Validation of Two Screening Tools for Detecting Delirium in Older Patients in the Post-Anaesthetic Care Unit: A Diagnostic Test Accuracy Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Participants
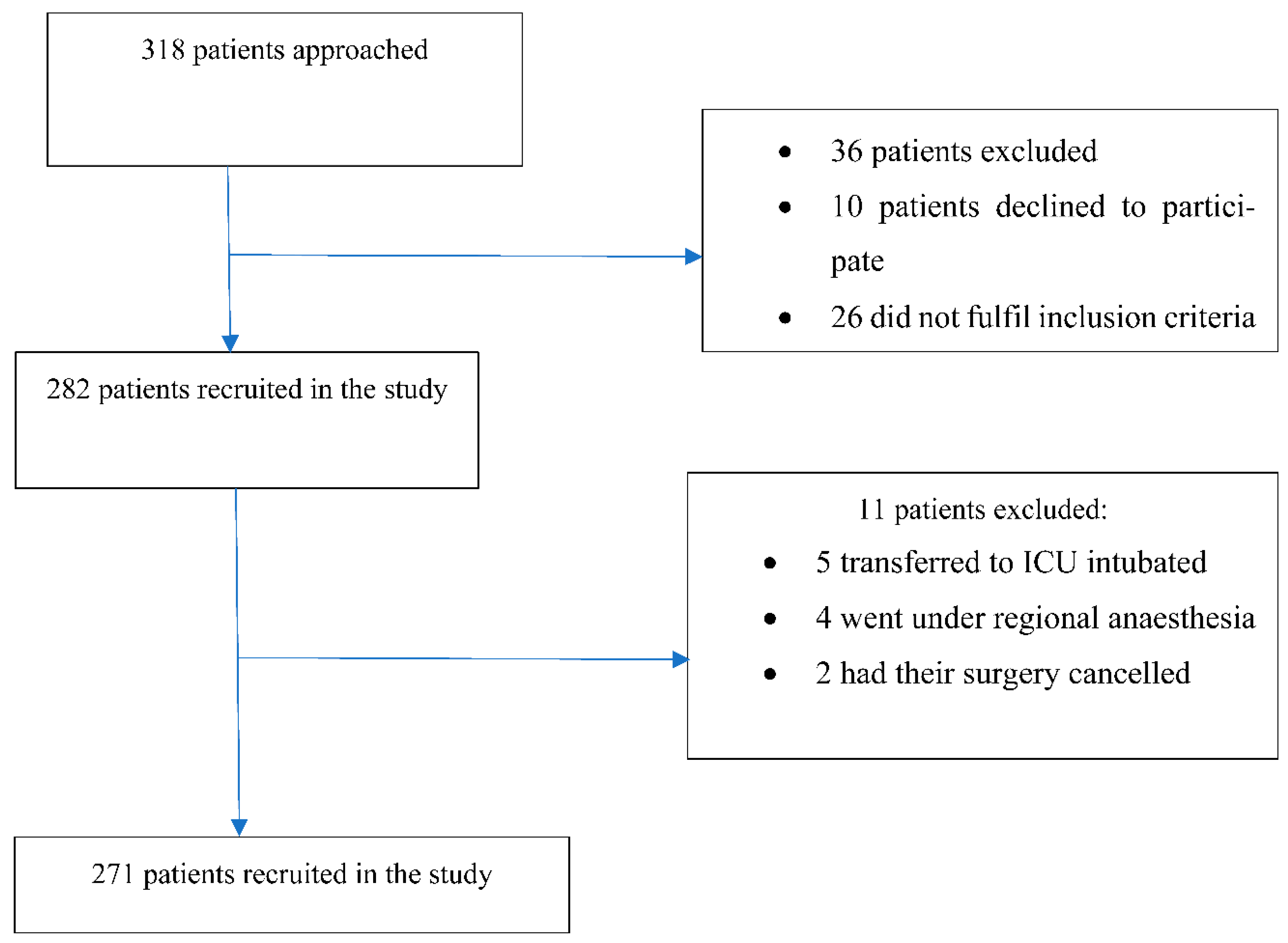
2.3. Sampling and Recruitment
2.4. Diagnostic Accuracy Procedure
2.4.1. Index Tests
The 4 A’s Test
The Three-Minute Diagnostic Interview for Confusion Assessment Method
Reference Standard
2.5. Randomisation
2.6. Sample Size Calculation
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
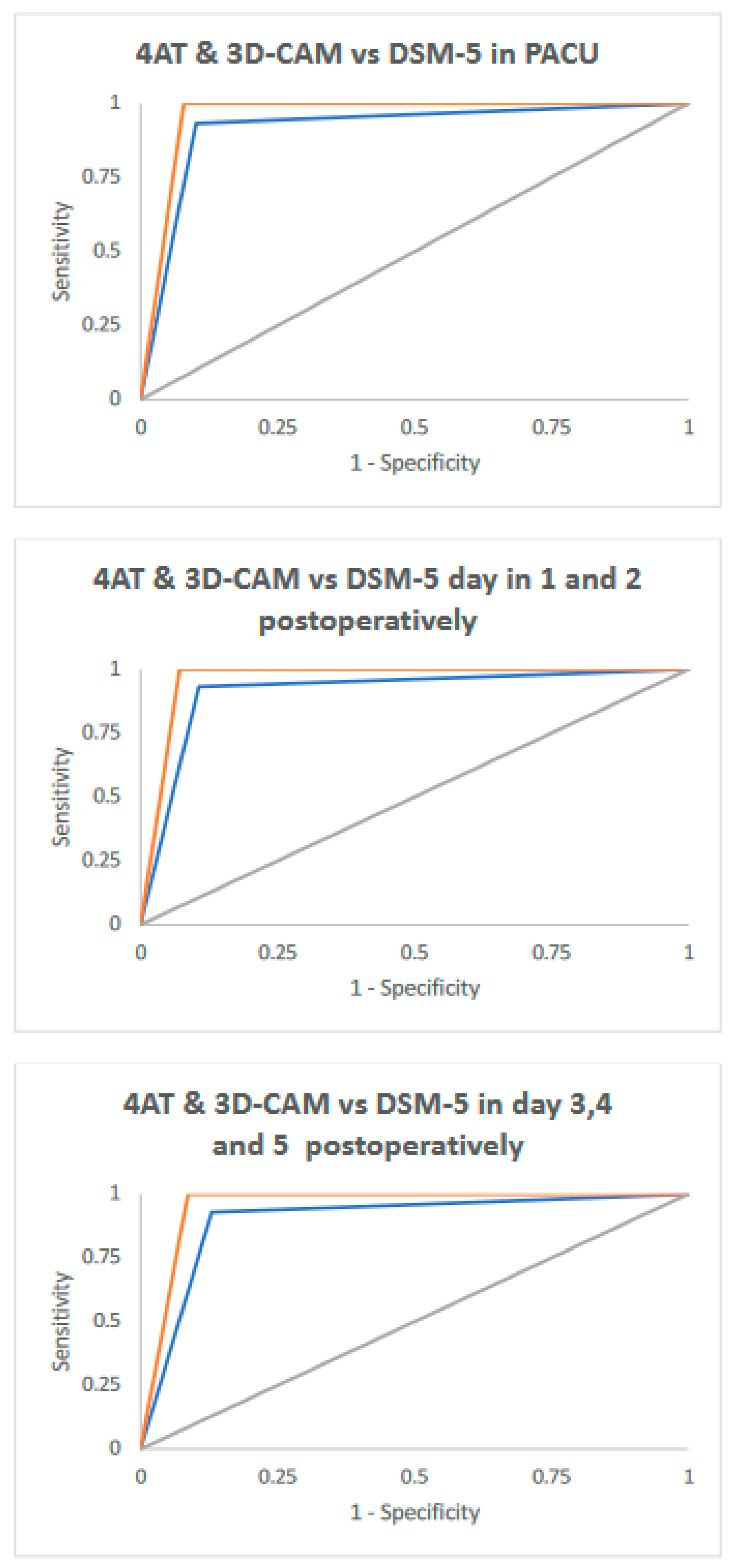
3.2. Comparison of Test Performance
3.3. Inter-Rater Reliability
3.4. Delirium Subtypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendry, K.; Quinn, T.J.; Evans, J.; Scortichini, V.; Miller, H.; Burns, J.; Cunnington, A.; Stott, D.J. Evaluation of delirium screening tools in geriatric medical inpatients: A diagnostic test accuracy study. Age Ageing 2016, 45, 832–837. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders: DSM–5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; p. 596. [Google Scholar]
- Robinson, T.N.; Raeburn, C.D.; Angles, E.M.; Tran, Z.V.; Brenner, L.A.; Moss, M. Postoperative delirium in the elderly: Risk factors and outcomes. Ann. Surg. 2009, 249, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Iseli, R.K.; Brand, C.; Telford, M.; LoGiudice, D. Delirium in elderly general medical inpatients: A prospective study. Intern. Med. J. 2007, 37, 806. [Google Scholar] [CrossRef] [PubMed]
- Witlox, J.; Eurelings, L.S.M.; de Jonghe, J.F.M.; Kalisvaart, K.J.; Eikelenboom, P.; van Gool, W.A. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia. JAMA J. Am. Med. Assoc. 2010, 304, 443. [Google Scholar] [CrossRef]
- Pezzullo, L.; Streatfeild, J.; Hickson, J.; Teodorczuk, A.; Agar, M.R.; Caplan, G.A. Economic impact of delirium in Australia: A cost of illness study. BMJ Open 2019, 9, e027514. [Google Scholar] [CrossRef] [Green Version]
- Saller, T.; MacLullich, A.M.J.; Schafer, S.T.; Crispin, A.; Neitzert, R.; Schule, C.; Dossow, V.; Hofmann-Kiefer, K.F. Screening for delirium after surgery: Validation of the 4 A’s test (4AT) in the post-anaesthesia care unit. Anaesthesia 2019, 74, 1260. [Google Scholar] [CrossRef]
- Neufeld, K.J.; Leoutsakos, J.S.; Sieber, F.E.; Joshi, D.; Wanamaker, B.L.; Rios-Robles, J.; Needham, D.M. Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br. J. Anaesth. 2013, 111, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in elderly people. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef] [Green Version]
