Carbothermal Synthesis of Sludge Biochar Supported Nanoscale Zero-Valent Iron for the Removal of Cd2+ and Cu2+: Preparation, Performance, and Safety Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Materials
2.3. Characterization of SB and SB-NZVI
2.4. Batch Removal Experiments
2.4.1. Single Heavy Metal Removal
2.4.2. Simultaneous Removal of Heavy Metals
2.5. Environmental Safety Risk Analysis
2.5.1. Speciation Analysis of Heavy Metals
2.5.2. Heavy Metal Leaching Test
3. Results and Discussion
3.1. Characterization of SB and SB-NZVI
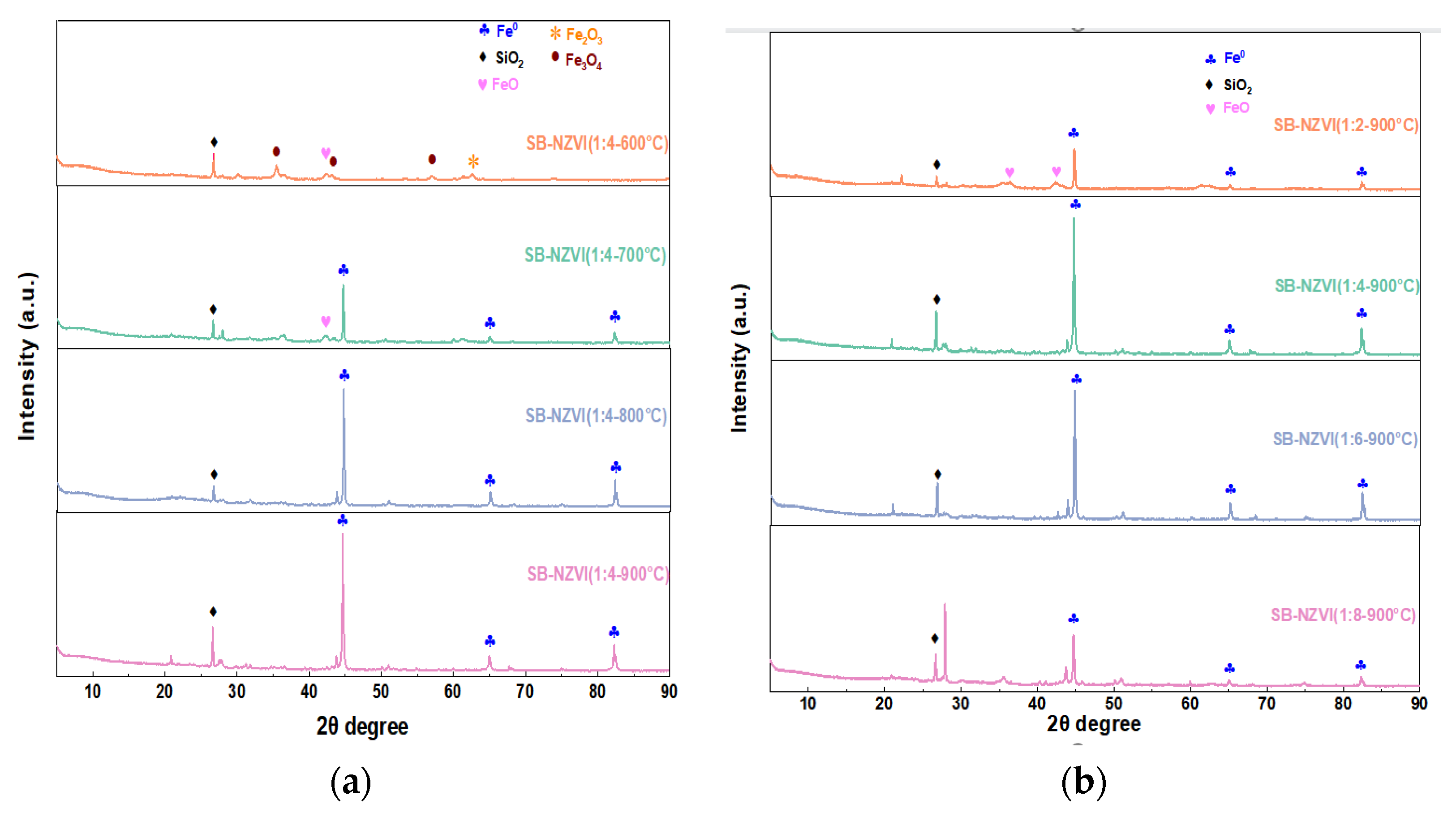
3.1.1. XRD Analysis
3.1.2. SEM Analysis
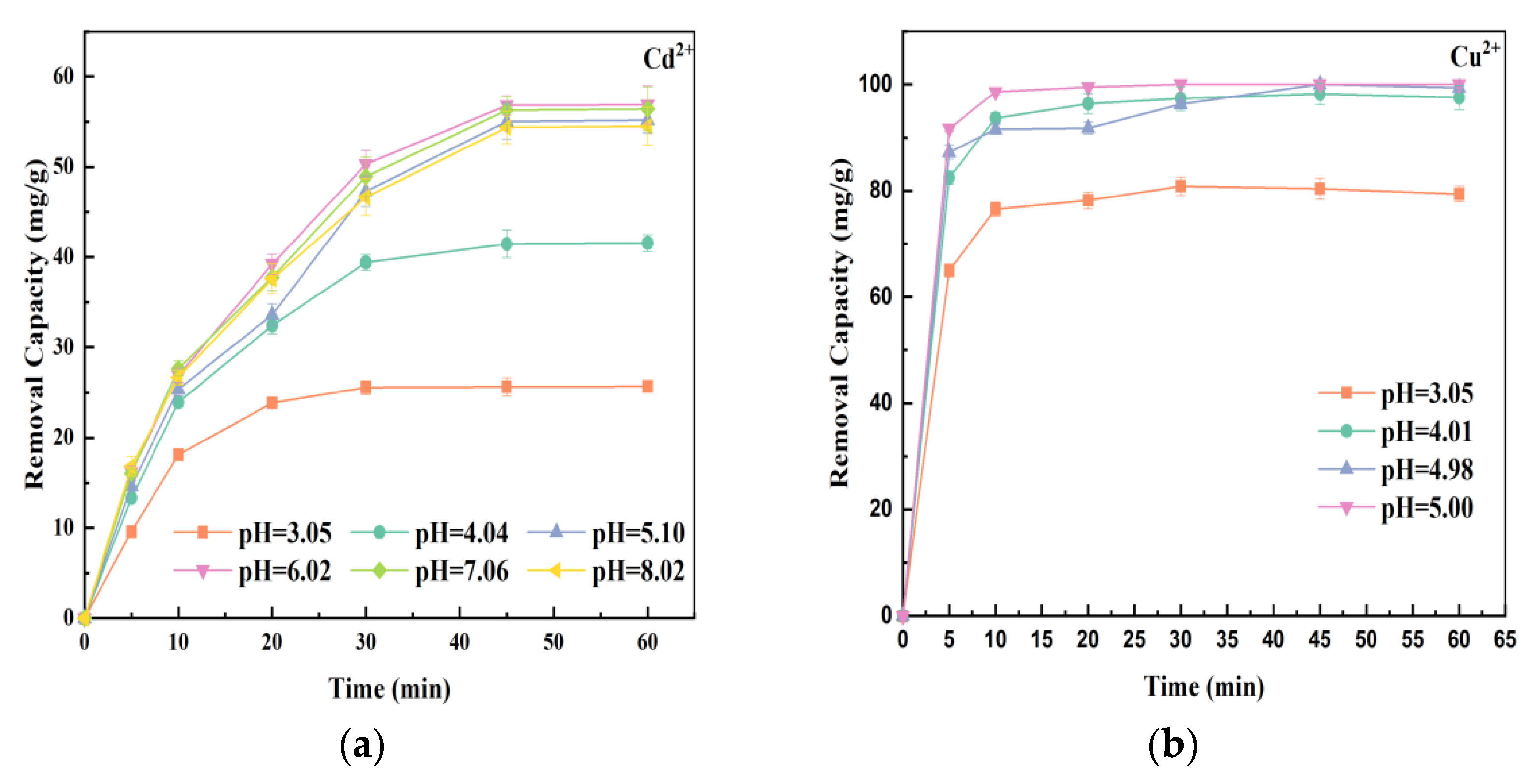
3.2. The Effect of Preparation Temperature on Heavy Metals Removal
3.3. The Effect of Mass Ratios of Fe to Sludge on Heavy Metals Removal
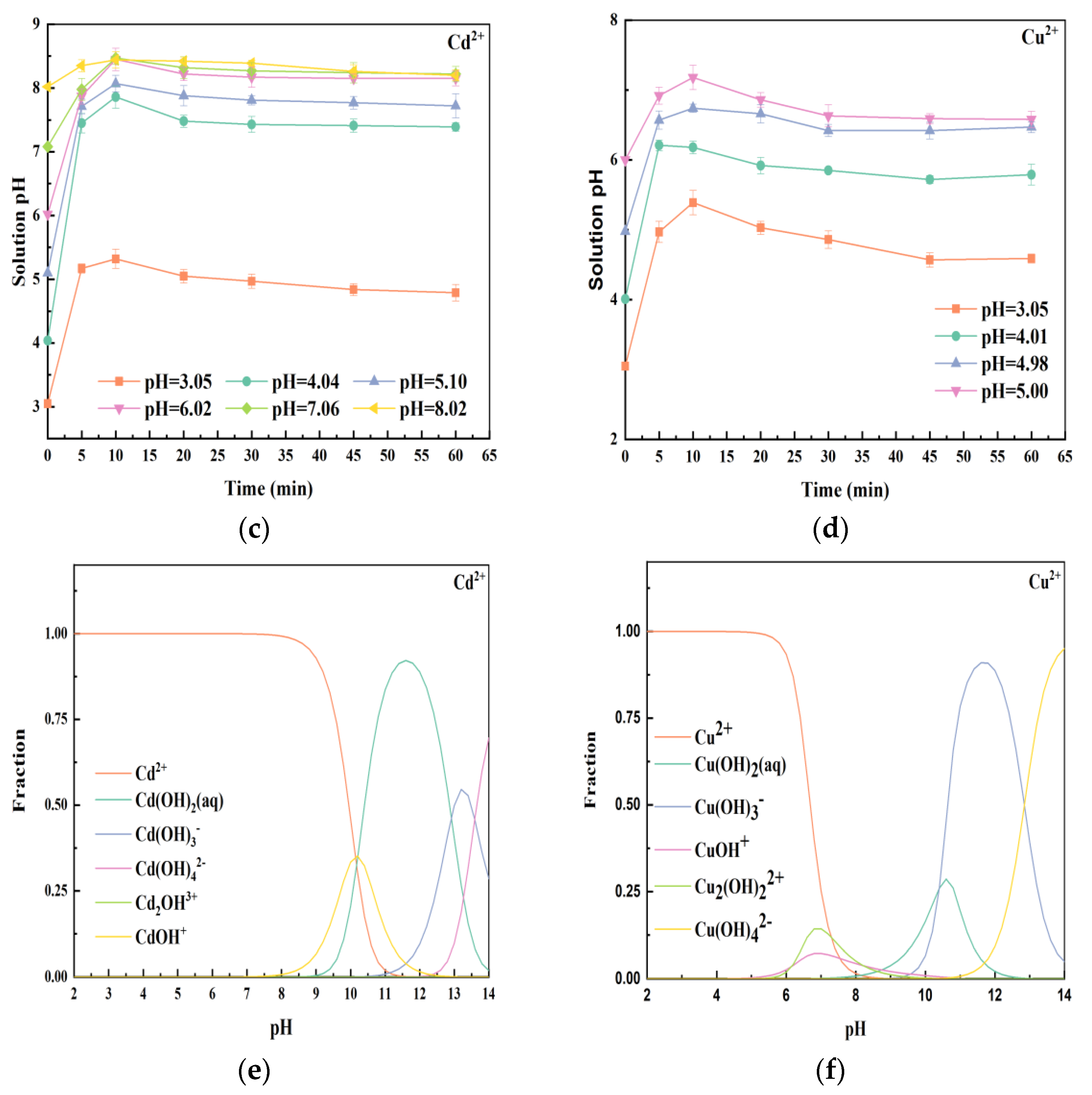
3.4. The Effect of Initial Solution pH on Heavy Metals Removal
3.5. Simultaneous Removal of Cd2+ and Cu2+
3.6. Mechanisms for Heavy Metals Removal
3.7. Environmental Safety Risk Analysis of SB-NZVI
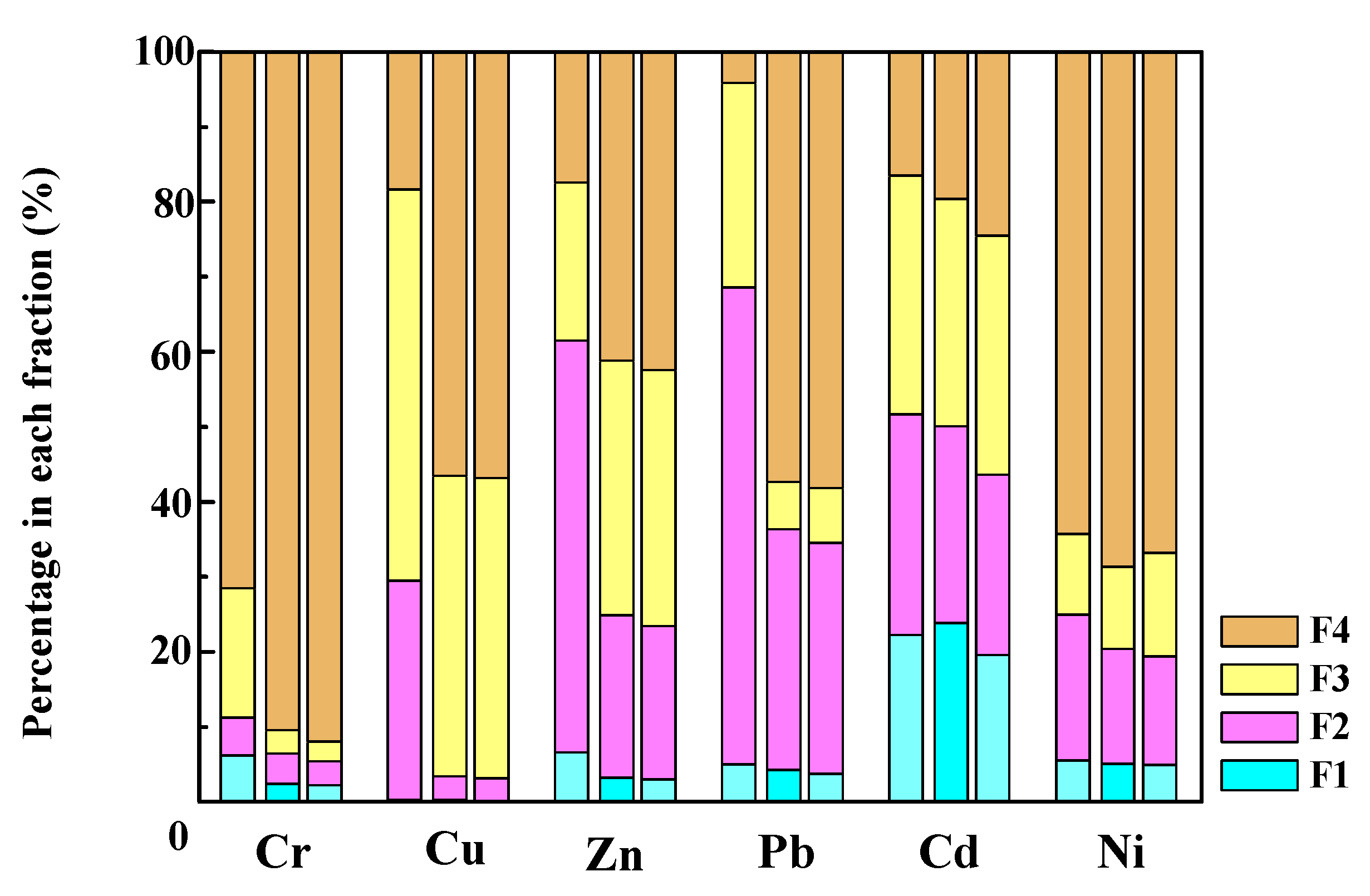
3.7.1. Speciation Analysis of Heavy Metals
3.7.2. Heavy Metal Leaching Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, F.; Du, Q.; Sui, L.; Cheng, K. One-step fabrication of artificial humic acid-functionalized colloid-like magnetic biochar for rapid heavy metal removal. Bioresour. Technol. 2021, 328, 124825. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.X.; Wang, Q.; Li, X.Q.; Chen, Z.Q.; Tang, Y.C. Enhanced organics and Cu2+ removal in electroplating wastewater by bioaugmentation. Chemosphere 2018, 212, 476–485. [Google Scholar] [CrossRef]
