Hypocalcemia Is a Common Risk Factor for Osteoporosis in Taiwanese Patients with Cushing’s Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
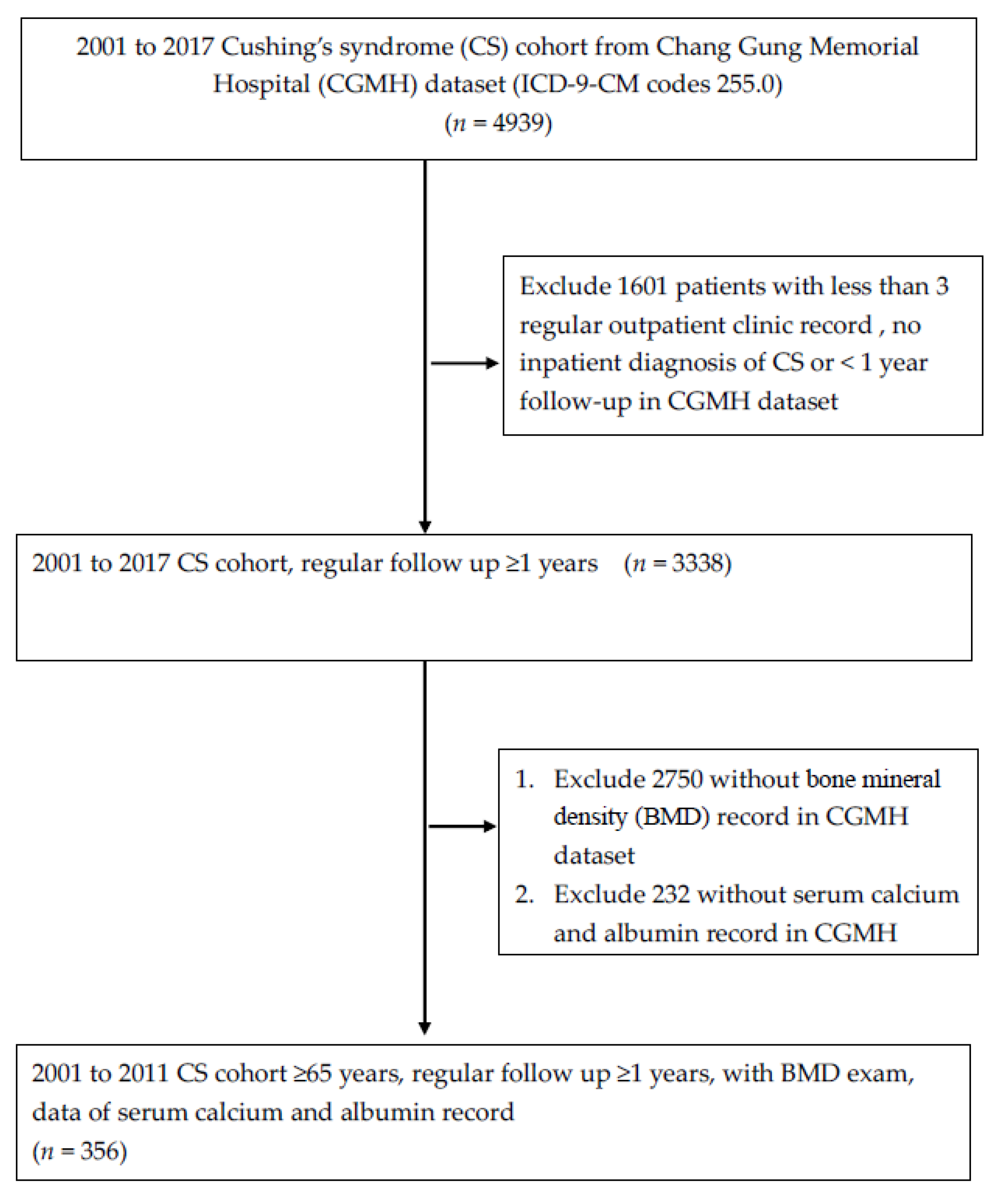
2.2. Selection of Patients with Cushing’s Syndrome
2.3. Definition of Steoporosis and Hypocalcemia
2.4. Statistical Analysis
3. Results
3.1. Study Process Flowchart
3.2. Different Characteristics and Comorbidities between Patients with and without Osteoporosis
3.3. Different Characteristics and Comorbidities between Patients with and without Heart Failure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.T.; Nieman, L.K.; Feelders, R.A. Cushing’s syndrome: Epidemiology and developments in disease management. Clin. Epidemiol. 2015, 7, 281–293. [Google Scholar] [PubMed]
- Liou, T.C.; Lam, H.C.; Ho, L.T. Cushing’s syndrome: Analysis of 188 cases. Taiwan Yi Xue Hui Za Zhi 1989, 88, 886–893. [Google Scholar] [PubMed]
- Su, D.H.; Chang, Y.C.; Chang, T.C.; Chang, C.C.; Tsai, K.S.; Huang, T.S. Characteristics of Cushing’s syndrome in Taiwanese. J. Formos. Med. Assoc. 2003, 102, 292–298. [Google Scholar] [PubMed]
- Tung, S.C.; Wang, P.W.; Liu, R.T.; Chen, J.F.; Hsieh, C.J.; Kuo, M.C.; Yang, J.W.; Lee, W.C.; Cheng, M.H.; Lee, T.C. Clinical Characteristics of Endogenous Cushing’s Syndrome at a Medical Center in Southern Taiwan. Int. J. Endocrinol. 2013, 2013, 685375. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, G.; Makras, P. Skeletal diseases in Cushing’s syndrome: Osteoporosis versus arthropathy. Neuroendocrinology 2010, 92 (Suppl. S1), 60–64. [Google Scholar] [CrossRef]
- Valassi, E.; Santos, A.; Yaneva, M.; Toth, M.; Strasburger, C.J.; Chanson, P.; Wass, J.A.; Chabre, O.; Pfeifer, M.; Feelders, R.A.; et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur. J. Endocrinol. 2011, 165, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.V.; Vieira Neto, L.; Madeira, M.; Alves Coelho, M.C.; de Mendonca, L.M.; Paranhos-Neto Fde, P.; Lima, I.C.B.; Gadelha, M.R.; Farias, M.L.F. Bone density and microarchitecture in endogenous hypercortisolism. Clin. Endocrinol. 2015, 83, 468–474. [Google Scholar] [CrossRef]
- Tauchmanova, L.; Pivonello, R.; Di Somma, C.; Rossi, R.; De Martino, M.C.; Camera, L.; Klain, M.; Salvatore, M.; Lombardi, G.; Colao, A. Bone demineralization and vertebral fractures in endogenous cortisol excess: Role of disease etiology and gonadal status. J. Clin. Endocrinol. Metab. 2006, 91, 1779–1784. [Google Scholar] [CrossRef]
