Catalytic Pyrolysis of Sawdust with Desulfurized Fly Ash for Pyrolysis Gas Upgrading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
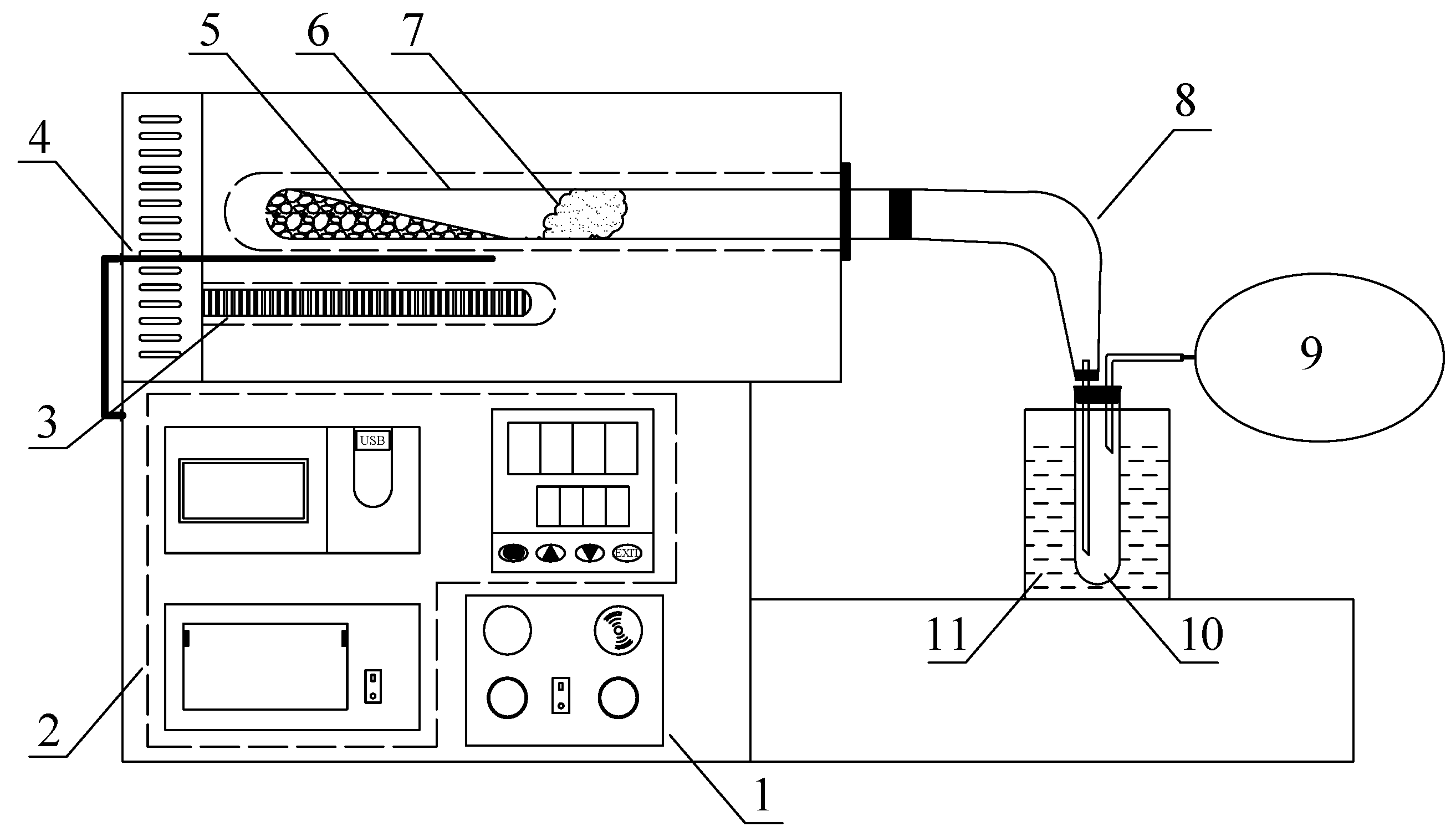
2.2. Pyrolysis Experiment
2.3. Thermogravimetric Analysis
2.4. Gas Chromatography Experiment
3. Results
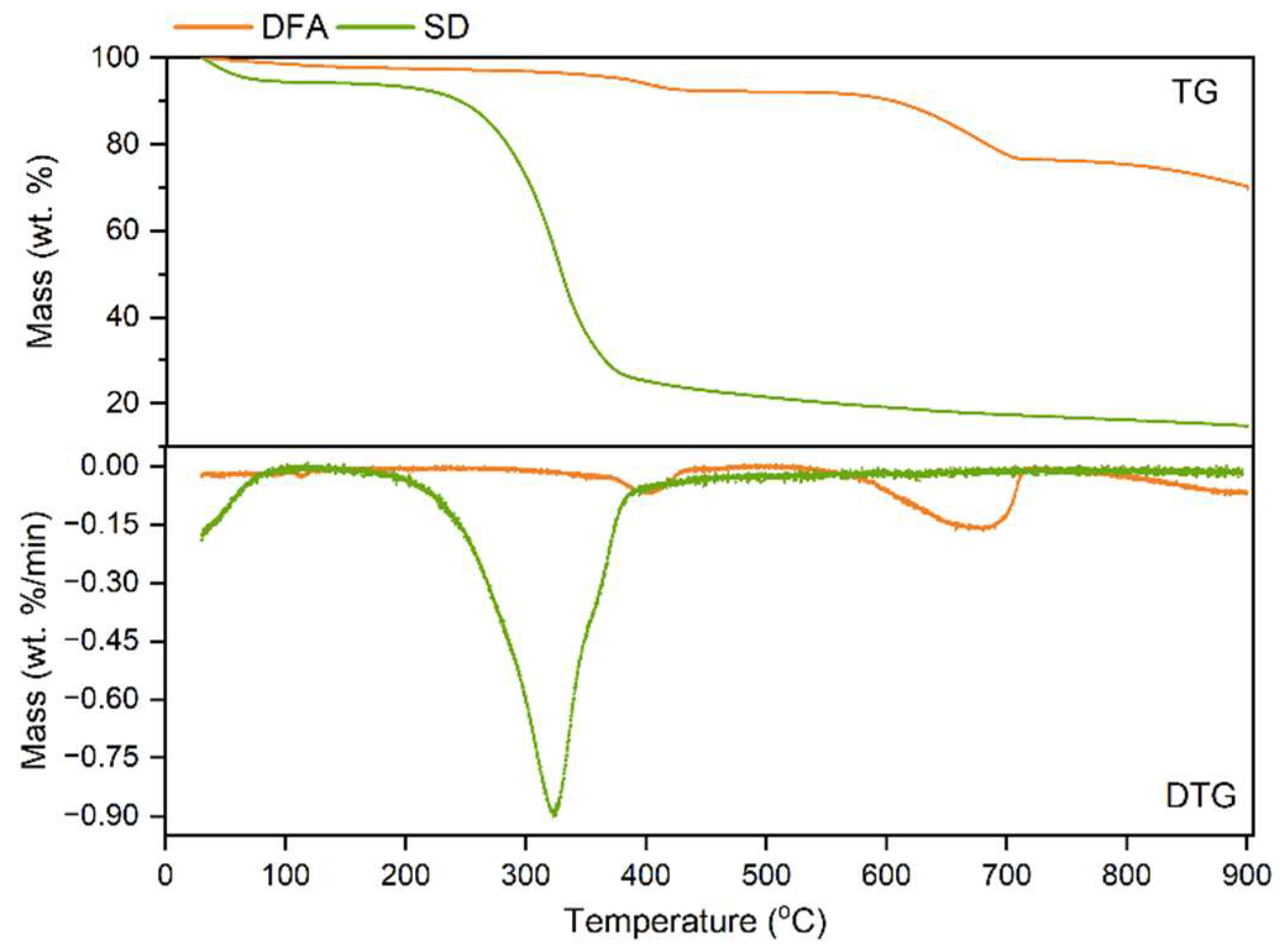
3.1. TG Analysis of SD and DFA
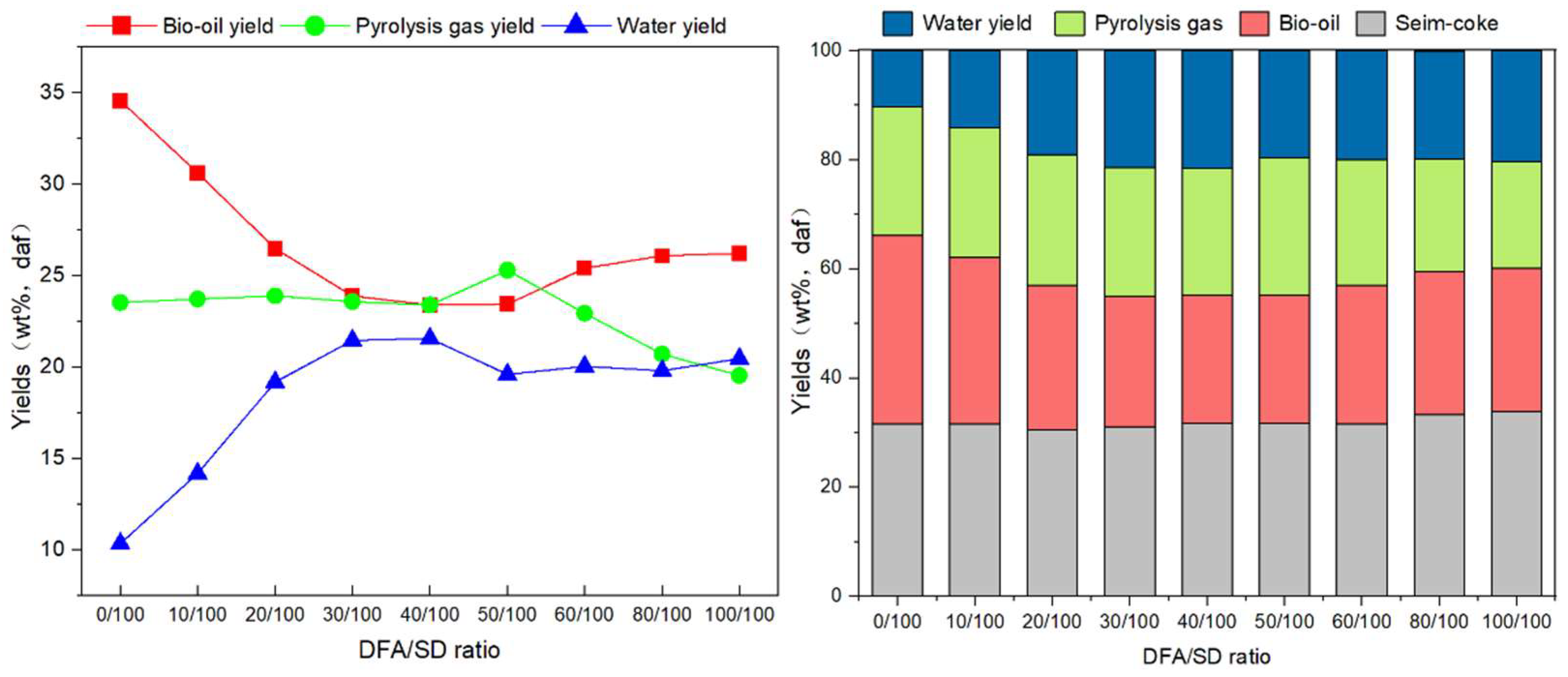
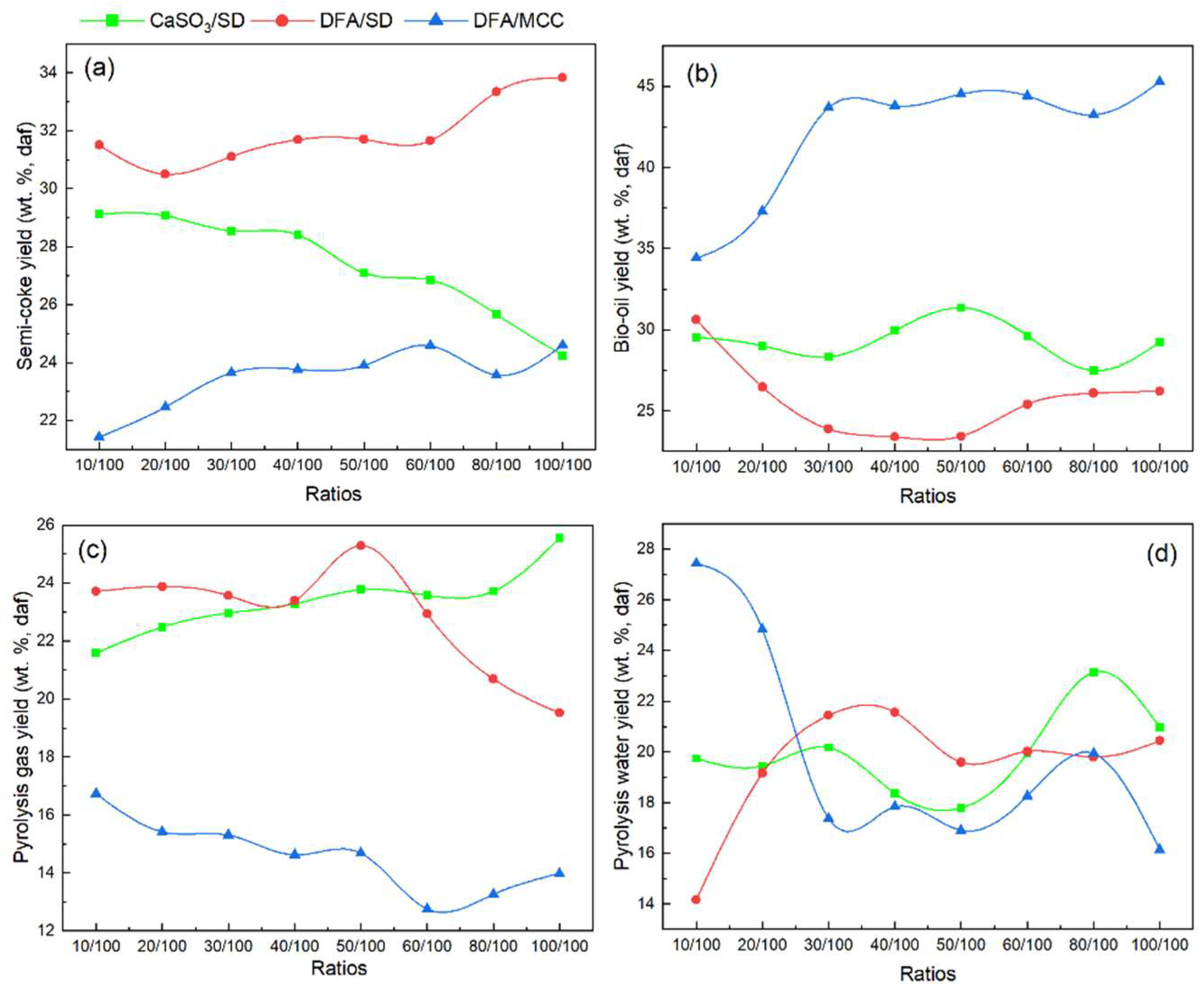
3.2. Product Distribution from DFA/SD Pyrolysis
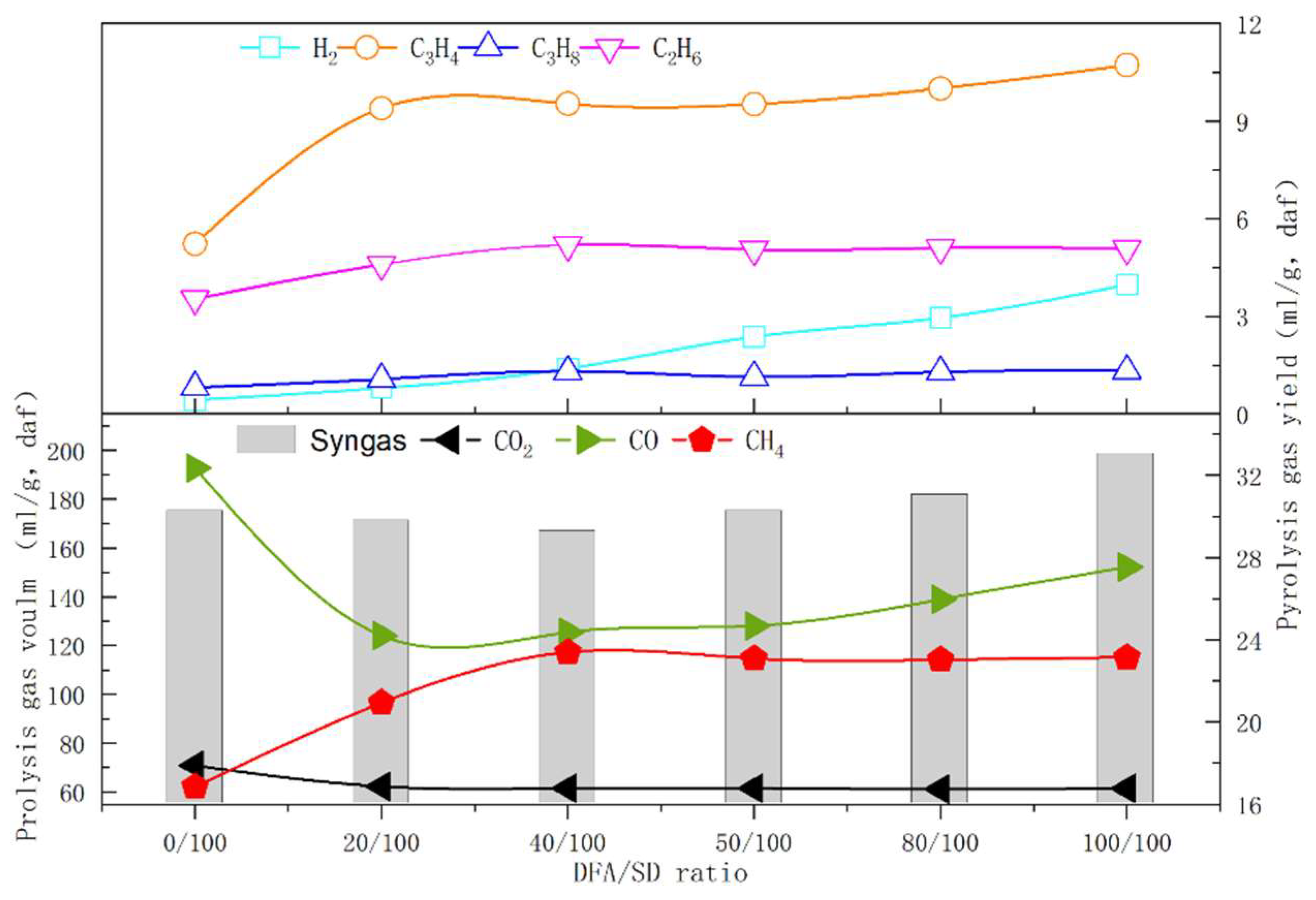
3.3. Effects of Material Components on Pyrolysis Gas Products
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kwon, E.E.; Cho, S.H.; Kim, S. Synergetic sustainability enhancement via utilization of carbon dioxide as carbon neutral chemical feedstock in the thermo-chemical processing of biomass. Environ. Sci. Technol. 2015, 49, 5028–5034. [Google Scholar] [CrossRef] [PubMed]
- Pileidis, F.D.; Titirici, M.M. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 2016, 9, 562–582. