Effect of Seed Sludge Type on Aerobic Granulation, Pollutant Removal and Microbial Community in a Sequencing Batch Reactor Treating Real Textile Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Operation
2.2. Wastewater Composition
2.3. Analytical Methods
2.4. Microbial Community Analysis
3. Results and Discussion
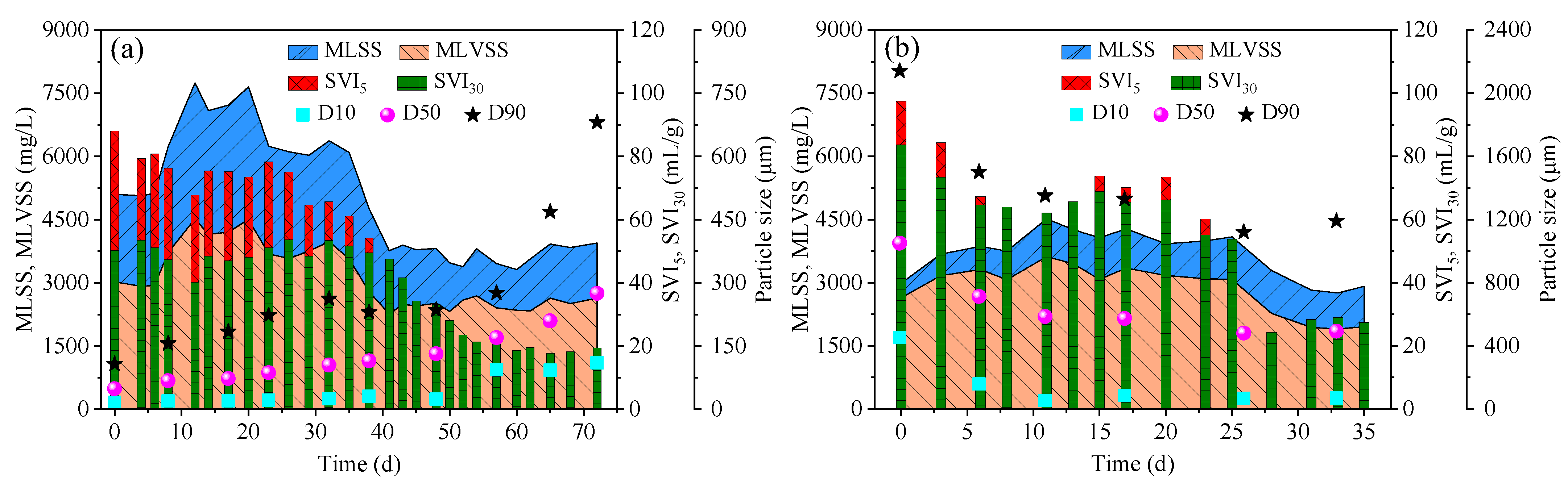
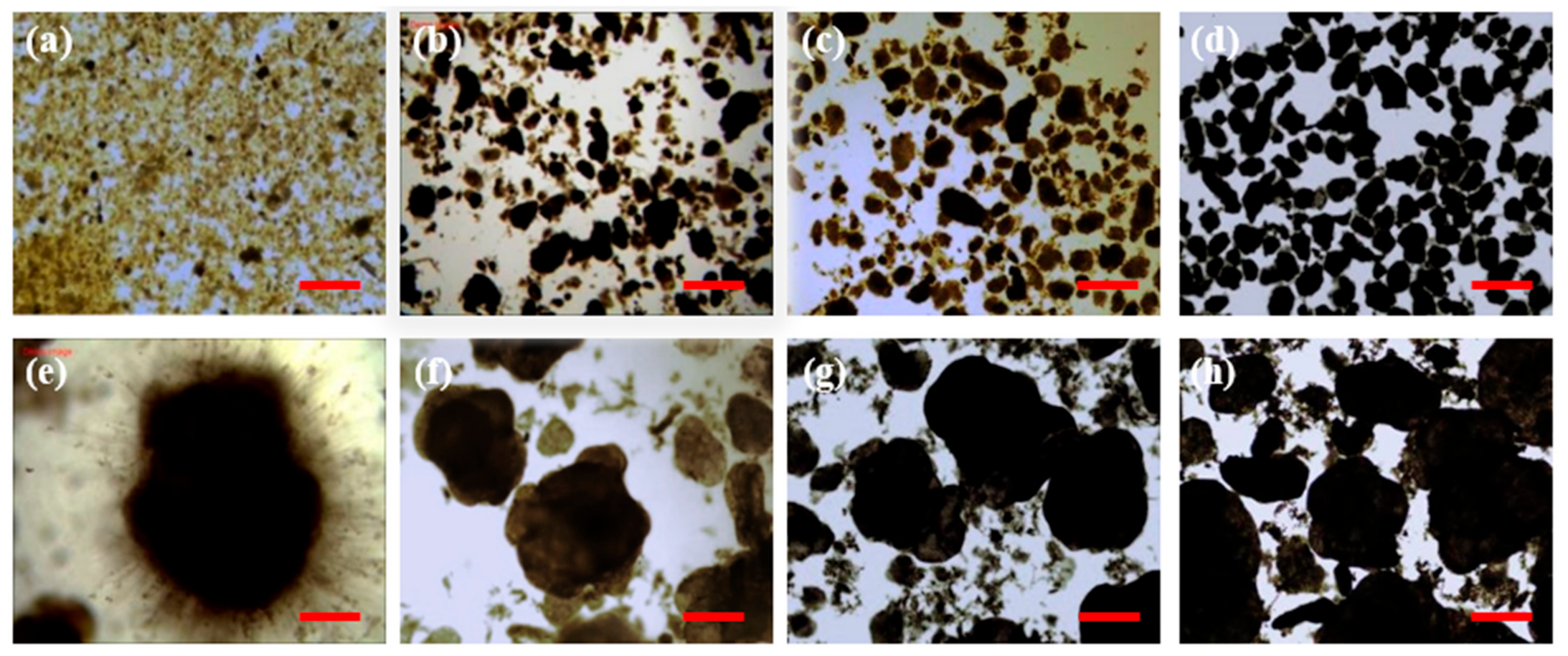
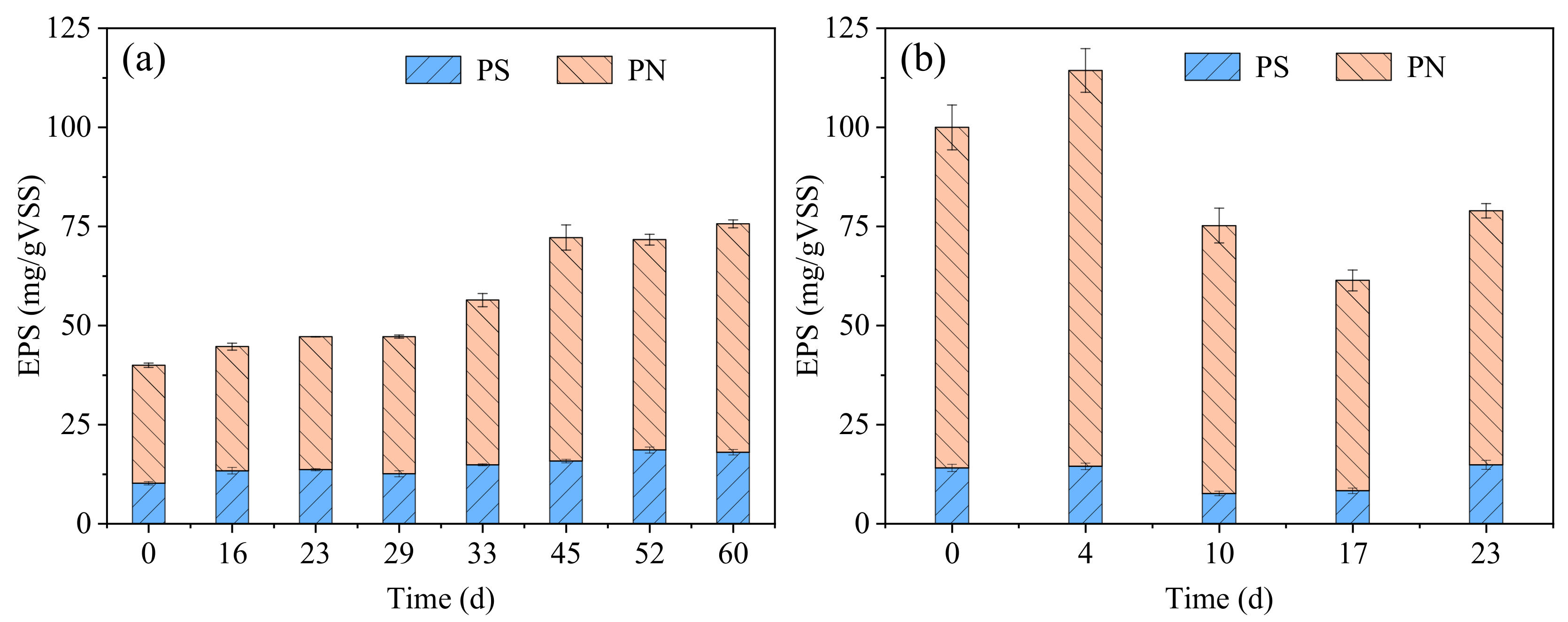
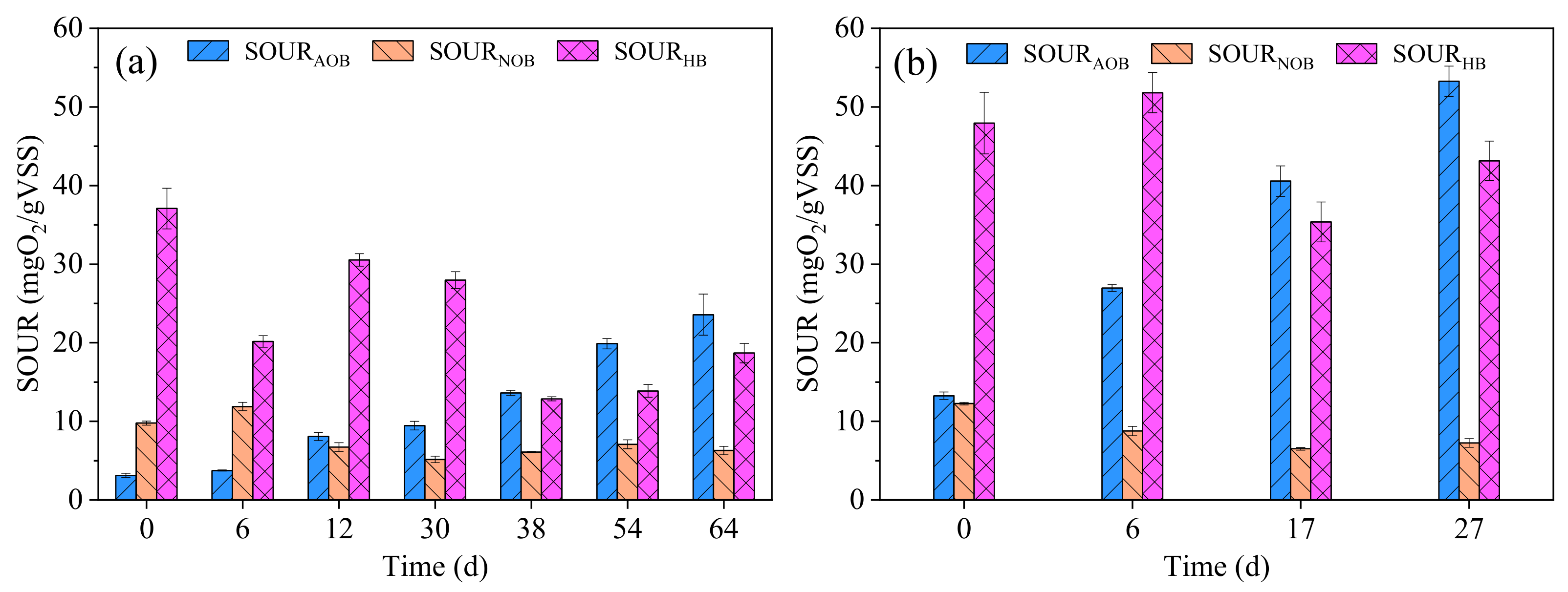
3.1. Evolution of Sludge Characterization
3.1.1. Formation of AGS in R1