- Wand, A.P.F.; Thoo, W.; Ting, V.; Baker, J.; Sciuriaga, H.; Hunt, G.E. Identification and rates of delirium in elderly medical inpatients from diverse language groups. Geriatr. Nurs. 2013, 34, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R. Still Predicting Delirium after All These Years. Anesth. Analg. 2020, 130, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann. Intern. Med. 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. Indicator Specification: Delirium Clinical Care Standard. Sydney: ACSQHC. 2016. Available online: https://www.safetyandquality.gov.au/our-work/clinical-care-standards/delirium-clinical-care-standard (accessed on 12 February 2020).
- National Institute for Health and Care Excellence. Delirium: Prevention, Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Aldwikat, R.K.; Manias, E.; Tomlinson, E.; Amin, M.; Nicholson, P. Delirium screening tools in the post-anaesthetic care unit: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2022, 34, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Olbert, M.; Eckert, S.; Morgeli, R.; Kruppa, J.; Spies, C.D. Validation of 3-minute diagnostic interview for CAM-defined Delirium to detect postoperative delirium in the recovery room: A prospective diagnostic study. Eur. J. Anaesthesiol. 2019, 36, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Group, S. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ Br. Med. J. 2015, 351, h5527. [Google Scholar]
- Bossuyt, P.M.; Korevaar, D.A.; Reitsma, J.B.; Bruns, D.E.; Bruns, D.E.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies1. Radiology 2015, 277, 826–832. [Google Scholar] [CrossRef] [Green Version]
- Melbourne Health. Melbourne Health Annual Report 2019; The Royal Melbourne Hospital: Melbourne, Australia, 2019. [Google Scholar]
- Lange, P.W.; Lamanna, M.; Watson, R.; Maier, A.B. Undiagnosed delirium is frequent and difficult to predict: Results from a prevalence survey of a tertiary hospital. J. Clin. Nurs. 2019, 28, 2537–2542. [Google Scholar] [CrossRef]
- Vreeswijk, R.; Kalisvaart, I.; Maier, A.B.; Kalisvaart, K.J. Development and validation of the delirium risk assessment score (DRAS). Eur. Geriatr. Med. 2020, 11, 307. [Google Scholar] [CrossRef]