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.K.; Hameed, B.H. Recent progress on biomass co-pyrolysis conversion into high-quality bio-oil. Bioresour. Technol. 2016, 221, 645–665. [Google Scholar] [CrossRef] [PubMed]
- Yiin, C.L.; Yusup, S.; Quitain, A.T.; Uemura, Y.; Sasaki, M.; Kida, T. Thermogravimetric analysis and kinetic modeling of low-transition-temperature mixtures pretreated bio-oil palm empty fruit bunch for possible maximum yield of pyrolysis bio-oil. Bioresour. Technol. 2018, 255, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Ruan, R. The effects of torrefaction on compositions of bio-bio-oil and syngas from biomass pyrolysis by microwave heating. Bioresour. Technol. 2013, 135, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Kong, S.; Liu, Y.; Zeng, H. Syngas production by two-stage method of biomass catalytic pyrolysis and gasification. Bioresour. Technol. 2012, 110, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Veses, A.; Aznar, M. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Xu, X. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 150479. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar]
- Deng, L.; Huang, X.; Tie, Y.; Jiang, J.; Zhang, K.; Ma, S.; Che, D. Experimental study on transformation of alkali and alkaline earth metals during biomass gasification. J. Energy Inst. 2022, 103, 117–127. [Google Scholar] [CrossRef]
- Qin, W.; Luo, L.; Chen, S.; Iqbal, T.; Xiao, X.; Dong, C. Efficient strategy of utilizing alkaline liquid waste boosting biomass chemical looping gasification to produce hydrogen. Fuel Process. Technol. 2021, 217, 106818. [Google Scholar] [CrossRef]
- Song, H.; Yang, G.; Xue, P.; Li, Y.; Zou, J.; Wang, S.; Chen, H. Recent development of biomass gasification for H2 rich gas production. Appl. Energy Combust. Sci. 2022, 10, 100059. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Gong, H.; Chen, L.; Yu, H. Study on the feasibility of using monolithic catalyst in the in-situ catalytic biomass pyrolysis for syngas production. Waste Manag. 2020, 120, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Saeaung, K.; Phusunti, N.; Phetwarotai, W.; Assabumrungrat, S.; Cheirsilp, B. Catalytic pyrolysis of petroleum-based and biodegradable plastic waste to obtain high-value chemicals. Waste Manag. 2021, 127, 101–111. [Google Scholar] [CrossRef]