3.1.2. Formation of AGS in R2
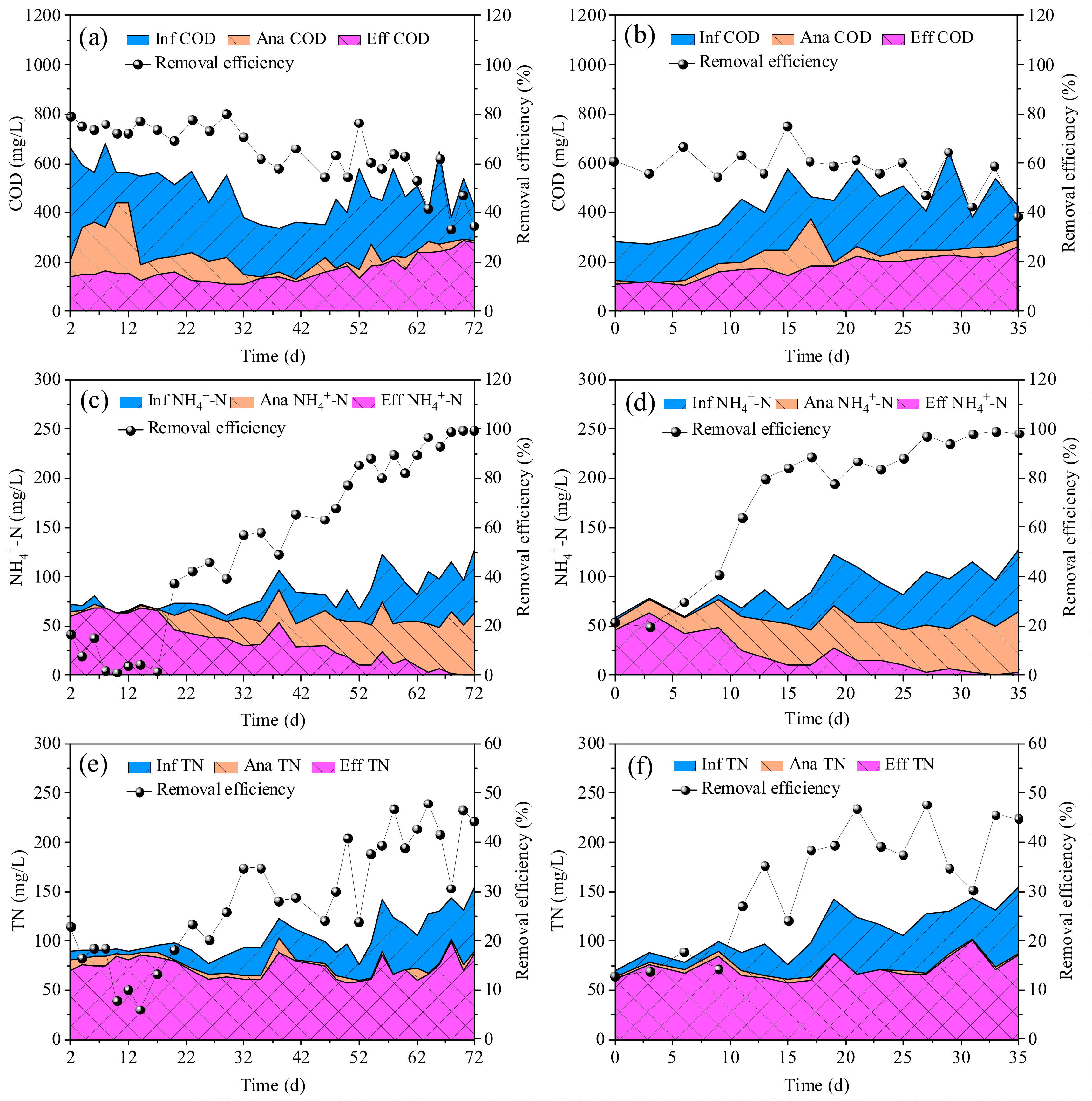
3.2. Removal of COD, NH4+-N and TN
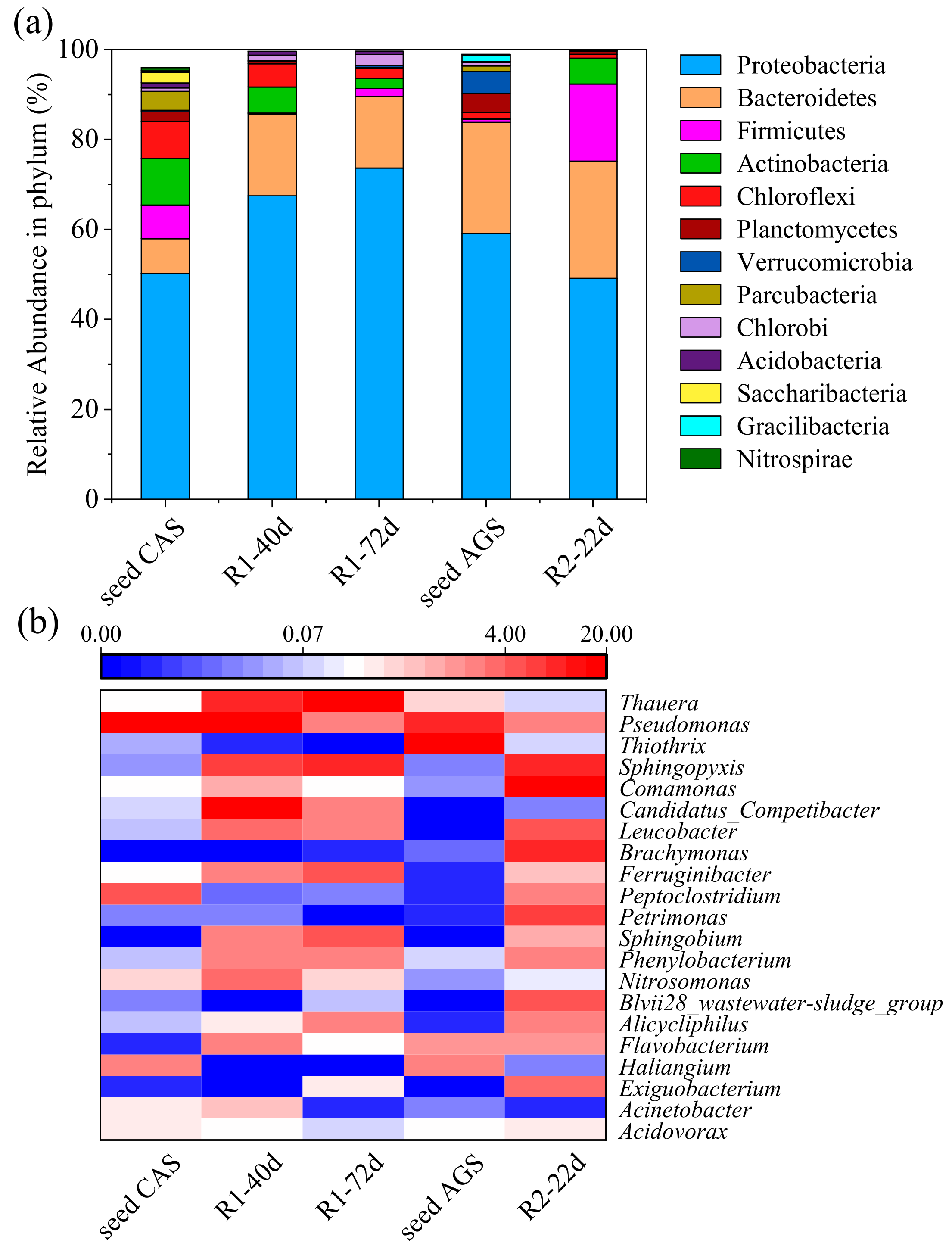
3.3. Microbial Community Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manavi, N.; Kazemi, A.S.; Bonakdarpour, B. The development of aerobic granules from conventional activated sludge under anaerobic-aerobic cycles and their adaptation for treatment of dyeing wastewater. Chem. Eng. J. 2017, 312, 375–384. [Google Scholar] [CrossRef]
