Disease Latency according to Asbestos Exposure Characteristics among Malignant Mesothelioma and Asbestos-Related Lung Cancer Cases in South Korea
Abstract
1. Introduction
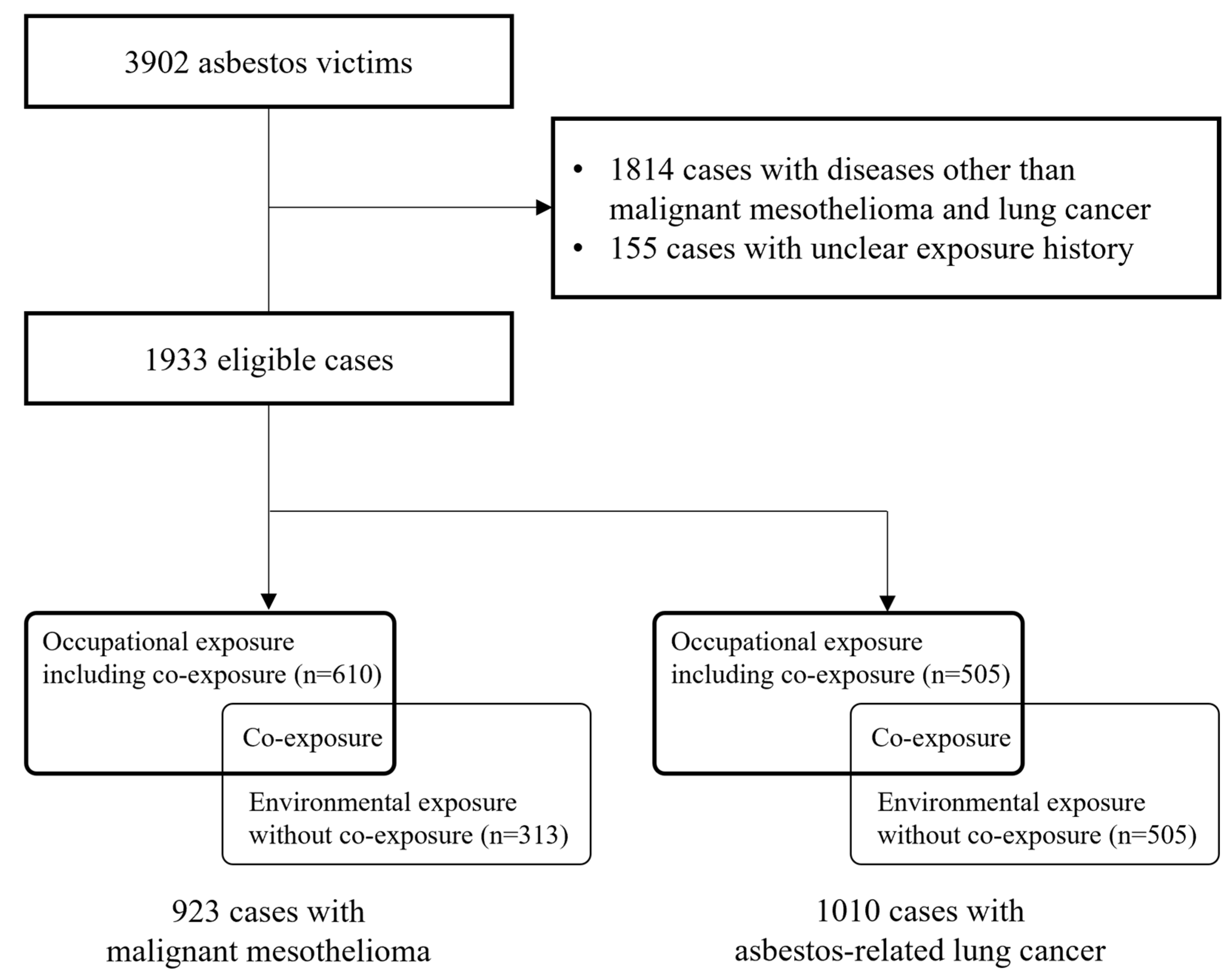
2. Materials and Methods
2.1. Data Source and Study Population
2.2. History of Asbestos Exposure
2.3. Latency Period
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteves Marques Janela, J.; Silva Pereira, P.J. História do Amianto no Mundo e em Portugal. CEM Cultura Espaço & Memória Revista do CITCEM. 2016, pp. 193–206. Available online: http://hdl.handle.net/10400.2/5984 (accessed on 15 October 2022).
