Filamentous Bacteria and Stalked Ciliates for the Stable Structure of Aerobic Granular Sludge Treating Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analytic Methods
3. Results
3.1. Granules from SBR1 (Synthetic Wastewater)
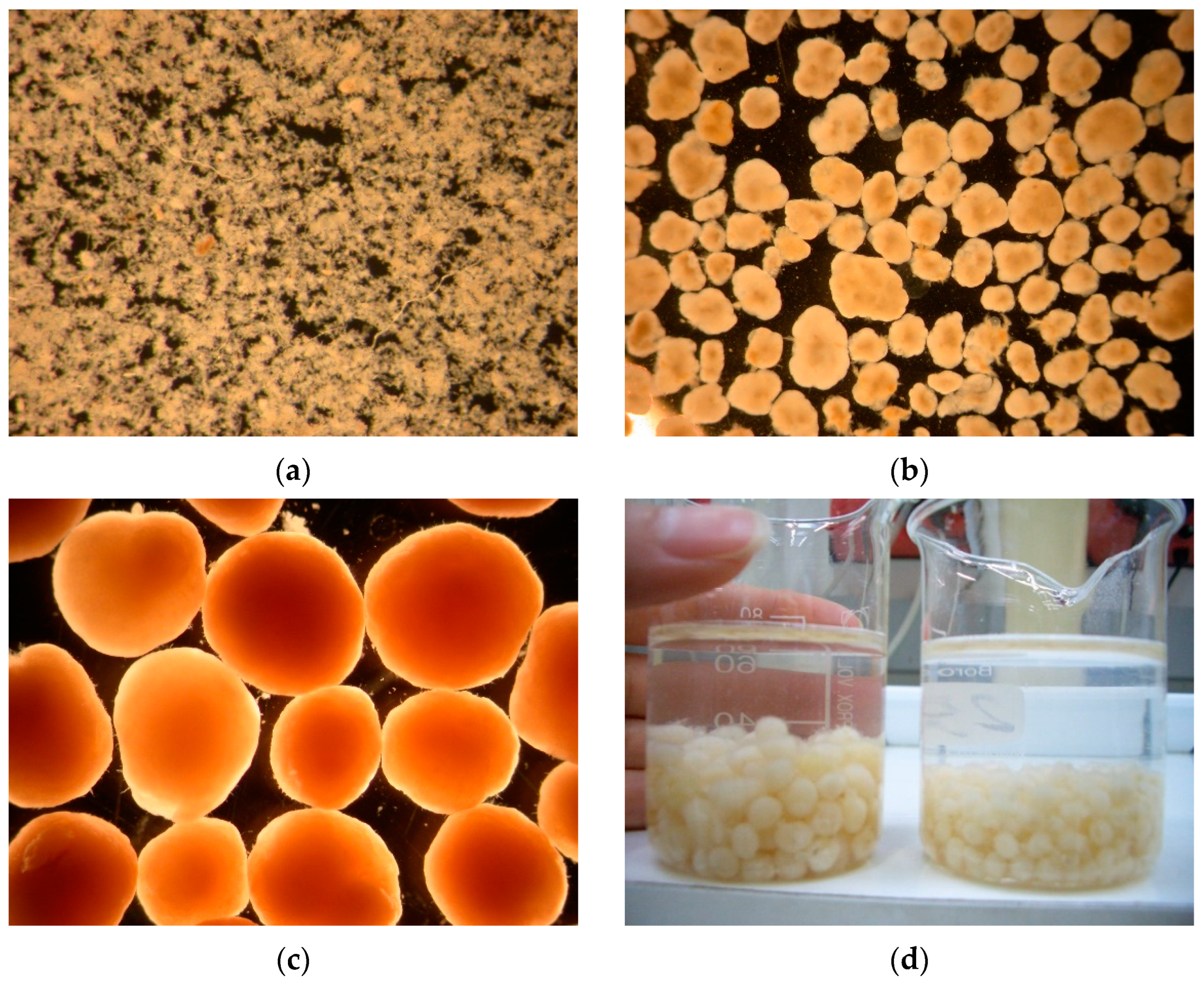
3.1.1. Morphology
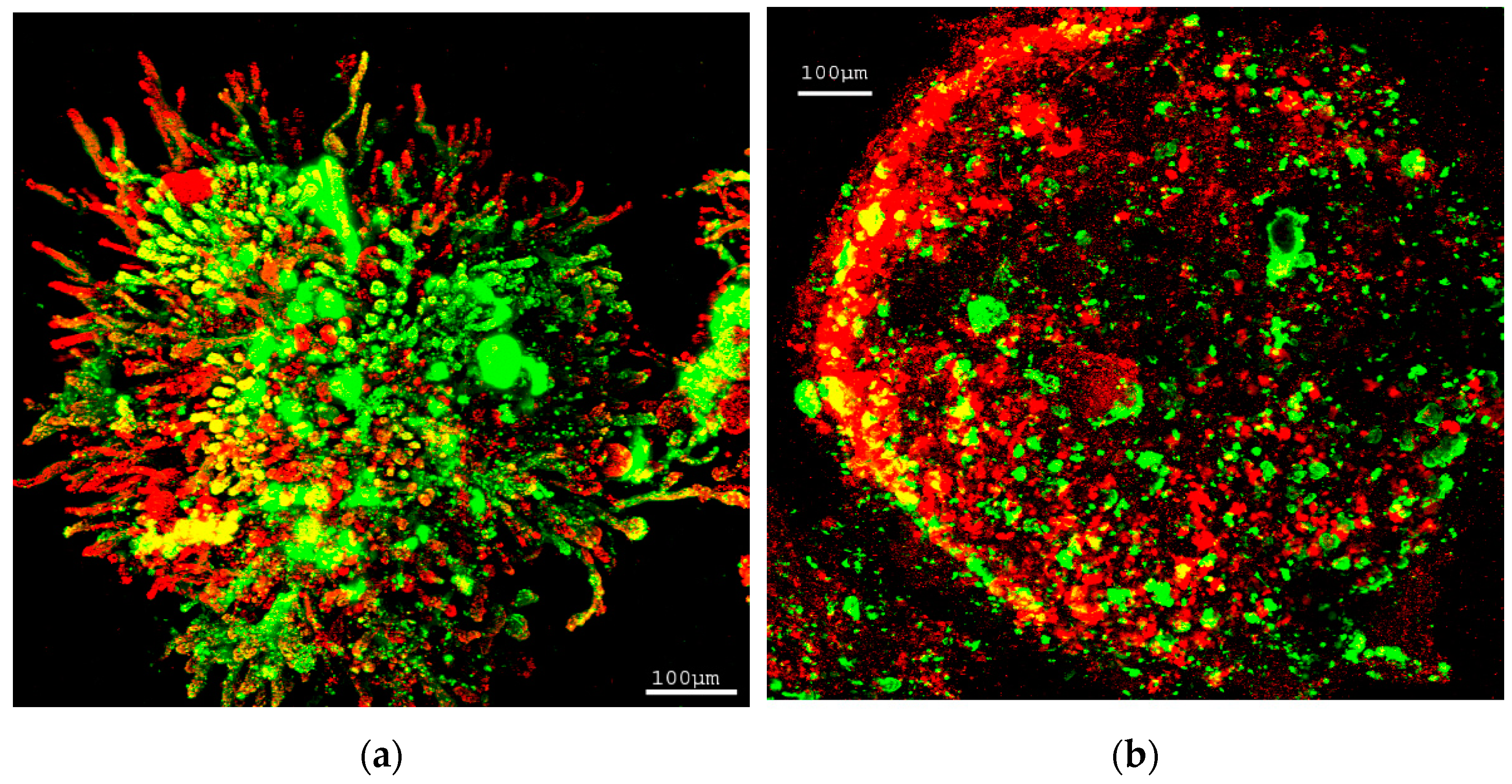
3.1.2. Granules Structure Analysis by CLSM
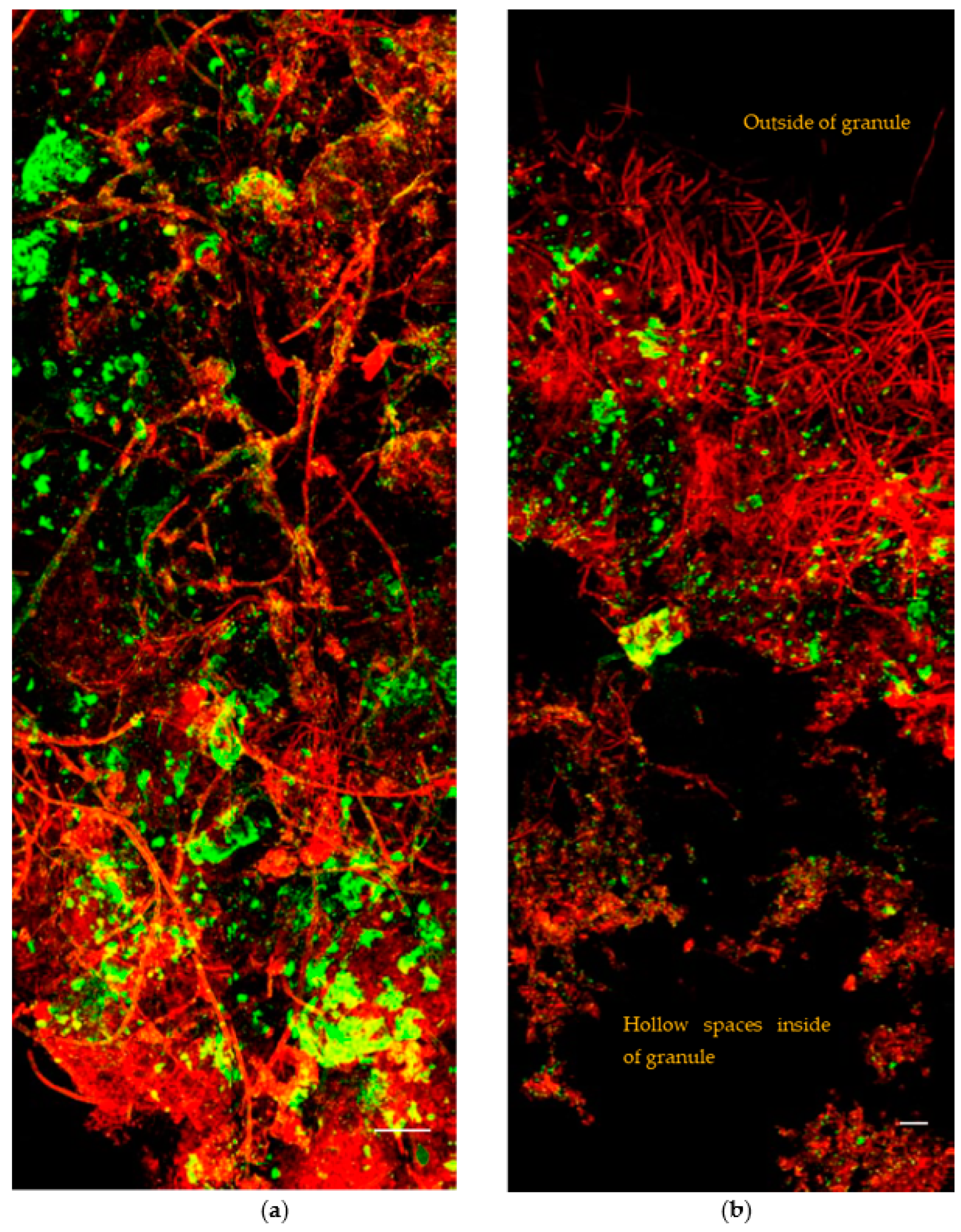
3.1.3. The Broken Granules Structure
3.2. Granules from SBR2 (Domestic Wastewater)
3.2.1. Morphology
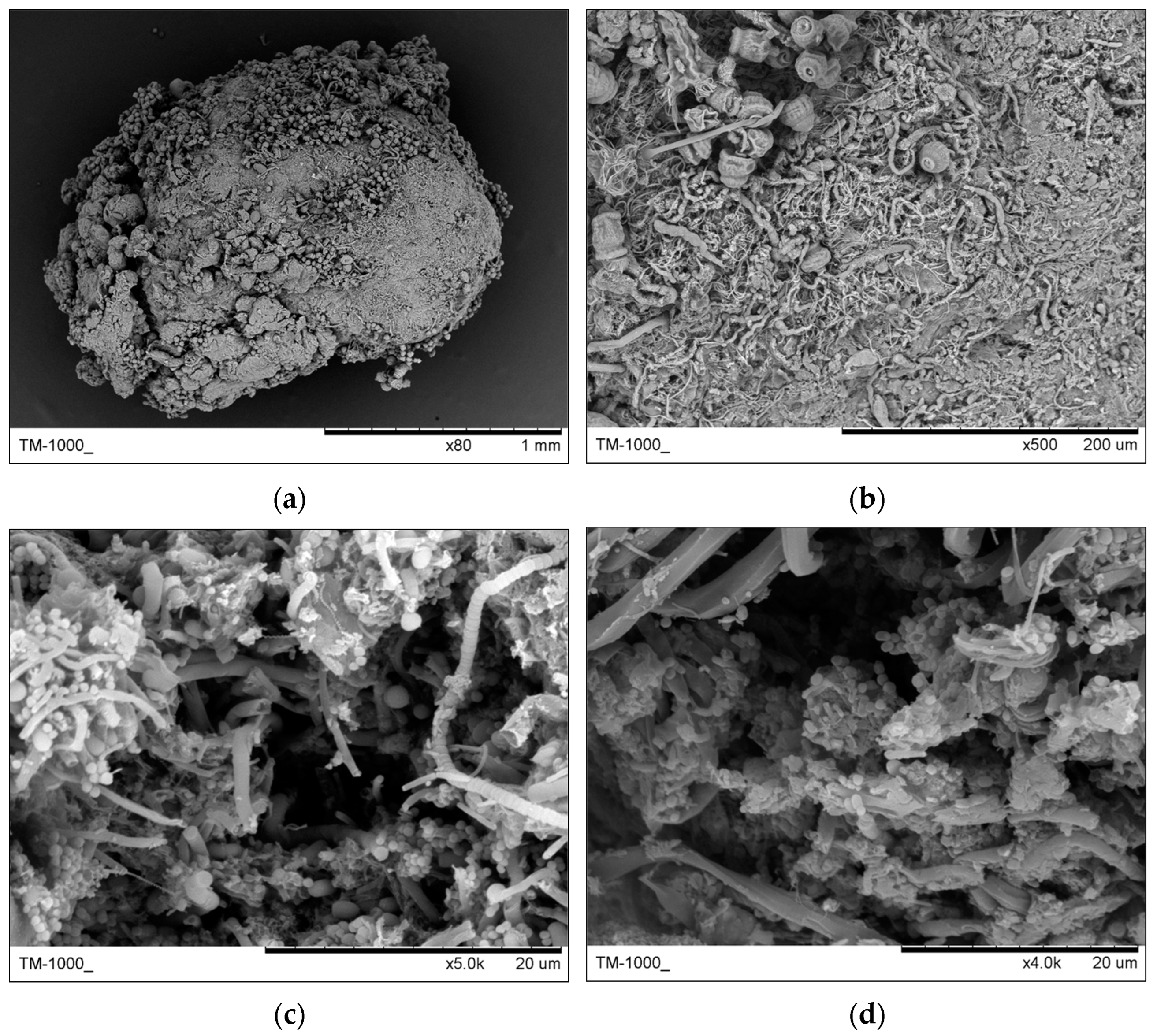
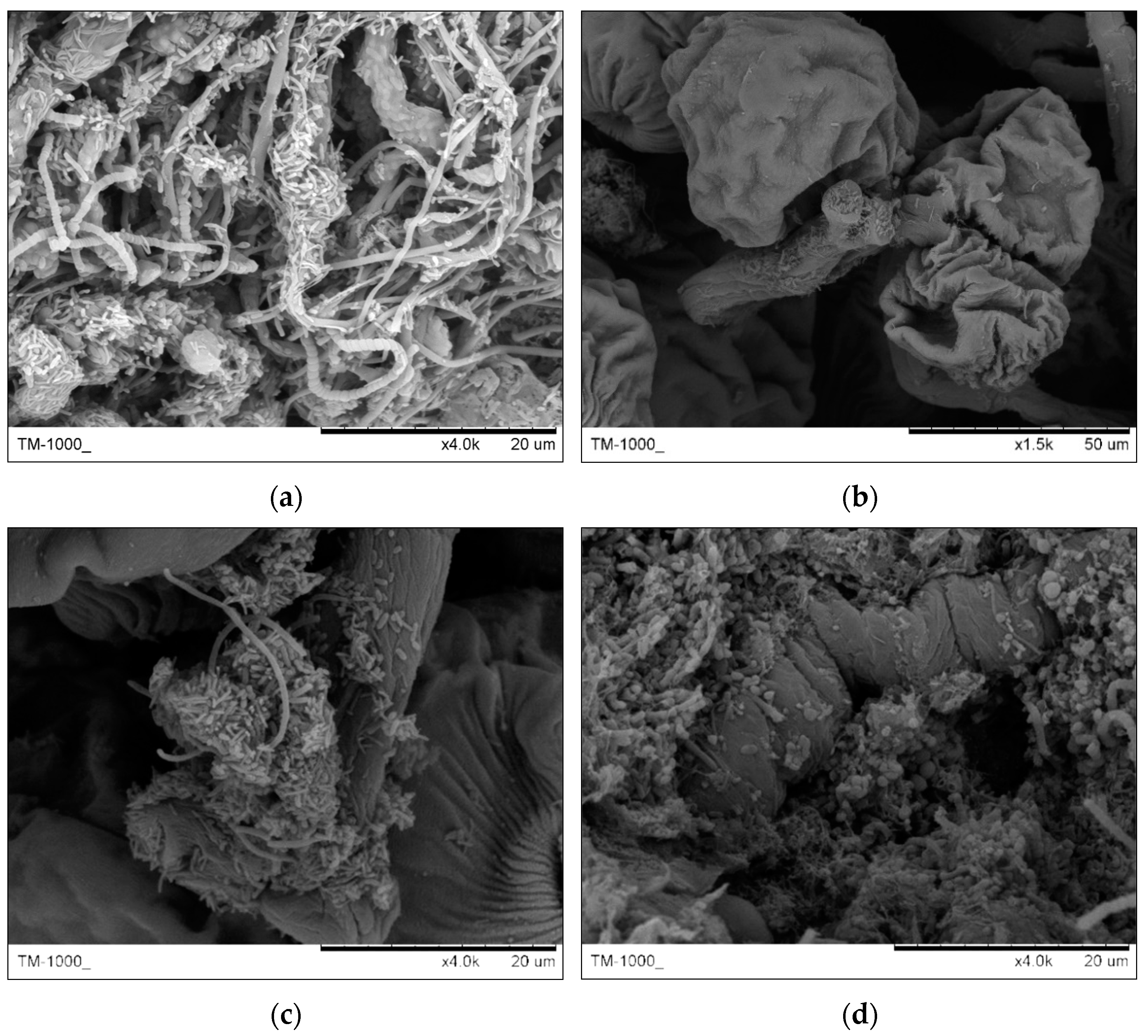
3.2.2. Granules Structure Analysis by SEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meena, M.; Yadav, G.; Sonigra, P.; Shah, M. A comprehensive review on application of bioreactor for industrial wastewater treatment. Lett. Appl. Microbiol. 2022, 74, 131–158. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Morgenroth, E.; Sherden, T.; Van Loosdrecht, M.; Heijnen, J.; Wilderer, P. Aerobic granular sludge in a sequencing batch reactor. Water Res. 1997, 31, 3191–3194. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Reddy, G.K.K. Aerobic granular sludge technology: Mechanisms of granulation and biotechnological applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef] [PubMed]
- van Loosdrecht, M.C.M.; Brdjanovic, D. Anticipating the next century of wastewater treatment. Science 2014, 344, 1452–1453. [Google Scholar] [CrossRef] [PubMed]