- Gayathri, R.; Gopinath, K.P.; Kumar, P.S. Adsorptive Separation of Toxic Metals from Aquatic Environment using Agro waste Biochar: Application in Electroplating Industrial Wastewater. Chemosphere 2020, 262, 128031. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yu, S.H.; Jiang, H.F.; Yao, Q.Z.; Fu, S.Q.; Zhou, G.T. Performance and mechanism of simultaneous removal of Cd(II) and Congo red from aqueous solution by hierarchical vaterite spherulites. Appl. Surf. Sci. 2018, 444, 224–234. [Google Scholar] [CrossRef]
- Kong, W.J.; Yue, Q.Y.; Li, Q.; Gao, B.Y. Adsorption of Cd2+ on GO/PAA hydrogel and preliminary recycle to GO/PAA-CdS as efficient photocatalyst. Sci. Total Environ. 2019, 668, 1165–1174. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Wu, L.; Zhang, Z. Highly efficient nickel (II) removal by sewage sludge biochar supported α-Fe2O3 and α-FeOOH: Sorption characteristics and mechanisms. PLoS ONE 2019, 14, e0218114. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Jugdaohsingh, R.; Matovic, V.; Bulat, Z.; Powell, J.J. Bone mineral health relates to environmental cadmium exposure- experimental and human data. Environ. Res. 2019, 176, 108539. [Google Scholar] [CrossRef]
- Gao, Y.; Kaziem, A.E.; Zhang, Y.; Xiao, Y.; Li, J. A hollow mesoporous silica and poly(diacetone acrylamide) composite with sustained-release and adhesion properties. Microporous Mesoporous Mater. 2017, 255, 15–22. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, C.; Sun, Y.C.; Zhang, Q.; Lv, M.W.; Guo, K.; Li, J.L. Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci. Total Environ. 2019, 689, 1160–1171. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Tan, Y.H.; Khalid, M.; Karri, R.R.; Walvekar, R.; Abdullah, E.C.; Nizamuddin, S.; Mazari, S.A. A comprehensive review on magnetic carbon nanotubes and carbon nanotube-based buckypaper for trmoval of heavy metals and dyes. J. Hazard Mater. 2021, 413, 125375. [Google Scholar] [CrossRef]