- Facina, T. Vigilância do Câncer Relacionado ao Trabalho e ao Ambiente. Rev. Bras. Cancerol. 2011, 57, 85–86. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Asbestos (Chrysotile, Amosite, Crocidolite, Tremolite, Actinolite, and Anthophyllite). IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100C, 1–309. [Google Scholar]
- Albin, M.; Magnani, C.; Krstev, S.; Rapiti, E.; Shefer, I. Asbestos and cancer: An overview of current trends in Europe. Environ. Health Perspect. 1999, 107 (Suppl. 2), 289–298. [Google Scholar]
- Hodgson, J.T.; Darnton, A. The quantitative risks of mesothelioma and lung cancer in relation to asbestos exposure. Ann. Occup. Hyg. 2000, 44, 565–601. [Google Scholar] [CrossRef]
- Pira, E.; Pelucchi, C.; Buffoni, L.; Palmas, A.; Turbiglio, M.; Negri, E.; Piolatto, P.G.; La Vecchia, C. Cancer mortality in a cohort of asbestos textile workers. Br. J. Cancer 2005, 92, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.W.; Crump, K.S. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit. Rev. Toxicol. 2008, 38 (Suppl. 1), 49–73. [Google Scholar] [CrossRef]
- Kamp, D.W. Asbestos-induced lung diseases: An update. Transl. Res. 2009, 153, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Thives, L.P.; Ghisi, E.; Júnior, J.J.T.; Vieira, A.S. Is asbestos still a problem in the world? A current review. J. Environ. Manag. 2022, 319, 115716. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global asbestos disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef]
- Ministry of Environment (MOE). Overview of Asbestos Management; MOE: Seoul, Republic of Korea, 2009; pp. 3–456. (In Korean).
- Choi, J.K.; Paek, D.M.; Paik, N.W. The production, the use, the number of workers and exposure level of asbestos in Korea. J. Korean Soc. Occup. Environ. Hyg. 1998, 8, 242–253. [Google Scholar]
- Ki, Y.-H.; Kim, J.-M.; Roh, Y.-M.; Chung, L.; Kim, Y.-S.; Sim, S.-H. A survey for some asbestos containing products in Korea. J. Environ. Health Sci. 2008, 34, 108–115. [Google Scholar] [CrossRef][Green Version]
- Kang, D.-M.; Kim, J.-E.; Lee, Y.-J.; Lee, H.-H.; Lee, C.-Y.; Moon, S.-J.; Kang, M.-S. Environmental health centers for asbestos and their health impact surveys and activities. Ann. Occup. Environ. Med. 2016, 28, 68. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, Y.-C.; Kim, Y.; Hong, W.-H. Predicting the mortality from asbestos-related diseases based on the amount of asbestos used and the effects of slate buildings in Korea. Sci. Total Environ. 2016, 542, 1–11. [Google Scholar] [CrossRef]
- Kwak, K.M.; Paek, D.; Hwang, S.-S.; Ju, Y.-S. Estimated future incidence of malignant mesothelioma in South Korea: Projection from 2014 to 2033. PLoS ONE 2017, 12, e0183404. [Google Scholar] [CrossRef]
- Kwak, K.; Cho, S.-I.; Paek, D. Future Incidence of Malignant Mesothelioma in South Korea: Updated Projection to 2038. Int. J. Environ. Res. Public Health 2021, 18, 6614. [Google Scholar] [CrossRef]