- Adav, S.S.; Lee, D.-J.; Show, K.-Y.; Tay, J.-H. Aerobic granular sludge: Recent advances. Biotechnol. Adv. 2008, 26, 411–423. [Google Scholar] [CrossRef]
- Show, K.-Y.; Lee, D.-J.; Tay, J.-H. Aerobic Granulation: Advances and Challenges. Appl. Biochem. Biotechnol. 2012, 167, 1622–1640. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.-K.H.; Meunier, C.; Henriet, O.; Mahillon, J.; Suárez-Ojeda, M.E.; Del Moro, G.; De Sanctis, M.; Di Iaconi, C.; Weissbrodt, D.G. An integrative review of granular sludge for the biological removal of nutrients and recalcitrant organic matter from wastewater. Chem. Eng. J. 2018, 336, 489–502. [Google Scholar] [CrossRef]
- Purba, L.D.A.; Ibiyeye, H.T.; Yuzir, A.; Mohamad, S.E.; Iwamoto, K.; Zamyadi, A.; Abdullah, N. Various applications of aerobic granular sludge: A review. Environ. Technol. Innov. 2020, 20, 101045. [Google Scholar] [CrossRef]
- Hu, P.; Shao, J.; Qian, G.; Adeleye, A.S.; Hao, T. Removal of tetracycline by aerobic granular sludge from marine aquaculture wastewater: A molecular dynamics investigation. Bioresour. Technol. 2022, 355, 127286. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Liu, Z.; Huang, X.; Fang, F.; Guo, J.; Yan, P. Effect of EPS and its forms of aerobic granular sludge on sludge aggregation performance during granulation process based on XDLVO theory. Sci. Total Environ. 2021, 795, 148682. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.; Wei, S.; Horn, H. Aerobic granules dwelling vorticella and rotifers in an SBR fed with domestic wastewater. Sep. Purif. Technol. 2013, 110, 127–131. [Google Scholar] [CrossRef]