- Ma, K.; Deng, J.; Ma, P.; Sun, C.; Zhou, Q.; Xu, J. A novel plant-internal route of recycling sulfur from the flue gas desulfurization (FGD) ash through sintering process: From lab-scale principles to industrial practices. J. Environ. Chem. Eng. 2021, 10, 106597. [Google Scholar] [CrossRef]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2018, 342, 436–445. [Google Scholar] [CrossRef]
- Obis, M.F.; Germain, P.; Troesch, O.; Spillemaecker, M.; Benbelkacem, H. Valorization of MSWI bottom ash for biogas desulfurization: Influence of biogas water content. Waste Manag. 2016, 60, 388–396. [Google Scholar] [CrossRef]
- Wang, T.; Wu, K.; Wu, M. Development of green binder systems based on flue gas desulfurization gypsum and fly ash incorporating slag or steel slag powders. Constr. Build. Mater. 2020, 265, 12075. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Potential of industrial de-dusting residues as a source of potassium for fertilizer production—A mini review. Resour. Conserv. Recycl. 2019, 143, 68–76. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of low-quality desulfurized ash from semi-dry flue gas desulfurization by mixing with hemihydrate gypsum. Fuel 2019, 255, 115783. [Google Scholar] [CrossRef]
- Buchireddy, P.R.; Peck, D.; Zappi, M.; Bricka, R.M. Catalytic Hot Gas Cleanup of Biomass Gasification Producer Gas via Steam Reforming Using Nickel-Supported Clay Minerals. Energies 2021, 14, 1875. [Google Scholar] [CrossRef]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. A Lewis. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tang, C.; Yang, L.; Li, X. Characteristics of small molecule compounds produced from the co-pyrolysis of cotton stalk and coal. Energies 2021, 14, 1469–1481. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Liu, Z.; Cai, N.; Xia, S.; Chen, W. Negative-carbon pyrolysis of biomass (NCPB) over CaO originated from carbide slag for on-line upgrading of pyrolysis gas and bio-oil. J. Anal. Appl. Pyrolysis 2021, 156, 105063. [Google Scholar] [CrossRef]