- Nielsen, L.S.; Baelum, J.; Rasmussen, J.; Dahl, S.; Olsen, K.E.; Albin, M.; Hansen, N.C.; Sherson, D. Occupational asbestos exposure and lung cancer—A systematic review of the literature. Arch. Environ. Occup. Health 2014, 69, 191–206. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Buncher, C.R. Latent period for malignant mesothelioma of occupational origin. J. Occup. Med. 1992, 34, 718–721. [Google Scholar]
- Marinaccio, A.; Binazzi, A.; Cauzillo, G.; Cavone, D.; De Zotti, R.; Ferrante, P.; Gennaro, V.; Gorini, G.; Menegozzo, M.; Mensi, C. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur. J. Cancer 2007, 43, 2722–2728. [Google Scholar] [CrossRef]
- Frost, G. The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005). Br. J. Cancer 2013, 109, 1965–1973. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, S.-K.; Lee, J.H.; Lim, M.H.; Kang, S.; Phee, Y.G. A study on the factors affecting asbestos exposure level from asbestos abatement in building demolition sites. J. Korean Soc. Occup. Environ. Hyg. 2009, 19, 8–15. [Google Scholar]
- Kang, D.-M. Health effects of environmental asbestos exposure. J. Environ. Health Sci. 2009, 35, 71–77. [Google Scholar] [CrossRef]
- Kang, D.-M.; Gu, D.-C.; Kim, K.-H. Asbestos-related diseases among asbestos textile factory workers and residents around the factory. J. Korean Med. Assoc. 2009, 52, 482–488. [Google Scholar] [CrossRef]
- Kang, D.-M.; Kim, J.-E.; Kim, Y.-K.; Lee, H.-H.; Kim, S.-Y. Occupational burden of Asbestos-related diseases in Korea, 1998–2013: Asbestosis, mesothelioma, lung Cancer, laryngeal Cancer, and ovarian Cancer. J. Korean Med. Sci. 2018, 33, e226. [Google Scholar] [CrossRef]
- Huh, D.-A.; Kang, M.-S.; Lee, J.; Choi, J.Y.; Moon, K.W.; Lee, Y.-J. Occupational and environmental asbestos exposure and the risk of lung cancer in Korea: A case-control study in South Chungcheong Province of Korea. PLoS ONE 2021, 16, e0249790. [Google Scholar] [CrossRef]
- Kwon, S.-C.; Lee, S.-S.; Kang, M.-S.; Huh, D.-A.; Lee, Y.-J. The Epidemiologic Characteristics of Malignant Mesothelioma Cases in Korea: Findings of the Asbestos Injury Relief System from 2011–2015. Int. J. Environ. Res. Public Health 2021, 18, 10007. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-S.; Lee, S.-S.; Kwon, S.-C.; Huh, D.-A.; Lee, Y.-J. Survival of Korean Patients with Malignant Pleural Mesothelioma Compensated for the Asbestos Injury Relief. Appl. Sci. 2021, 11, 9713. [Google Scholar] [CrossRef]
- Bianchi, C.; Bianchi, T. Malignant mesothelioma: Global incidence and relationship with asbestos. Ind. Health 2007, 45, 379–387. [Google Scholar] [CrossRef]
- Hilliard, A.; Lovett, J.; McGavin, C. The rise and fall in incidence of malignant mesothelioma from a British Naval Dockyard, 1979–1999. Occup. Med. 2003, 53, 209–212. [Google Scholar] [CrossRef]
- Ministry of Employment and Labor (MOEL). Investigation on the Actual Condition of Hazardous Environment at Asbestos Handling Workplaces; MOEL: Seoul, Republic of Korea, 1984; pp. 254–263. (In Korean).