- Sato, A.; Takeda, R.; Oyanagi, R.; Nishihara, R.; Murakami, R. Reduction of cadmium uptake in spinach (Spinacia oleracea L.) by soil amendment with animal waste compost. J. Hazard Mater. 2010, 181, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Ngueagni, P.T.; Woumfo, E.D.; Kumar, P.S.; Siewe, M.; Vieillard, J.; Brun, N.; Nkuigue, P.F. Adsorption of Cu(II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J. Mol. Liq. 2019, 298, 112023. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Kumar, P.S.; Varjani, S.; Reshma, B. Simultaneous removal of Cu(II) and Reactive Green 6 dye from wastewater using immobilized mixed fungal biomass and its recovery. Chemosphere 2021, 271, 129519. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar]
- Chen, L.S.; Ni, R.; Yuan, T.J.; Gao, Y.; Kong, W.J.; Zhang, P.; Yue, Q.Y.; Gao, B.Y. Effects of green synthesis, magnetization, and regeneration on ciprofloxacin removal by bimetallic nZVI/Cu composites and insights of degradation mechanism. J. Hazard Mater. 2020, 382, 121008. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, J.; Liu, Q.; Liu, X.; Gao, B. A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol. Environ. Int. 2020, 138, 105639. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, X.; Guo, Y.; Lin, Z.; Liu, B. The removal of heavy metal cations by sulfidated nanoscale zero-valent iron (S-nZVI): The reaction mechanisms and the role of sulfur. J. Hazard Mater. 2021, 404, 124057. [Google Scholar] [CrossRef]
