Assessment of Standing Multi-Frequency Bioimpedance Analyzer to Measure Body Composition of the Whole Body and Limbs in Elite Male Wrestlers
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Considerations
2.3. Procedure
2.4. Bioimpedance Analysis
2.5. Dual-Energy X-ray Absorptiometry
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horswill, C.A. Applied physiology of amateur wrestling. Sport. Med. 1992, 14, 114–143. [Google Scholar] [CrossRef] [PubMed]
- Lansky, R.C. Wrestling and Olympic-style lifts: In-season maintenance of power and anaerobic endurance. Strength Cond. J. 1999, 21, 21–27. [Google Scholar] [CrossRef]
- Vardar, S.A.; Tezel, S.; Öztürk, L.; Kaya, O. The relationship between body composition and anaerobic performance of elite young wrestlers. J. Sport. Sci. Med. 2007, 6, 34–38. [Google Scholar]
- Kelly, J.M.; Goreney, B.A.; Kalm, K.K. The effects of a collegiate wrestling season on body composition, cardiovascular fitness and muscular strength and endurance. Med. Sci. Sport. 1978, 10, 119–124. [Google Scholar]
- Roemmich, J.N.; Sinning, W.E. Sport-seasonal changes in body composition, growth, power and strength of adolescent wrestlers. Int. J. Sport. Med. 1996, 17, 92–99. [Google Scholar] [CrossRef]
- Roemmich, J.N.; Sinning, W.E. Weight loss and wrestling training: Effects on nutrition, growth, maturation, body composition, and strength. J. Appl. Physiol. 1997, 82, 1751–1759. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Raymond-Pope, C.J. New frontiers of body composition in sport. Int. J. Sport. Med. 2021, 42, 588–601. [Google Scholar] [CrossRef]
- Roelofs, E.; Bockin, A.; Bosch, T.; Oliver, J.; Bach, C.W.; Carbuhn, A.; Stanforth, P.R.; Dengel, D.R. Body composition of National Collegiate Athletic Association (NCAA) Division I female soccer athletes through competitive seasons. Int. J. Sport. Med. 2020, 41, 766–770. [Google Scholar] [CrossRef]
- Czeck, M.A.; Raymond-Pope, C.J.; Stanforth, P.R.; Carbuhn, A.; Bosch, T.A.; Bach, C.W.; Oliver, J.M.; Dengel, D.R. Total and regional body composition of NCAA Division I collegiate female softball athletes. Int. J. Sport. Med. 2019, 40, 645–649. [Google Scholar] [CrossRef]
- Chiarlitti, N.A.; Delisle-Houde, P.; Reid, R.E.; Kennedy, C.; Andersen, R.E. Importance of body composition in the national hockey league combine physiological assessments. J. Strength Cond. Res. 2018, 32, 3135–3142. [Google Scholar] [CrossRef]
- Bosch, T.A.; Carbuhn, A.; Stanforth, P.R.; Oliver, J.M.; Keller, K.A.; Dengel, D.R. Body composition and bone mineral density of Division I collegiate football players: A consortium of college athlete research study. J. Strength Cond. Res. 2019, 33, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Prokop, N.W.; Reid, R.E.; Andersen, R.E. Seasonal changes in whole body and regional body composition profiles of elite collegiate ice-hockey players. J. Strength Cond. Res. 2016, 30, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Milanese, C.; Cavedon, V.; Corradini, G.; De Vita, F.; Zancanaro, C. Seasonal DXA-measured body composition changes in professional male soccer players. J. Sport. Sci. 2015, 33, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.A.; Burruss, T.P.; Weir, N.L.; Fielding, K.A.; Engel, B.E.; Weston, T.D.; Dengel, D.R. Abdominal body composition differences in NFL football players. J. Strength Cond. Res. 2014, 28, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Allison, D.B.; Kotler, D.P.; Ross, R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am. J. Clin. Nutr. 2002, 75, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Berker, D.; Kopoaral, S.; Isik, S.; Paşaoğlu, L.; Aydin, Y.; Erol, K.; Delibaşi, T.; Güler, S. Compatibliity of different methods for the measurement of visceral fat in different body mass index strata. Diagn. Interv. Radiol. 2010, 16, 99–105. [Google Scholar] [CrossRef]
- Bonora, E.; Micciolo, R.; Ghiatas, A.A.; Lancaster, J.L.; Alyassin, A.; Muggeo, M.; Defronzo, R.A. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism 1995, 44, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J. Human body composition: In vivo methods. Physiol. Rev. 2000, 80, 649–680. [Google Scholar] [CrossRef] [PubMed]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kyttä, J.; Cancino, W.; Rinta-Kiikka IPertuz, S.; Arponen, O. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; Ahlgren, A.; Romu, T.; Widholm, P.; Leinhard, O.D.; West, J. Reproducibility and repeatability of MRI-based body composition analysis. Magn. Reson. Med. 2000, 84, 3146–3156. [Google Scholar] [CrossRef]
- Sanfilippo, J.; Krueger, D.; Heiderscheit, B.; Binkley, N. Dual energy X-ray absorptiometry body composition in NCAA Division I athletes: Exploration of mass distribution. Sport. Health 2019, 11, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 21, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Gobbo, L.A.; Stagi, S.; Cyrino, L.T.; Toselli, S. Bioelectrical impedance analysis versus reference methods in the assessment of body composition in athletes. Eur. J. Appl. Physiol. 2022, 122, 561–589. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Blue, M.N.M.; Hirsch, K.T.; Saylor, H.E.; Gould, L.M.; Nelson, A.G.; Smith-Ryan, A.E. Validation of InBody 770 bioelectrical impedance analysis compared to four-compartment model criterion in young adults. Clin. Physiol. Funct. Imaging 2021, 41, 317–325. [Google Scholar] [CrossRef]
- Antnio, J.; Kenyon, M.; Ellerbroek, A.; Carson, C.; Burgess, V.; Tyler-Palmer, D.; Mike, J.; Roberts, J.; Angeli, G.; Peacock, C. Comparison of dual-energy X-ray absorptiometry (DXA) versus a multi-frequency bioelectrical impedance (InBody 770) device for body composition assessment after a 4-week hypoenergetic diet. J. Funct. Morphol. Kinesiol. 2019, 4, 23. [Google Scholar] [CrossRef]
- Antonio, J.; Kenyon, M.; Ellerbroek, A.; Carson, C.; Yuler-Palmer, D.; Burgess, V.; Angeli, G.; Silver, T.; Jiannine, L.; Peacock, C. Body composition assessment: A comparison of the Bod Pod, InBody 770, and DXA. J. Exerc. Nutr. 2019, 2, 11. [Google Scholar]
- McLester, C.N.; Nickerson, B.S.; KLliszczweicz McLester, J.R. Reliability and agreement of various InBody body composition analyzers as compared to Duaal-energy X-ray absorptiometry in healthy men and women. J. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, M.; Yoshida, T.; Miyachi, M.; Arai, H. Validating muscle mass cutoffs of four international sarcopenia-working groups in Japanese people using DXA and BIA. J. Cachexia Sarcopenia Muscle 2021, 12, 1000–1010. [Google Scholar] [CrossRef]