- Kim, S.H. Clinical Characteristics and Long-Term Follow-Up of Asbestos in Workers at One Asbestos Textile Factory in Busan. Master’s Thesis, Inje University, Gyeongsangnam-do, Republic of Korea, 2019. [Google Scholar]
- Choi, J.K.; Paek, D.M.; Paik, N.W.; Hisanaga, N.; Sakai, K. A study on several minerals contaminated with asbestiform fibers in Korea. J. Korean Soc. Occup. Environ. Hyg. 1998, 8, 254–263. [Google Scholar]
- Aboagye-Sarfo, P.; Reid, A.; de Klerk, N.; Samuels, L.; Franklin, P.; Musk, M. Determinants of latency periods of lung cancer (lc) and malignant mesothelioma (mm) in former workers and residents exposed to crocidolite at Wittenoom, Western Australia. Am. J. Respir. Crit. Care Med. 2011, 183, A4812. [Google Scholar]
- Tossavainen, A. Asbestos, asbestosis, and cancer: The Helsinki criteria for diagnosis and attribution. Scand. J. Work. Environ. Health 2010, 23, 311–316. [Google Scholar] [CrossRef]
- Bitchatchi, E.; Kayser, K.; Perelman, M.; Richter, E.D. Mesothelioma and asbestosis in a young woman following occupational asbestos exposure: Short latency and long survival: Case Report. Diagn. Pathol. 2010, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Ohar, J.A.; Cheung, M.; Talarchek, J.; Howard, S.E.; Howard, T.D.; Hesdorffer, M.; Peng, H.; Rauscher, F.J.; Testa, J.R. Germline BAP1 Mutational Landscape of Asbestos-Exposed Malignant Mesothelioma Patients with Family History of CancerGermline BAP1 Mutations in Mesothelioma Patients. Cancer Res. 2016, 76, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Casalone, E.; Ferrante, D.; Aspesi, A.; Morleo, G.; Biasi, A.; Sculco, M.; Mancuso, G.; Guarrera, S.; Righi, L. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017, 405, 38–45. [Google Scholar] [CrossRef]
- Panou, V.; Røe, O.D. Inherited genetic mutations and polymorphisms in malignant mesothelioma: A comprehensive review. Int. J. Mol. Sci. 2020, 21, 4327. [Google Scholar] [CrossRef]
- Scherpereel, A.; Opitz, I.; Berghmans, T.; Psallidas, I.; Glatzer, M.; Rigau, D.; Astoul, P.; Bölükbas, S.; Boyd, J.; Coolen, J. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur. Respir. J. 2020, 55, 1900953. [Google Scholar] [CrossRef]
| Variable | Malignant Mesothelioma | Lung Cancer |
|---|---|---|
| n (%) | n (%) | |
| Total | 923 (100.0) | 1010 (100.0) |
| Sex | ||
| Male | 616 (66.7) | 721 (71.4) |
| Female | 307 (33.3) | 289 (28.6) |
| Age | ||
| <60 | 112 (12.1) | 67 (6.6) |
| 60–69 | 206 (22.3) | 244 (24.2) |
| 70–79 | 289 (31.3) | 363 (35.9) |
| 80–89 | 235 (25.5) | 270 (26.7) |
| ≥90 | 81 (8.8) | 66 (6.5) |
| Smoking status | ||
| Never | 80 (8.7) | 149 (14.8) |
| Past smoker | 68 (7.4) | 109 (10.8) |
| Current smoker | 21 (2.3) | 40 (4.0) |
| Unknown | 754 (81.7) | 712 (70.5) |
| Exposure modalities | ||
| Occupational | 610 (66.1) | 505 (50.0) |
| Environmental | 313 (33.9) | 505 (50.0) |
| Diagnosis year | ||
| <2000 | 10 (1.1) | 1 (0.1) |
| 2000–2004 | 83 (9.0) | 14 (1.4) |
| 2005–2009 | 174 (18.9) | 55 (5.4) |
| 2010–2014 | 254 (27.5) | 213 (21.1) |