- Barrios-Hernández, M.L.; Buenaño-Vargas, C.; García, H.; Brdjanovic, D.; van Loosdrecht, M.C.; Hooijmans, C.M. Effect of the co-treatment of synthetic faecal sludge and wastewater in an aerobic granular sludge system. Sci. Total Environ. 2020, 741, 140480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Piché-Choquette, S.; Sellamuthu, B. Roles of bacterial and epistylis populations in aerobic granular SBRs treating domestic and synthetic wastewaters. Chem. Eng. J. 2018, 351, 952–958. [Google Scholar] [CrossRef]
- Barrios-Hernández, M.L.; Bettinelli, C.; Mora-Cabrera, K.; Vanegas-Camero, M.-C.; Garcia, H.; van de Vossenberg, J.; Prats, D.; Brdjanovic, D.; van Loosdrecht, M.C.M.; Hooijmans, C.M. Unravelling the removal mechanisms of bacterial and viral surrogates in aerobic granular sludge systems. Water Res. 2021, 195, 116992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tay, J.-H. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res. 2002, 36, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tay, J.-H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2004, 22, 533–563. [Google Scholar] [CrossRef]
- Su, K.; Wang, C.; Zhang, S.; Liu, S. Lotka–Volterra equation based modeling of aerobic granulation process in sequencing batch reactors. Int. Biodeterior. Biodegrad. 2016, 115, 49–54. [Google Scholar] [CrossRef]