- Hurt, R.T.; Ebbert, J.O.; Crohan, I.; Nanda, S.; Schroeder, D.R.; Teigen, L.M.; Velapati, S.R.; Mundi, M.S. The comparison of segmental multifequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry for estimating fat free mass and percentage body fat in an ambulatory population. JPEN J. Paraenter. Enteral. Nutr. 2021, 45, 1231–1238. [Google Scholar] [CrossRef]
- Carrion, B.M.; Wells, A.; Mayhew, J.L.; Koch, A.J. Concordance among bioelectrical impedance analysis measures of percent body fat in athletic young adults. Int. J. Exerc. Sci. 2019, 12, 324–331. [Google Scholar]
- Raymond, C.J.; Dengel, D.R.; Bosch, T.A. Total and segmental body composition examination in collegiate football players using multifrequency bioelectrical impedance analysis and dual X-ray absorptiometry. J. Strength Cond. Res. 2018, 32, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Blue, M.N.M.; Hirsch, K.R.; Peterjohn, A.M.; Smith-Ryan, A.E. Appendicular body composition analysis: Validity of bioelectrical impedance analysis compared with dual-energy X-ray absorptiometry in division I college athletes. J. Strength Cond. Res. 2019, 33, 2920–2925. [Google Scholar] [CrossRef]
- Munguia-Izquierdo, D.; Suarez-Arrones, L.; Salvo, V.D.; Paredes-Hernadez, V.; Alcazar, J.; Ara, I.; Kreider, R.; Mendez-Villanueva, A. Validation of field methods to assess body fat percentage in elite youth soccer players. Int. J. Sport. Med. 2018, 39, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Utter, A.; Lambeth, P.G. Evaluation of multifrequency bioelectrical impedance analysis in assessing body composition of wrestlers. Med. Sci. Sport Exerc. 2010, 42, 361–367. [Google Scholar] [CrossRef]
- Bazzocchi, A.; Ponti, F.; Albisnni, U.; Battista, G.; Guglielmi, G. DXA: Technical aspects and application. Eur. J. Radiol. 2016, 85, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Esco, M.R.; Snarr, R.L.; Leatherwood, M.D.; Chamberlain, N.A.; Redding, M.L.; Flatt, A.A.; Moon, J.R.; Williford, H.N. Comparison of total and segmental body composition using DXA and multifrequency bioimpedance in collegiate female athletes. J. Strength Cond. Res. 2015, 29, 918–925. [Google Scholar] [CrossRef]
- Sardinha, L.B.; Correia, I.R.; Magalhaes, J.P.; Judice, P.B.; Silva, A.M.; Hetherington-Rauth, M. Development and validation of BIA prediction equations of upper and lower limb lean soft tissue in athletes. Eur. J. Clin. Nutr. 2020, 74, 1646–1652. [Google Scholar] [CrossRef]
- Hong, S.; Oh, H.J.; Cho, H.; Kim, J.G.; Lim, S.K.; Kim, E.K.; Pyo, E.Y.; Oh, K.; Kim, Y.T.; WILSON, K.; et al. Characteristics of body fat, body fat percentage and other body composition for Koreans from KNHANES IV. J. Korean Med. Sci. 2011, 26, 1599–1605. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef]
- Aloui, A.; Chtourou, H.; Souissi, N. Weight reduction cycles and effects in Tawkwondo. In Performance Optimization in Taekwondo: From Laboratory to Field; Haddad, M., Ed.; OMICS International: Foster City, CA, USA; pp. 131–136.