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, Y.; Liang, Y.; Li, H.; Liang, W.J. Degradation mechanism of Norfloxacin in Water using Persulfate Activated by BC@nZVI/Ni. Chem. Eng. J. 2020, 389, 124276. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Creamer, A.E.; He, F. Adsorptive removal of arsenate from aqueous solutions by biochar supported zero-valent iron nanocomposite: Batch and continuous flow tests. J. Hazard Mater. 2017, 322, 172–181. [Google Scholar] [CrossRef]
- Liu, X.; Lai, D.; Wang, Y. Performance of Pb(II) Removal by an Activated Carbon Supported Nanoscale Zero-Valent Iron Composite at Ultralow Iron Content. J. Hazard Mater. 2018, 361, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Woo, Y.C.; Yao, M.; Lim, S.; Tijing, L.D.; Shon, H.K. Nanoscale zero-valent iron (nZVI) immobilization onto graphene oxide (GO)-incorporated electrospun polyvinylidene fluoride (PVDF) nanofiber membrane for groundwater remediation via gravity-driven membrane filtration. Sci. Total Environ. 2019, 688, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, S.; Lu, X.Q.; Chen, Z.L. Removal of Pb(II) from water using synthesized kaolin supported nanoscale zero-valent iron. Chem. Eng. J. 2010, 163, 243–248. [Google Scholar] [CrossRef]
- Diao, Z.H.; Chu, W. FeS2 assisted degradation of atrazine by bentonite- supported nZVI coupling with hydrogen peroxide process in water: Performance and mechanism. Sci. Total Environ. 2020, 754, 142155. [Google Scholar] [CrossRef]