- Kawamata, A.; Iihara, M.; Okamoto, T.; Obara, T. Bone mineral density before and after surgical cure of Cushing’s syndrome due to adrenocortical adenoma: Prospective study. World J. Surg. 2008, 32, 890–896. [Google Scholar] [CrossRef]
- Vestergaard, P.; Lindholm, J.; Jorgensen, J.O.; Hagen, C.; Hoeck, H.C.; Laurberg, P.; Rejnmark, L.; Brixen, K.; Kristensen, L.O.; Feldt-Rasmussen, U.; et al. Increased risk of osteoporotic fractures in patients with Cushing’s syndrome. Eur. J. Endocrinol. 2002, 146, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tomita, A. Glucocorticoid-induced osteoporosis--mechanisms and preventions. Nihon Rinsho 1998, 56, 1574–1578. [Google Scholar] [PubMed]
- Guo, W.; Li, F.; Zhu, C.; Wang, B.; Wang, K.; Dai, C.; Jia, H.; Wei, H.; He, Q.; Cui, J.; et al. Effect of hypercortisolism on bone mineral density and bone metabolism: A potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J. Int. Med. Res. 2018, 46, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.R.; Lyles, K.W. Glucocorticoid-induced osteoporosis: Mechanisms for bone loss; evaluation of strategies for prevention. J. Gerontol. 1990, 45, M153–M158. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Huybers, S.; Naber, T.H.; Bindels, R.J.; Hoenderop, J.G. Prednisolone-induced Ca2+ malabsorption is caused by diminished expression of the epithelial Ca2+ channel TRPV6. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G92–G97. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Recent developments in intestinal calcium absorption. Nutr. Rev. 2009, 67, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, G.; Angeli, A.; Bilezikian, J.P.; Canalis, E.; Giustina, A. Glucocorticoid-induced osteoporosis: An update. Trends Endocrinol. Metab. 2006, 17, 144–149. [Google Scholar] [CrossRef]
- Manelli, F.; Giustina, A. Glucocorticoid-induced osteoporosis. Trends Endocrinol. Metab. 2000, 11, 79–85. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.R.; Mosekilde, L.; Foldspang, A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: A pragmatic population-based 3-year intervention study. J. Bone Miner. Res. 2004, 19, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016, 27, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Szent-Gyorgyi, A.G. Calcium regulation of muscle contraction. Biophys. J. 1975, 15, 707–723. [Google Scholar] [CrossRef]