- Bešlija, T.; Čular, D.; Kezić, A.; Tomljanović, M.; Ardigò, L.P.; Dhabhi, W.; Padulo, J. Height-based model for the categorization of athletes in combat sports. Appl. Sport Sci. 2020, 21, 471–480. [Google Scholar] [CrossRef]
| Light Weight (n = 12) | Middle Weight (n = 40) | Heavy Weight (n = 14) | All (n = 66) | |
|---|---|---|---|---|
| Age (year) | 20.1 ± 1.1 (18.7, 21.1) | 20.6 ± 1.1 (18.7, 22.3) | 21.1 ± 1.0 (19.4, 22.3) | 20.6 ± 1.1 (18.7, 22.3) |
| Weight (kg) | 60.6 ± 2.7 (56.4, 63.9) | 71.8 ± 5.6 (64.1, 83.8) | 103.1 ± 13.0 (84.0, 127.9) | 76.4 ± 16.3 (56.4, 127.9) |
| Height (cm) | 164.2 ± 4.5 (156.5, 171.0) | 170.4 ± 4.3 (162.0, 181.0) | 176.7 ± 7.0 (170.0, 196.0) | 170.6 ± 6.3 (156.5, 196.0) |
| BMI (kg/m2) | 22.5 ± 1.2 (20.7, 24.5) | 24.8 ± 2.1 (20.9, 29.1) | 33.0 ± 3.2 (27.5, 37.5) | 26.1 ± 4.3 (20.7,37.5) |
| Percent Body Fat (%) | 10.7 ± 3.7 (5.1, 17.7) | 15.7 ± 7.2 (8.1, 36.2) | 30.3 ± 5.0 (21.8, 37.1) | 17.9 ± 9.2 (5.1, 37.1) |
| FFM (kg) | 54.5 ± 2.9 (50.2, 60.8) | 60.8 ± 4.8 (49.4, 74.3) | 71.4 ± 6.1 (65.0, 86.1) | 61.9 ± 7.3 (49.4, 86.1) |
| LSTM (kg) | 51.4 ± 2.7 (47.6, 57.3) | 57.3 ± 4.9 (46.1, 70.4) | 67.5 ± 6.1 (60.8, 82.2) | 58.4 ± 7.0 (46.1, 82.2) |
| TBW (kg) | 40.2 ± 2.1 (36.9, 44.8) | 44.8 ± 3.5 (36.4, 54.8) | 52.6 ± 4.5 (47.9, 63.4) | 43.0 ± 5.4 (36.4, 63.4) |
| Training experience (year) | 7.9 ± 1.9 (6.7, 9.3) | 8.2 ± 1.6 (6.9, 8.9) | 8.8 ± 1.3 (5.9, 9.5) | 8.5 ± 2.1 (6.7, 9.5) |