| 2015–2019 | 293 (31.7) | 530 (52.5) |
| ≥2020 | 109 (11.8) | 197 (19.5) |
| Variable | n | Mean Latency (±SD) | p-Value | Median Latency | Range (min–max) | 5–95 Percentile |
|---|---|---|---|---|---|---|
| Malignant mesothelioma | ||||||
| Total | 923 | 33.7 (±13.8) | 34.0 | 8.0–84.0 | 14.0–57.8 | |
| Sex | ||||||
| Male | 616 | 33.6 (±13.3) | 0.619 | 33.0 | 8.0–77.0 | 14.0–56.0 |
| Female | 307 | 34.1 (±14.5) | 34.0 | 8.0–84.0 | 15.0–61.0 | |
| Age | ||||||
| <60 | 112 | 25.3 a (±10.9) | <0.001 | 25.0 | 8.0–51.0 | 11.7–44.4 |
| 60–69 | 206 | 29.9 b (±11.4) | 30.0 | 10.0–62.0 | 13.0–49.0 | |
| 70–79 | 289 | 35.2 c (±13.0) | 36.0 | 8.0–72.0 | 15.0–57.5 | |
| 80–89 | 235 | 38.6 d (±14.2) | 38.0 | 11.0–84.0 | 16.0–68.0 | |
| ≥90 | 81 | 36.3 cd (±15.5) | 37.0 | 10.0–75.0 | 13.1–65.6 | |
| Lung cancer | ||||||
| Total | 1010 | 40.1 (±16.3) | 39.0 | 7.0–94.0 | 15.0–73.5 | |
| Sex | ||||||
| Male | 721 | 40.7 (±16.1) | 0.076 | 40.0 | 7.0–87.0 | 15.0–73.0 |
| Female | 289 | 38.7 (±16.7) | 37.0 | 11.0–94.0 | 15.5–74.0 | |
| Age | ||||||
| <60 | 67 | 29.4 a (±10.7) | <0.001 | 28.0 | 11.0–54.0 | 14.0–45.2 |
| 60–69 | 244 | 32.6 a (±13.3) | 34.0 | 10.0–65.0 | 14.0–56.0 | |
| 70–79 | 363 | 41.2 b (±14.8) | 40.0 | 7.0–75.0 | 18.0–69.0 | |
| 80–89 | 270 | 45.8 c (±17.1) | 43.0 | 10.0–84.0 | 20.0–78.0 | |
| ≥90 | 66 | 49.3 c (±19.4) | 46.5 | 13.0–94.0 | 18.0–86.0 |
| Variable | Malignant Mesothelioma | Lung Cancer | ||||
|---|---|---|---|---|---|---|
| N | Estimate (95% CI) | p-Value | N | Estimate (95% CI) | p-Value | |
| Sex | ||||||
| Male | 616 | 34.2 (33.1, 35.3) | 0.100 | 721 | 40.0 (39.6, 40.4) | 0.394 |
| Female | 307 | 35.0 (34.0, 36.0) | 289 | 40.2 (39.7, 40.8) | ||
| Age | ||||||
| <60 | 112 | 15.3 (13.8, 16.9) | <0.001 | 67 | 23.6 (22.4, 24.8) | <0.001 |
| 60–69 | 206 | 27.6 (26.5, 28.8) | 244 | 32.5 (31.9, 33.2) | ||
| 70–79 | 289 | 36.6 (35.5, 37.6) | 363 | 40.6 (40.0, 41.2) | ||
| 80–89 | 235 | 43.9 (42.6, 45.1) | 270 | 48.4 (47.8, 49.1) | ||
| ≥90 | 81 | 49.5 (47.8, 51.2) | 66 | 54.8 (53.6, 56.0) | ||
| Smoking status | ||||||
| Never | 80 | 35.4 (33.9, 36.8) | 0.011 | 149 | 40.3 (39.7, 40.9) | 0.875 |
| Past smoker | 68 | 36.4 (33.6, 39.1) | 109 | 39.9 (39.2, 40.7) | ||
| Current smoker | 21 | 33.3 (31.7, 34.8) | 40 | 40.0 (38.9, 41.2) | ||
| Unknown | 754 | 33.4 (32.9, 33.9) | 712 | 40.2 (39.9, 40.5) | ||
| Lifetime smoking (in pack years) | ||||||
| <10 | 93 | 35.8 (34.4, 37.1) | 0.019 | 174 | 40.3 (39.7, 40.9) | 0.502 |
| 10–30 | 27 | 33.5 (31.1, 35.9) | 52 | 39.5 (38.4, 40.5) | ||
| 30–50 | 32 | 33.0 (30.8, 35.2) | 52 | 39.9 (38.9, 41.0) | ||
| ≥50 | 17 | 33.6 (30.6, 36.7) | 20 | 41.1 (39.5, 42.8) | ||
| Unknown | 754 | 33.4 (32.9, 33.9) | 712 | 40.2 (39.9, 40.5) | ||
| Exposure modalities | ||||||
| Occupational | 610 | 33.4 (32.4, 34.4) | <0.001 | 505 | 39.5 (39.0, 40.0) | <0.001 |
| Environmental | 313 | 35.8 (34.7, 36.9) | 505 | 40.7 (40.2, 41.2) | ||
| Type of job | ||||||
| Building 1 | 313 | 32.7 (31.6, 33.7) | <0.001 | 190 | 37.7 (36.9, 38.5) | 0.009 |