- Pan, Z.; Guo, T.; Sheng, J.; Feng, H.; Yan, A.; Li, J. Adding waste iron shavings in reactor to develop aerobic granular sludge and enhance removal of nitrogen and phosphorus. J. Environ. Chem. Eng. 2021, 9, 106620. [Google Scholar] [CrossRef]
- de Sousa Rollemberg, S.L.; Barros, A.R.M.; Firmino, P.I.M.; dos Santos, A.B. Aerobic granular sludge: Cultivation parameters and removal mechanisms. Bioresour. Technol. 2018, 270, 678–688. [Google Scholar] [CrossRef]
- van Dijk, E.J.H.; Haaksman, V.A.; van Loosdrecht, M.C.M.; Pronk, M. On the mechanisms for aerobic granulation-model based evaluation. Water Res. 2022, 216, 118365. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, Q.; Wan, C.; Zhang, C.; Tan, X.; Liu, X. Aggregation performance and adhesion behavior of microbes in response to feast/famine condition: Rapid granulation of aerobic granular sludge. Environ. Res. 2022, 208, 112780. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Han, Z.; Shao, X.; Zhao, Z.; Zhang, H.; Lin, T.; Yang, S.; Zhou, C. Response of aerobic granular sludge under polyethylene microplastics stress: Physicochemical properties, decontamination performance, and microbial community. J. Environ. Manag. 2022, 323, 116215. [Google Scholar] [CrossRef]