- Huang, J.; Liu, C.; Tong, H.; Li, W.; Wu, D. A density functional theory study on formation mechanism of CO, CO2 and CH4 in pyrolysis of lignin. Comput. Theor. Chem. 2014, 1045, 1–9. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar]
- Dong, C.Q.; Zhang, Z.F.; Lu, Q.; Yang, Y.P. Characteristics and mechanism study of analytical fast pyrolysis of poplar wood. Energy Convers. Manag. 2012, 57, 49–59. [Google Scholar] [CrossRef]


| Proximate Analysis (wt.%) | Ultimate Analysis (wt%, daf) | ||
|---|---|---|---|
| Moisture (ad 1) | 4.22 | C | 48.44 |
| Ash (d 1) | 0.75 | H | 6.14 |
| Volatile Matter (daf 1) | 75.85 | N | 0.69 |
| Fixed Carbon (daf) | 19.18 | S | 0.09 |
| -- | -- | O 2 | 44.64 |
| Sample | Content (%) | Sample | Content (%) |
|---|---|---|---|
| CaO | 58.54 | Cl | 1.65 |
| SO3 | 21.24 | F | 1.20 |
| SiO2 | 7.77 | TiO2 | 0.36 |
| Al2O3 | 3.80 | K2O | 0.31 |
| MgO | 2.66 | Na2O | 0.18 |