- Usher-Smith Juliet, A.; Sharp Stephen, J.; Griffin Simon, J. The spectrum effect in tests for risk prediction, screening, and diagnosis. BMJ Br. Med. J. 2016, 353, i3139. [Google Scholar] [CrossRef] [Green Version]
- Mander, G.T.W.; Munn, Z. Understanding diagnostic test accuracy studies and systematic reviews: A primer for medical radiation technologists. J. Med. Imaging Radiat. Sci. 2021, 52, 286–294. [Google Scholar] [CrossRef]
- Radtke, F.M.; Franck, M.; Schneider, M.; Luetz, A.; Seeling, M.; Heinz, A.; Wernecke, K.D.; Spies, C.D. Comparison of three scores to screen for delirium in the recovery room. Br. J. Anaesth. 2008, 101, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Aldwikat, R.; Manias, E.; Nicholson, P. Incidence and risk factors for acute delirium in older patients with a hip fracture: A retrospective cohort study. Nurs. Health Sci. 2020, 22, 958–966. [Google Scholar] [CrossRef]
- Bellelli, G.; Morandi, A.; Davis, D.H.J.; Mazzola, P.; Turco, R.; Gentile, S.; Ryan, T.; Cash, H.; Guerini, F.; Torpilliesi, T.; et al. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing 2014, 43, 496–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcantonio, E.R.; Ngo, L.H.; O’Connor, M.; Jones, R.N.; Crane, P.K.; Metzger, E.D.; Inouye, S.K. 3D-CAM: Derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: A cross-sectional diagnostic test study. Ann. Intern. Med. 2014, 161, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SAS Institute Inc. SAS/Stat User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2009. [Google Scholar]
- Fenn Buderer, N.M. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad. Emerg. Med. 1996, 3, 895–900. [Google Scholar] [CrossRef]
- Munoz, S.R.; Bangdiwala, S.I. Interpretation of Kappa- and B statistics measures of agreement. J. Appl. Stat. 1997, 24, 105. [Google Scholar] [CrossRef]
- De, J.; Wand, A.P.F.; Smerdely, P.I.; Hunt, G.E. Validating the 4A’s test in screening for delirium in a culturally diverse geriatric inpatient population. Int. J. Geriatr. Psychiatry 2017, 32, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Palihnich, K.; Inouye, S.; Marcantonio, E. The 3D CAM Training Manual for Research; Hospital Elder Life Program: Boston, MA, USA, 2014. [Google Scholar]