| Production 2 | 54 | 31.3 (29.7, 33.0) | 42 | 36.7 (35.4, 38.0) | ||
| Maintenance 3 | 143 | 33.3 (32.1, 34.6) | 135 | 37.9 (37.1, 38.8) | ||
| Mining 4 | 17 | 34.9 (32.3, 37.6) | 72 | 38.4 (37.4, 39.5) | ||
| Others | 83 | 33.6 (32.2, 35.0) | 66 | 37.3 (36.3, 38.3) | ||
| Type of exposure source | ||||||
| Asbestos mines | 37 | 40.4 (38.2, 42.5) | <0.001 | 151 | 43.5 (42.6, 44.3) | 0.238 |
| Asbestos industries | 162 | 33.0 (31.7, 34.3) | 255 | 41.3 (40.6, 42.1) | ||
| Shipyards | 25 | 37.3 (35.1, 39.5) | 71 | 42.5 (41.4, 43.6) | ||
| Asbestos-containing building | 62 | 36.2 (34.6, 37.9) | 18 | 42.4 (40.6, 44.3) | ||
| Others | 27 | 39.2 (37.0, 41.4) | 10 | 42.4 (40.0, 44.8) | ||
| Variable | Malignant Mesothelioma | Lung Cancer | ||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Occupational exposure | ||||
| Exposure duration (years) | −0.068 (−0.156, 0.019) | 0.126 | 0.001 (−0.049, 0.052) | 0.960 |
| Age of first exposure (years) | −1.023 (−1.121, −0.925) | <0.001 | −0.990 (−1.044, −0.936) | <0.001 |
| Lifetime smoking (in pack years) | −0.020 (−0.059, 0.020) | 0.325 | −0.004 (−0.030, 0.023) | 0.775 |
| Environmental exposure | ||||
| Distance (km) | 0.854 (−0.071, 1.779) | 0.069 | 0.397 (−0.085, 0.878) | 0.106 |
| Exposure duration (years) | −0.018 (−0.135, 0.099) | 0.755 | 0.000 (−0.045, 0.046) | 0.987 |
| Age of first exposure (years) | −0.985 (−1.123, −0.847) | <0.001 | −0.960 (−1.014, −0.906) | <0.001 |
| Lifetime smkoing (in pack years) | −0.017 (−0.066, 0.032) | 0.484 | −0.001 (−0.041, 0.039) | 0.974 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, D.-A.; Chae, W.-R.; Choi, Y.-H.; Kang, M.-S.; Lee, Y.-J.; Moon, K.-W. Disease Latency according to Asbestos Exposure Characteristics among Malignant Mesothelioma and Asbestos-Related Lung Cancer Cases in South Korea. Int. J. Environ. Res. Public Health 2022, 19, 15934. https://doi.org/10.3390/ijerph192315934
Huh D-A, Chae W-R, Choi Y-H, Kang M-S, Lee Y-J, Moon K-W. Disease Latency according to Asbestos Exposure Characteristics among Malignant Mesothelioma and Asbestos-Related Lung Cancer Cases in South Korea. International Journal of Environmental Research and Public Health. 2022; 19(23):15934. https://doi.org/10.3390/ijerph192315934
Chicago/Turabian StyleHuh, Da-An, Woo-Ri Chae, Yun-Hee Choi, Min-Sung Kang, Yong-Jin Lee, and Kyong-Whan Moon. 2022. "Disease Latency according to Asbestos Exposure Characteristics among Malignant Mesothelioma and Asbestos-Related Lung Cancer Cases in South Korea" International Journal of Environmental Research and Public Health 19, no. 23: 15934. https://doi.org/10.3390/ijerph192315934
APA StyleHuh, D.-A., Chae, W.-R., Choi, Y.-H., Kang, M.-S., Lee, Y.-J., & Moon, K.-W. (2022). Disease Latency according to Asbestos Exposure Characteristics among Malignant Mesothelioma and Asbestos-Related Lung Cancer Cases in South Korea. International Journal of Environmental Research and Public Health, 19(23), 15934. https://doi.org/10.3390/ijerph192315934