| Fe2O3 | 2.16 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Tang, C.; An, X.; Wang, Y.; Zhou, S.; Huang, C. Catalytic Pyrolysis of Sawdust with Desulfurized Fly Ash for Pyrolysis Gas Upgrading. Int. J. Environ. Res. Public Health 2022, 19, 15755. https://doi.org/10.3390/ijerph192315755
Song J, Tang C, An X, Wang Y, Zhou S, Huang C. Catalytic Pyrolysis of Sawdust with Desulfurized Fly Ash for Pyrolysis Gas Upgrading. International Journal of Environmental Research and Public Health. 2022; 19(23):15755. https://doi.org/10.3390/ijerph192315755
Chicago/Turabian StyleSong, Jinling, Chuyang Tang, Xinyuan An, Yi Wang, Shankun Zhou, and Chunhong Huang. 2022. "Catalytic Pyrolysis of Sawdust with Desulfurized Fly Ash for Pyrolysis Gas Upgrading" International Journal of Environmental Research and Public Health 19, no. 23: 15755. https://doi.org/10.3390/ijerph192315755
APA StyleSong, J., Tang, C., An, X., Wang, Y., Zhou, S., & Huang, C. (2022). Catalytic Pyrolysis of Sawdust with Desulfurized Fly Ash for Pyrolysis Gas Upgrading. International Journal of Environmental Research and Public Health, 19(23), 15755. https://doi.org/10.3390/ijerph192315755