- Weir, C.J.; Lees, K.R. Comparison of stratification and adaptive methods for treatment allocation in an acute stroke clinical trial. Stat. Med. 2003, 22, 705–726. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Grimes, D.A. Allocation concealment in randomised trials: Defending against deciphering. Lancet 2002, 359, 614. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, D.F.; Feinstein, A.R. Problems of Spectrum and Bias in Evaluating the Efficacy of Diagnostic Tests. N. Engl. J. Med. 1978, 299, 926–930. [Google Scholar] [CrossRef]
| All Participants (n = 271) | No Delirium (n = 227) | Delirium (n = 44) | p Value | |
|---|---|---|---|---|
| Mean age (years) (SD) | 76.9 (7.9) | 75.5 (7.9) | 83.8 (8.5) | 0.0005 |
| Gender | 0.423 | |||
| Male n (%) | 132 (49%) | 113 (50%) | 19 (43%) | |
| Female n (%) | 139 (51%) | 114 (50%) | 25 (57%) | |
| Dementia n (%) | 10 (3.7%) | 0 (0%) | 10 (22.7%) | 0.001 |
| MiniCog score < 3 (preoperatively) (%) | 87 (32%) | 45 (20%) | 42 (95.5%) | 0.001 |
| Mean Katz-ADL score (preoperatively) (SD) | 5.3 (0.43) | 5.7 (0.53) | 4.95 (1.29) | 0.001 |
| Mean Charlson comorbidity index score | 5.22 (2.1) | 5.0 (2.2) | 6.4 (2.0) | 0.005 |
| Type of surgery | 0.085 | |||
| Orthopaedic (%) | 101 (37%) | 72 (31.7%) | 29 (66%) | |
| Urology (%) | 35 (12.9%) | 33 (14.5%) | 2 (4.5%) | |
| ENT (%) | 21 (7.7%) | 21 (9.2%) | 0 (0%) | |
| Lap-abdomen (%) | 20 (7.4%) | 19 (8.3%) | 1 (2.2%) | |
| Colorectal (%) | 18 (6.6%) | 15 (6.6%) | 3 (6.8%) | |
| Plastic (%) | 17 (6.3%) | 14 (6.1%) | 3 (6.8%) | |
| Thoracic (%) | 16 (5.9%) | 13 (5.7%) | 3 (6.8%) | |
| Reno-vascular (%) | 13 (4.7%) | 13 (5.7%) | 0 (0%) | |
| Vascular (%) | 13 (4.7%) | 12 (5.2%) | 1 (2.2%) | |
| Breast (%) | 11 (4.1%) | 11 (4.8%) | 0 (0%) | |
| Hepatobiliary (%) | 4 (1.4%) | 3 (1.3%) | 1 (2.2%) | |
| Cardiothoracic (%) | 3 (1.1%) | 2 (0.8%) | 1 (2.2%) | |
| Median duration of anaesthesia (min) (IQR) | 138 min (88–196 min) | 139 min (83–211 min) | 136 min (98–167 min) | 0.890 |
| Tool | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | AUROC (95% CI) | |
|---|---|---|---|---|---|---|
| In PACU | 4AT | 93.2% (81.3–98.6) | 89.9% (85.2–93.5) | 64.1% (51.1–75.7) | 98.6% (95.8–99.7) | 0.92 (0.87–0.96) |
| 3D-CAM | 100.0% (92.0–100.0) | 93.0% (87.8–95.2) | 71.0% (58.1–81.8) | 100.0% (98.3–100.0) | 0.96 (0.94–0.98) | |
| Day 1 post-operatively | 4AT | 93.3% (81.7–98.6) | 89.4% (84.6–93.1) | 63.6% (50.9–75.1) | 98.5% (95.8–99.7) | 0.91 (0.87–0.96) |
| 3D-CAM | 100.0% (92.1–100.0) | 93.0% (88.8–95.9) | 73.8% (60.9–84.2) | 100.0% (98.3–100.0) | 0.96 (0.95–0.98) | |