- Guo, T.; Ji, Y.; Zhao, J.; Horn, H.; Li, J. Coupling of Fe-C and aerobic granular sludge to treat refractory wastewater from a membrane manufacturer in a pilot-scale system. Water Res. 2020, 186, 116331. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.; Feng, S.; Wang, D.; Li, J.; Shi, W. Unveiling significance of Ca2+ ion for start-up of aerobic granular sludge reactor by distinguishing its effects on physicochemical property and bioactivity of sludge. Environ. Res. 2022, 212, 113299. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, L.-B.; Cai, A.; Huang, G.-X.; Horn, H. Aerobic Sludge Granulation in a Full-Scale Sequencing Batch Reactor. BioMed Res. Int. 2014, 2014, 268789. [Google Scholar] [CrossRef] [PubMed]
- Alves, O.I.; Araújo, J.M.; Silva, P.M.; Magnus, B.S.; Gavazza, S.; Florencio, L.; Kato, M.T. Formation and stability of aerobic granular sludge in a sequential batch reactor for the simultaneous removal of organic matter and nutrients from low-strength domestic wastewater. Sci. Total Environ. 2022, 843, 156988. [Google Scholar] [CrossRef]
- Kent, T.R.; Bott, C.B.; Wang, Z.-W. State of the art of aerobic granulation in continuous flow bioreactors. Biotechnol. Adv. 2018, 36, 1139–1166. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Pan, J.; Wu, S.; Qian, M.; He, Z.; Wang, B.; Li, J. Rapid control of activated sludge bulking and simultaneous acceleration of aerobic granulation by adding intact aerobic granular sludge. Sci. Total Environ. 2019, 674, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.; Jun, L.; Kai, X.; Sellamuthu, B. Role of adding dried sludge micropowder in aerobic granular sludge reactor with extended filamentous bacteria. Bioresour. Technol. Rep. 2019, 5, 51–58. [Google Scholar]