- Ma, Y.; Li, M.; Li, P.; Yang, L.; Zhang, Z. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour. Technol. 2021, 319, 124199. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zhang, Y.; Liu, S.; Wang, C.; Chen, W.; Liu, C.; Chen, Z.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2019, 297, 122381. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Li, Z.; Tang, X.; Xu, J. Simultaneous adsorption of Cd(II) and As(III) by a novel biochar-supported nanoscale zero-valent iron in aqueous systems. Sci. Total Environ. 2019, 708, 134823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ruan, Y.; Liang, A.; Shih, K.; Diao, Z.; Su, M.; Chen, D.; Lu, H.; Kong, L. Carbothermal reduction for preparing nZVI/BC to extract uranium: Insight into the iron species dependent uranium adsorption behavior. J. Clean Prod. 2019, 239, 117873. [Google Scholar] [CrossRef]
- Zhan, J.; Sunkara, B.; Tang, J.; Wang, Y.; He, J.; Mcpherson, G.L.; John, V.T. Carbothermal Synthesis of Aerosol-Based Adsorptive-Reactive Iron–Carbon Particles for the Remediation of Chlorinated Hydrocarbons. Ind. Eng. Chem. Res. 2011, 50, 13021–13029. [Google Scholar] [CrossRef]
- Bleyl, S.; Georgi, A.; Kopinke, F.D. Carbo-Iron—An Fe/AC composite—As alternative to nano-iron for groundwater treatment. Water Res. 2012, 46, 3817–3826. [Google Scholar]
- Nistico, R.; Carlos, L. High yield of nano zero-valent iron (nZVI) from carbothermal synthesis using lignin-derived substances from municipal biowaste. J. Anal. Appl. Pyrol. 2019, 140, 239–244. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.M.; Yang, Q.; Zeng, G.M.; Zhang, Y.; Liao, D.X.; Liu, J.J.; Hu, J.M.; Guo, L. Total concentrations and speciation of heavy metals in municipal sludge from Changsha, Zhuzhou and Xiangtan in middle-south region of China. J. Hazard Mater. 2008, 160, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, F.; Zhang, Y.; Lan, Y.; Cheng, K. Performance of lead ion removal by the three-dimensional carbon foam supported nanoscale zero-valent iron composite. J. Clean Prod. 2020, 294, 125350. [Google Scholar] [CrossRef]