- Valek, M.; Roblova, L.; Raska, I., Jr.; Schaffelhoferova, D.; Palecek, T. Hypocalcaemic cardiomyopathy: A description of two cases and a literature review. ESC Heart Fail. 2020, 7, 1291–1301. [Google Scholar] [CrossRef]
- Gu, Z.; Yuanyuan, Y.; Lingyu, Z.; Cong, C. Assessment of the risk of incident heart failure in patients with osteoporosis: A systematic review and meta-analysis of eligible cohort studies. Pol. Arch. Intern. Med. 2020, 130, 934–941. [Google Scholar] [CrossRef]
- Dasgupta, Y.; Golovine, K.; Nieborowska-Skorska, M.; Luo, L.; Matlawska-Wasowska, K.; Mullighan, C.G.; Skorski, T. Drugging DNA repair to target T-ALL cells. Leuk. Lymphoma 2018, 59, 1746–1749. [Google Scholar] [CrossRef]
- Yu, T.M.; Lin, C.L.; Shu, K.H.; Liu, Y.L.; Chen, C.H.; Huang, S.T.; Kao, C.H. Increased risk of cardiovascular events in end-stage renal disease patients with osteoporosis: A nationwide population-based cohort study. Osteoporos. Int. 2015, 26, 785–793. [Google Scholar] [CrossRef]
- Swann, W.B.; Jetten, J. Identity fusion ”in the wild”: Moving toward or away from a general theory of identity fusion? Behav. Brain Sci. 2018, 41, e218. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Berghout, B.P.; Fani, L.; Heshmatollah, A.; Koudstaal, P.J.; Ikram, M.A.; Zillikens, M.C.; Ikram, M.K. Vitamin D Status and Risk of Stroke: The Rotterdam Study. Stroke 2019, 50, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Huang, T.S.; Fu, T.S.; Sun, C.C.; Chao, A.S.; Tsai, T.L. Secular trends in incidence of osteoporosis in Taiwan: A nationwide population-based study. Biomed. J. 2018, 41, 314–320. [Google Scholar] [CrossRef] [PubMed]
| All Patients | Without Osteoporosis | With Osteoporosis | p Value | |
|---|---|---|---|---|
| N | 356 | 149 | 207 | |
| Age (years) | 68.6 ± 12.6 | 63.9 ± 11.3 | 72.2 ± 11.9 | <0.001 |
| Male | 90 (25.1) | 55 (36.7) | 35 (16.8) | <0.001 |
| Smoking | 21 (5.9) | 11 (7.3) | 10 (4.8) | 0.356 |
| Alcohol | 6 (1.7) | 3 (2.0) | 3 (1.4) | 0.698 |
| BMI (kg/m2) | 25.8 ± 5.1 | 26.6 ± 5.0 | 25.3 ± 5.0 | 0.022 |