- Li, J.; Garny, K.; Neu, T.; He, M.; Lindenblatt, C.; Horn, H. Comparison of some characteristics of aerobic granules and sludge flocs from sequencing batch reactors. Water Sci. Technol. 2007, 55, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Staudt, C.; Horn, H.; Hempel, D.C.; Neu, T. Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol. Bioeng. 2004, 88, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. One-photon versus two-photon laser scanning microscopy and digital image analysis of microbial biofilms. Meth. Microbiol. 2005, 34, 89–136. [Google Scholar]
- Ren, T.-T.; Liu, L.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q.; Zhang, M.-C.; Zhu, J.-R. Calcium spatial distribution in aerobic granules and its effects on granule structure, strength and bioactivity. Water Res. 2008, 42, 3343–3352. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Lan, G.-H.; Zeng, P. Size-dependent calcium carbonate precipitation induced microbiologically in aerobic granules. Chem. Eng. J. 2016, 285, 341–348. [Google Scholar] [CrossRef]
- Wan, C.L.; Lee, D.J.; Yang, X.; Wang, Y.Y.; Wang, X.Z.; Liu, X. Calcium precipitate induced aerobic granulation. Bioresour. Technol. 2015, 176, 32–37. [Google Scholar] [CrossRef]
- Tay, J.-H.; Ivanov, V.; Pan, S.; Tay, S.T.-L. Specific layers in aerobically grown microbial granules. Lett. Appl. Microbiol. 2002, 34, 254–257. [Google Scholar] [CrossRef]