- Magalhaes, F.; Pereira, M.C.; Fabris, J.D.; Bottrel, S.; Sansiviero, M.; Amaya, A.; Tancredi, N.; Lago, R.M. Novel highly reactive and regenerable carbon/iron composites prepared from tar and hematite for the reduction of Cr(VI) contaminant. J. Hazard Mater. 2009, 165, 1016–1022. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Assadi, A.A.; Bousselmi, L. Chemical treatment of orange tree sawdust for a cationic dye enhancement removal from aqueous solutions: Kinetic, equilibrium and thermodynamic studies. Desalin. Water Treat. 2015, 57, 22107–22119. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Lu, C.; Werner, D. Reductive sequestration of chromate by hierarchical FeS@Fe0 particles. Water Res. 2016, 102, 73–81. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, L.; Wu, R.R.; Zhang, S.M.; Xiao, R.B. Adsorption Behavior and Relative Distribution of Cd2+ Adsorption Mechanisms by the Magnetic and Nonmagnetic Biochars Derived from Chicken Manure. Int. J. Environ. Res. Public Health 2020, 17, 1602. [Google Scholar] [CrossRef]
- Singh, P.; Pal, P.; Mondal, P.; Saravanan, G.; Bhowmick, S. Kinetics and mechanism of arsenic removal using sulfide-modified nanoscale zerovalent iron. Chem. Eng. J. 2021, 412, 128667. [Google Scholar] [CrossRef]
- Fang, Y.; Wen, J.; Zhang, H.; Wang, Q.; Hu, X. Enhancing Cr(Ⅵ) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 2020, 260, 114021. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Gao, Y.; Yue, Q.Y.; Kong, W.J.; Jiang, W.Q. Degradation of chlortetracycline with simultaneous removal of copper (II) from aqueous solution using wheat straw-supported nanoscale zero-valent iron. Chem. Eng. J. 2019, 379, 122384. [Google Scholar] [CrossRef]