| Creatinine (mg/dL) | 0.91 (0.69, 1.39) | 0.88 (0.70, 1.37) | 0.95 (0.66, 1.50) | 1.000 |
| eGFR (mg/mL/1.73 m2) | 70.7 ± 44.7 | 71.7 ± 35.3 | 70.0 ± 50.4 | 0.713 |
| AST (U/L) | 26.0 (20.0, 35.0) | 26.0 (20.0, 35.0) | 26.0 (21.0, 34.8) | 0.236 |
| ALT (U/L) | 21.0 (14.0, 32.0) | 22.0 (16.8, 34.2) | 20.0 (13.0, 31.0) | 0.607 |
| Calcium (mg/dL) | 9.1 ± 1.0 | 9.3 ± 0.8 | 9.0 ± 1.0 | <0.001 |
| Hypocalcemia | 47 (13.1) | 8 (5.3) | 39 (18.8) | <0.001 |
| Diabetes | 204 (57.0) | 81 (54.0) | 123 (59.1) | 0.387 |
| Hypertension | 277 (77.4) | 111 (74.0) | 166 (79.8) | 0.203 |
| Chronic kidney disease | 75 (20.9) | 32 (21.3) | 43 (20.7) | 0.896 |
| Stroke | 69 (19.3) | 22 (14.7) | 47 (22.6) | 0.077 |
| Peripheral artery disease | 9 (2.5) | 1 (0.7) | 8 (3.8) | 0.086 |
| Coronary artery disease | 44 (12.3) | 19 (12.7) | 25 (12.0) | 0.872 |
| Heart failure | 73 (20.4) | 22 (14.7) | 51 (24.5) | 0.024 |
| Hyperparathyroidism | 46 (12.8) | 20 (13.3) | 26 (12.5) | 0.873 |
| Hyperthyroidism | 1 (0.3) | 0 (0.0) | 1 (0.5) | 1.000 |
| Hypogonadism | 28 (7.8) | 9 (6.0) | 19 (9.1) | 0.322 |
| Univariate Analysis | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| BMI | 0.95 (0.91–0.99) * | 0.96 (0.92–1.00) | 0.96 (0.92–1.00) | 0.96 (0.92–1.01) |
| Calcium | 0.67 (0.53–0.84) & | 0.70 (0.55–0.90) & | 0.68 (0.53–0.89) & | 0.70 (0.54–0.91) & |
| Heart failure | 1.89 (1.09–3.28) * | 1.37 (0.75–2.50) | 1.62 (1.86–3.05) | 1.47 (0.77–2.81) |
| Normocalcemia (n = 311) | Hypocalcemia (n = 47) | p Value | |
|---|---|---|---|
| Age (years) | 67.9 ± 12.6 | 73.1 ± 11.8 | 0.008 |
| Male | 84 (27.0) | 6 (12.8) | 0.036 |
| Smoking | 20 (6.4) | 1 (2.1) | 0.334 |
| Alcohol | 5 (1.6) | 1 (2.1) | 0.573 |
| BMI (kg/m2) | 26.1 ± 5.0 | 24.3 ± 5.4 | 0.027 |
| Creatinine (mg/dL) | 0.88 (0.69, 1.28) | 1.52 (0.85, 2.94) | <0.001 |
| eGFR (mg/mL/1.73 m2) | 70.0 (48.8, 95.8) | 41.9 (15.2, 75.2) | 0.002 |
| AST (U/L) | 26.0 (21.0, 35.3) | 26.0 (19.0, 33.0) | 0.188 |
| ALT (U/L) | 21.0 (14.0, 33.0) | 20.0 (15.0, 29.0) | 0.146 |
| Calcium (mg/dL) | 9.4 ± 0.8 | 7.5 ± 0.5 | <0.001 |
| Diabetes | 169 (54.3) | 35 (74.5) | 0.011 |
| Hypertension | 233 (74.9) | 44 (93.6) | 0.003 |
| Chronic kidney disease | 58 (18.6) | 17 (36.2) | 0.011 |
| Stroke | 50 (16.1) | 19 (40.4) | <0.001 |
| Peripheral artery disease | 6 (1.9) | 3 (6.4) | 0.101 |