| Limit of Agreement | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MFBIA | DXA | ρ | CCC | p 1 | Cb | Bias | Lower | Upper | Regression Line | Trend | |
| FFMWB (kg) | 61.97 ± 8.10 | 61.89 ± 7.27 | 0.958 | 0.953 | 0.790 | 0.994 | 0.077 | −4.523 | 4.683 | Y = −6.956 + 0.109x | ** |
| LSTMWB (kg) | 58.55 ± 7.59 | 58.40 ± 7.02 | 0.954 | 0.951 | 0.519 | 0.997 | 0.151 | −4.332 | 4.635 | Y = −4.475 + 0.079x | * |
| BFMWB (kg) | 14.02 ± 9.47 | 14.09 ± 10.16 | 0.982 | 0.979 | 0.772 | 0.997 | −0.07 | −3.963 | 3.802 | Y = 0.930 − 0.710x | ** |
| PBFWB (%) | 17.11 ± 7.51 | 17.91 ± 9.20 | 0.962 | 0.938 | * | 0.975 | −0.795 | −6.362 | 4.771 | Y = 2.822 − 0.206x | *** |
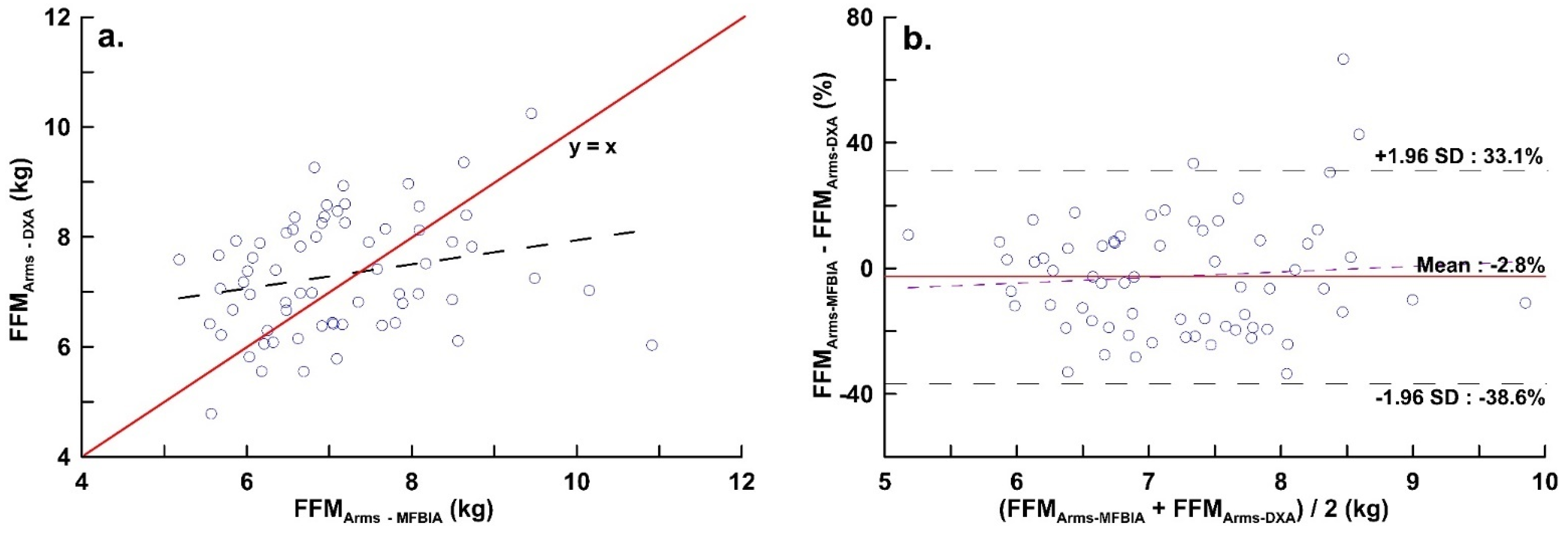
| FFMRA (kg) | 3.58 ± 0.58 | 3.69 ± 0.57 | 0.238 | 0.233 | 0.186 | 0.979 | −0.117 | −1.526 | 1.285 | Y = −0.268 + 0.041x | 0.834 |
| FFMLA (kg) | 3.54 ± 0.57 | 3.61 ± 0.53 | 0.224 | 0.221 | 0.420 | 0.990 | −0.069 | −1.424 | 1.283 | Y = −0.439 + 0.103x | 0.604 |
| FFMTK (kg) | 27.36 ± 3.47 | 29.83 ± 4.27 | 0.929 | 0.755 | *** | 0.813 | −2.467 | −5.714 | 0.777 | Y = 3.689 − 0.215x | *** |
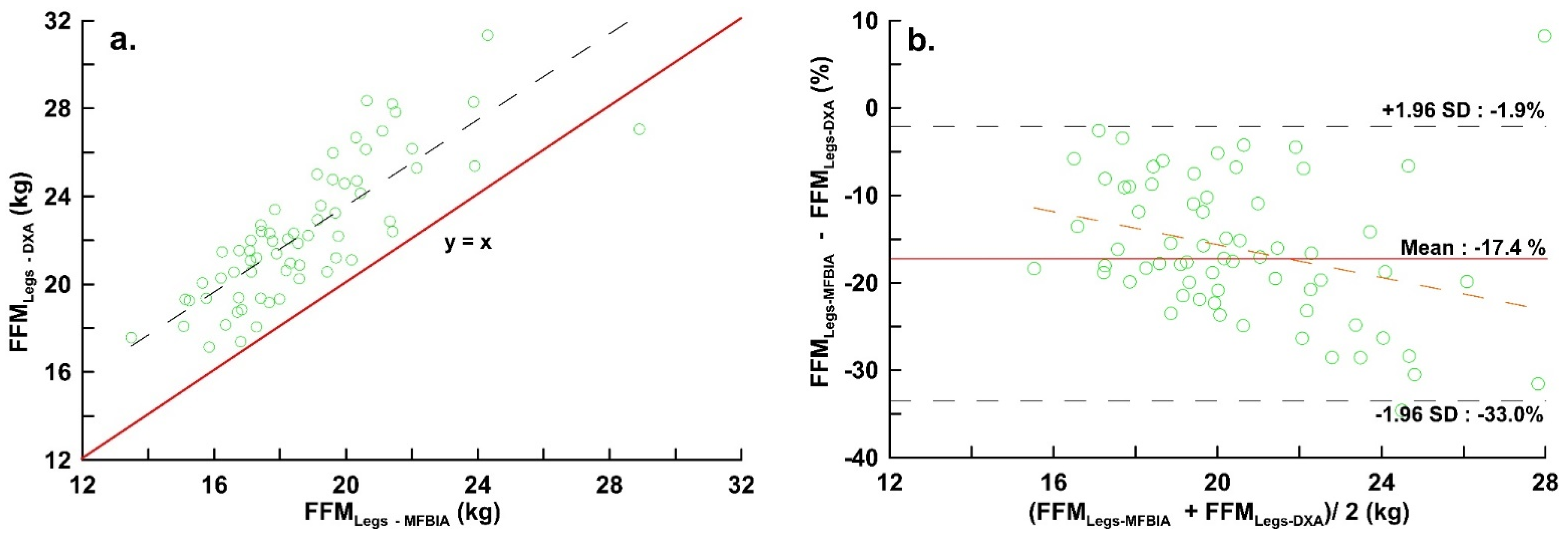
| FFMRL (kg) | 9.39 ± 1.30 | 11.19 ± 1.61 | 0.795 | 0.440 | *** | 0.554 | −1.796 | −3.715 | 0.121 | Y = 0.645 − 2.372x | *** |
| FFMLL (kg) | 9.32 ± 1.27 | 11.12 ± 2.32 | 0.811 | 0.434 | *** | 0.536 | −1.798 | −3.546 | −0.054 | Y = 0.247 − 0.200x | * |
| FFMArms (kg) | 7.12 ± 1.15 | 7.31 ± 1.06 | 0.237 | 0.233 | 0.273 | 0.983 | −0.186 | −2.877 | 2.504 | Y = −1.129 + 0.130x | 0.506 |