- Tang, L.; Feng, H.; Tang, J.; Zeng, G.; Deng, Y.; Wang, J.; Liu, Y.; Zhou, Y. Treatment of arsenic in acid wastewater and river sediment by Fe@Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism. Water Res. 2017, 117, 175. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, F.; Cui, J.; Yang, S.; Fang, L. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: Synergistic effects and mechanisms. J. Hazard Mater. 2020, 395, 122623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Dai, C.; Zhou, X.; Zhang, W. Sequestration of Cd(II) with nanoscale zero-valent iron (nZVI): Characterization and test in a two-stage system. Chem. Eng. J. 2014, 244, 218–226. [Google Scholar] [CrossRef]
- Liu, F.; Shan, C.; Zhang, X.; Zhang, Y.; Zhang, W.; Pan, B. Enhanced removal of EDTA-chelated Cu(II) by polymeric anion-exchanger supported nanoscale zero-valent iron. J. Hazard Mater. 2014, 321, 290–298. [Google Scholar] [CrossRef]
- Chen, F.; Hu, Y.; Dou, X.; Chen, D.; Dai, X. Chemical forms of heavy metals in pyrolytic char of heavy metal-implanted sewage sludge and their impacts on leaching behaviors. J. Anal. Appl. Pyrol. 2015, 116, 152–160. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The fate of Cu, Zn, Pb and Cd during the pyrolysis of sewage sludge at different temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef]




| Samples | Heavy Metals (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Cr | Cu | Zn | Pb | Cd | Ni | |
| sludge | 0.78 ± 0.01 | 1.76 ± 0.12 | 16.96 ± 0.61 | 7.91 ± 0.25 | 0.68 ± 0.05 | 3.66 ± 0.09 |