- de Kreuk, M.; Kishida, N.; Tsuneda, S.; van Loosdrecht, M. Behavior of polymeric substrates in an aerobic granular sludge system. Water Res. 2010, 44, 5929–5938. [Google Scholar] [CrossRef]
- Pronk, M.; Abbas, B.; Al-Zuhairy, S.H.K.; Kraan, R.; Kleerebezem, R.; Van Loosdrecht, M.C.M. Effect and behaviour of different substrates in relation to the formation of aerobic granular sludge. Appl. Microbiol. Biotechnol. 2015, 99, 5257–5268. [Google Scholar] [CrossRef]
- Martins, A.M.; Karahan, Ö.; van Loosdrecht, M.C. Effect of polymeric substrate on sludge settleability. Water Res. 2011, 45, 263–273. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Peng, D.; Hou, Y.; Pei, L.; Yu, L. Extended filaments of bulking sludge sink in the floc layer with particulate substrate. Chemosphere 2013, 93, 2725–2731. [Google Scholar] [CrossRef]
- Martins, A.M.; Heijnen, J.J.; van Loosdrecht, M.C. Effect of feeding pattern and storage on the sludge settleability under aerobic conditions. Water Res. 2003, 37, 2555–2570. [Google Scholar] [CrossRef]
- Beun, J.; Hendriks, A.; van Loosdrecht, M.; Morgenroth, E.; Wilderer, P.; Heijnen, J. Aerobic granulation in a sequencing batch reactor. Water Res. 1999, 33, 2283–2290. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.-W.; Qin, L.; Liu, Y.-Q.; Tay, J.-H. Selection pressure-driven aerobic granulation in a sequencing batch reactor. Appl. Microbiol. Biotechnol. 2004, 67, 26–32. [Google Scholar] [CrossRef]