| FFMLegs (kg) | 18.71 ± 2.56 | 22.30 ± 3.10 | 0.809 | 0.440 | *** | 0.544 | −3.594 | −7.173 | −0.015 | Y = 0.705 − 0.209x | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-K.; Ho, C.-Y.; Lai, C.-L.; Taun, C.-Y.; Hsieh, K.-C. Assessment of Standing Multi-Frequency Bioimpedance Analyzer to Measure Body Composition of the Whole Body and Limbs in Elite Male Wrestlers. Int. J. Environ. Res. Public Health 2022, 19, 15807. https://doi.org/10.3390/ijerph192315807
Lai Y-K, Ho C-Y, Lai C-L, Taun C-Y, Hsieh K-C. Assessment of Standing Multi-Frequency Bioimpedance Analyzer to Measure Body Composition of the Whole Body and Limbs in Elite Male Wrestlers. International Journal of Environmental Research and Public Health. 2022; 19(23):15807. https://doi.org/10.3390/ijerph192315807
Chicago/Turabian StyleLai, Yeong-Kang, Chu-Ying Ho, Chung-Liang Lai, Chih-Yang Taun, and Kuen-Chang Hsieh. 2022. "Assessment of Standing Multi-Frequency Bioimpedance Analyzer to Measure Body Composition of the Whole Body and Limbs in Elite Male Wrestlers" International Journal of Environmental Research and Public Health 19, no. 23: 15807. https://doi.org/10.3390/ijerph192315807
APA StyleLai, Y.-K., Ho, C.-Y., Lai, C.-L., Taun, C.-Y., & Hsieh, K.-C. (2022). Assessment of Standing Multi-Frequency Bioimpedance Analyzer to Measure Body Composition of the Whole Body and Limbs in Elite Male Wrestlers. International Journal of Environmental Research and Public Health, 19(23), 15807. https://doi.org/10.3390/ijerph192315807