| SB | 0.02 ± 0.01 | 0.25 ± 0.05 | 6.36 ± 0.25 | 7.26 ± 0.37 | 0.71 ± 0.02 | 2.88 ± 0.14 |
| SB-NZVI | ND | 0.17 ± 0.04 | 5.68 ± 0.08 | 6.93 ± 0.19 | 0.62 ± 0.03 | 2.71 ± 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Tian, C.; Yang, Y.; Shao, Y.; Zhang, T.; Shi, X.; Zhang, W.; Zhu, Y. Carbothermal Synthesis of Sludge Biochar Supported Nanoscale Zero-Valent Iron for the Removal of Cd2+ and Cu2+: Preparation, Performance, and Safety Risks. Int. J. Environ. Res. Public Health 2022, 19, 16041. https://doi.org/10.3390/ijerph192316041
Shao Y, Tian C, Yang Y, Shao Y, Zhang T, Shi X, Zhang W, Zhu Y. Carbothermal Synthesis of Sludge Biochar Supported Nanoscale Zero-Valent Iron for the Removal of Cd2+ and Cu2+: Preparation, Performance, and Safety Risks. International Journal of Environmental Research and Public Health. 2022; 19(23):16041. https://doi.org/10.3390/ijerph192316041
Chicago/Turabian StyleShao, Yingying, Chao Tian, Yanfeng Yang, Yanqiu Shao, Tao Zhang, Xinhua Shi, Weiyi Zhang, and Ying Zhu. 2022. "Carbothermal Synthesis of Sludge Biochar Supported Nanoscale Zero-Valent Iron for the Removal of Cd2+ and Cu2+: Preparation, Performance, and Safety Risks" International Journal of Environmental Research and Public Health 19, no. 23: 16041. https://doi.org/10.3390/ijerph192316041
APA StyleShao, Y., Tian, C., Yang, Y., Shao, Y., Zhang, T., Shi, X., Zhang, W., & Zhu, Y. (2022). Carbothermal Synthesis of Sludge Biochar Supported Nanoscale Zero-Valent Iron for the Removal of Cd2+ and Cu2+: Preparation, Performance, and Safety Risks. International Journal of Environmental Research and Public Health, 19(23), 16041. https://doi.org/10.3390/ijerph192316041





