0.01 | 30.60 vs. 27.76 | 0.01 | 43.29 vs. 27.76 | <0.01 | |
| BW | UC | 57.50 vs. 38.12 | 0.330 | 38.12 vs. 44.25 | 0.203 | 57.50 vs. 44.25 | 0.554 |
| WFH | 54.44 vs. 38.32 | 0.015 | 38.32 vs. 45.76 | 0.180 | 54..4 vs. 45.76 | 0.267 | |
| BMI | 54.60 vs. 37.63 | 0.032 | 37.63 vs. 44.66 | 0.211 | 54.60 vs. 44.66 | 0.336 | |
| IBW% | 52.50 vs. 37.74 | 0.213 | 37.74 vs. 41.66 | 0.243 | 52.50 vs. 41.66 | 0.444 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadenczki, O.; Dezsofi, A.; Cseh, A.; Szucs, D.; Vass, N.; Nemes, E.; Tarnok, A.; Szakos, E.; Guthy, I.; Kovacs, M.; et al. Disease Activity Is Associated with Obesity in Newly Diagnosed Pediatric Patients with Ulcerative Colitis. Int. J. Environ. Res. Public Health 2022, 19, 16091. https://doi.org/10.3390/ijerph192316091
Kadenczki O, Dezsofi A, Cseh A, Szucs D, Vass N, Nemes E, Tarnok A, Szakos E, Guthy I, Kovacs M, et al. Disease Activity Is Associated with Obesity in Newly Diagnosed Pediatric Patients with Ulcerative Colitis. International Journal of Environmental Research and Public Health. 2022; 19(23):16091. https://doi.org/10.3390/ijerph192316091
Chicago/Turabian StyleKadenczki, Orsolya, Antal Dezsofi, Aron Cseh, Daniel Szucs, Noemi Vass, Eva Nemes, Andras Tarnok, Erzsebet Szakos, Ildiko Guthy, Marta Kovacs, and et al. 2022. "Disease Activity Is Associated with Obesity in Newly Diagnosed Pediatric Patients with Ulcerative Colitis" International Journal of Environmental Research and Public Health 19, no. 23: 16091. https://doi.org/10.3390/ijerph192316091
APA StyleKadenczki, O., Dezsofi, A., Cseh, A., Szucs, D., Vass, N., Nemes, E., Tarnok, A., Szakos, E., Guthy, I., Kovacs, M., Karoliny, A., Czelecz, J., Kiss, C., & Müller, K. E. (2022). Disease Activity Is Associated with Obesity in Newly Diagnosed Pediatric Patients with Ulcerative Colitis. International Journal of Environmental Research and Public Health, 19(23), 16091. https://doi.org/10.3390/ijerph192316091