- Madoni, P. Protozoa in wastewater treatment processes: A minireview. Ital. J. Zool. 2011, 78, 3–11. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Pan, Z.; Guo, T.; Feng, H.; Yan, A.; Ni, Y.; Li, J. Filamentous Bacteria and Stalked Ciliates for the Stable Structure of Aerobic Granular Sludge Treating Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 15747. https://doi.org/10.3390/ijerph192315747
Liang Y, Pan Z, Guo T, Feng H, Yan A, Ni Y, Li J. Filamentous Bacteria and Stalked Ciliates for the Stable Structure of Aerobic Granular Sludge Treating Wastewater. International Journal of Environmental Research and Public Health. 2022; 19(23):15747. https://doi.org/10.3390/ijerph192315747
Chicago/Turabian StyleLiang, Yifan, Zengrui Pan, Tao Guo, Hongbo Feng, Anqi Yan, Yongjiong Ni, and Jun Li. 2022. "Filamentous Bacteria and Stalked Ciliates for the Stable Structure of Aerobic Granular Sludge Treating Wastewater" International Journal of Environmental Research and Public Health 19, no. 23: 15747. https://doi.org/10.3390/ijerph192315747
APA StyleLiang, Y., Pan, Z., Guo, T., Feng, H., Yan, A., Ni, Y., & Li, J. (2022). Filamentous Bacteria and Stalked Ciliates for the Stable Structure of Aerobic Granular Sludge Treating Wastewater. International Journal of Environmental Research and Public Health, 19(23), 15747. https://doi.org/10.3390/ijerph192315747