| Day 2 post-operatively | 4AT | 93.3% (81.7–98.6) | 89.4% (84.6–93.1) | 63.6% (50.9–75.1) | 98.5% (95.8–99.7) | 0.91 (0.87–0.96) |
| 3D-CAM | 100.0% (92.1–100.0) | 93.0% (88.8–95.9) | 73.8% (60.9–84.2) | 100.0% (98.3–100.0) | 0.96 (0.95–0.98) | |
| Day 3 post-operatively | 4AT | 92.9% (80.5–98.5) | 87.0% (80.9–91.8) | 65.0% (51.6–76.9) | 97.9% (94.0–99.6) | 0.90 (0.85–0.95) |
| 3D-CAM | 100.0% (91.6–100.0) | 91.4% (85.9–95.2) | 75.0% (61.6–85.6) | 100.0% (97.5–100.0) | 0.96 (0.94–0.98) | |
| Day 4 post-operatively | 4AT | 92.9% (80.5–98.5) | 87.0% (80.9–91.8) | 65.0% (51.6–76.9) | 97.9% (94.0–99.6) | 0.90 (0.85–0.95) |
| 3D-CAM | 100.0% (91.6–100.0) | 91.4% (85.9–95.2) | 75.0% (61.6–85.6) | 100.0% (97.5–100.0) | 0.96 (0.94–0.98) | |
| Day 5 post-operatively | 4AT | 92.9% (80.5–98.5) | 87.0% (80.9–91.8) | 65.0% (51.6–76.9) | 97.9% (94.0–99.6) | 0.90 (0.85–0.95) |
| 3D-CAM | 100.0% (91.6–100.0) | 91.4% (85.9–95.2) | 75.0% (61.6–85.6) | 100.0% (97.5–100.0) | 0.96 (0.94–0.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldwikat, R.K.; Manias, E.; Holmes, A.; Tomlinson, E.; Nicholson, P. Validation of Two Screening Tools for Detecting Delirium in Older Patients in the Post-Anaesthetic Care Unit: A Diagnostic Test Accuracy Study. Int. J. Environ. Res. Public Health 2022, 19, 16020. https://doi.org/10.3390/ijerph192316020
Aldwikat RK, Manias E, Holmes A, Tomlinson E, Nicholson P. Validation of Two Screening Tools for Detecting Delirium in Older Patients in the Post-Anaesthetic Care Unit: A Diagnostic Test Accuracy Study. International Journal of Environmental Research and Public Health. 2022; 19(23):16020. https://doi.org/10.3390/ijerph192316020
Chicago/Turabian StyleAldwikat, Rami K., Elizabeth Manias, Alex. Holmes, Emily Tomlinson, and Patricia Nicholson. 2022. "Validation of Two Screening Tools for Detecting Delirium in Older Patients in the Post-Anaesthetic Care Unit: A Diagnostic Test Accuracy Study" International Journal of Environmental Research and Public Health 19, no. 23: 16020. https://doi.org/10.3390/ijerph192316020
APA StyleAldwikat, R. K., Manias, E., Holmes, A., Tomlinson, E., & Nicholson, P. (2022). Validation of Two Screening Tools for Detecting Delirium in Older Patients in the Post-Anaesthetic Care Unit: A Diagnostic Test Accuracy Study. International Journal of Environmental Research and Public Health, 19(23), 16020. https://doi.org/10.3390/ijerph192316020