| Coronary artery disease | 35 (11.3) | 9 (19.1) | 0.150 |
| Heart failure | 54 (17.4) | 19 (40.5) | 0.001 |
| Osteoporosis | 169 (54.3) | 39 (83.0) | <0.001 |
| Hyperparathyroidism | 34 (10.9) | 12 (25.5) | 0.009 |
| Hyperthyroidism | 1 (0.3) | 0 (0.0) | 1.000 |
| Hypogonadism | 27 (8.3) | 1 (2.1) | 0.150 |
| Univariate Analysis | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| BMI | 0.93 (0.97–0.99) * | 0.93 (0.87–1.00) | 0.94 (0.87–1.01) | 0.95 (0.88–1.02) |
| eGFR | 0.99 (0.98–0.99) & | 0.99 (0.98–0.99) & | 0.99 (0.98–0.99) * | 0.99 (0.98–1.00) |
| Diabetes | 2.45 (1.23–4.90) * | 2.41 (1.19–4.87) * | 2.15 (1.05–4.41) * | 1.55 (0.70–3.42) |
| Hypertension | 4.91 (1.48–16.26) & | 4.63 (1.39–15.44) * | 4.44 (1.32–14.9) * | 2.77 (0.77–10.05) |
| Stroke | 3.54 (1.84–6.83) & | 3.10 (1.57–6.14) & | 3.11 (1.55–6.25) & | 2.58 (1.21–5.46) * |
| Heart failure | 3.23 (1.68–6.20) & | 2.71 (1.39–5.28) & | 2.50 (1.25–5.00) * | 2.14 (1.02–4.47) * |
| Osteoporosis | 4.10 (1.85–9.05) & | 2.99 (1.29–6.93) & | 3.05 (1.31–7.12) * | 3.04 (1.24–7.41) * |
| Hyperparathyroidism | 2.79 (1.33–5.89) & | 3.04 (1.41–6.58) & | 2.83 (1.25–6.38) * | 2.00 (0.80–5.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-N.; Tsai, J.-R.; Chen, J.-F.; Shen, F.-C. Hypocalcemia Is a Common Risk Factor for Osteoporosis in Taiwanese Patients with Cushing’s Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 16064. https://doi.org/10.3390/ijerph192316064
Chen Y-N, Tsai J-R, Chen J-F, Shen F-C. Hypocalcemia Is a Common Risk Factor for Osteoporosis in Taiwanese Patients with Cushing’s Syndrome. International Journal of Environmental Research and Public Health. 2022; 19(23):16064. https://doi.org/10.3390/ijerph192316064
Chicago/Turabian StyleChen, Yung-Nien, Jia-Ruei Tsai, Jung-Fu Chen, and Feng-Chih Shen. 2022. "Hypocalcemia Is a Common Risk Factor for Osteoporosis in Taiwanese Patients with Cushing’s Syndrome" International Journal of Environmental Research and Public Health 19, no. 23: 16064. https://doi.org/10.3390/ijerph192316064
APA StyleChen, Y.-N., Tsai, J.-R., Chen, J.-F., & Shen, F.-C. (2022). Hypocalcemia Is a Common Risk Factor for Osteoporosis in Taiwanese Patients with Cushing’s Syndrome. International Journal of Environmental Research and Public Health, 19(23), 16064. https://doi.org/10.3390/ijerph192316064