- Singh, A.; Pal, D.B.; Mohammad, A.; Alhazmi, A.; Haque, S.; Yoon, T.; Srivastava, N.; Gupta, V.K. Biological remediation technologies for dyes and heavy metals in wastewater treatment: New insight. Bioresour. Technol. 2022, 343, 126154. [Google Scholar] [CrossRef] [PubMed]
- Kee, T.C.; Bay, H.H.; Lim, C.K.; Muda, K.; Ibrahim, Z. Development of bio-granules using selected mixed culture of decolorizing bacteria for the treatment of textile wastewater. Desalin. Water Treat. 2015, 54, 132–139. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Li, Y.; Wang, J. Enhanced biological nitrogen removal under low dissolved oxygen in an anaerobic-anoxic-oxic system: Kinetics, stoichiometry and microbial community. Chemosphere 2021, 263, 128184. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.R.; Lei, L.R.; Li, Y.M.; Chen, Y.C. A review of aerobic granular sludge (AGS) treating recalcitrant wastewater: Refractory organics removal mechanism, application and prospect. Sci. Total Environ. 2021, 782, 146852. [Google Scholar] [CrossRef]
- Li, J.; Ding, L.B.; Cai, A.; Huang, G.X.; Horn, H. Aerobic sludge granulation in a full-scale sequencing batch reactor. BioMed. Res. Int. 2014, 2014, 268789. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.T.; Yu, F.F.; Pan, J.Y.; Pan, B.J.; Wu, S.Y.; Qian, M.J.; Li, J. Rapid start-up of an aerobic granular sludge system for nitrogen and phosphorus removal through seeding chitosan-based sludge aggregates. Sci. Total Environ. 2021, 762, 144171. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.T.; Ye, L.; Ren, H.Q.; Zhang, X.X. Microbial community structure and function in aerobic granular sludge. Appl. Microbiol. Biotechnol. 2018, 102, 3967–3979. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.T.; Tao, Y.Q.; Li, J.; Wu, S.Y.; Ni, Y.J. Cultivating aerobic granular sludge in a developed continuous-flow reactor with two-zone sedimentation tank treating real and low-strength wastewater. Bioresour. Technol. 2018, 247, 776–783. [Google Scholar] [CrossRef]
- Muda, K.; Aris, A.; Salim, M.R.; Ibrahim, Z.; Yahya, A.; van Loosdrecht, M.C.; Ahmad, A.; Nawahwi, M.Z. Development of granular sludge for textile wastewater treatment. Water Res. 2010, 44, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Muda, K.; Aris, A.; Salim, M.R.; Ibrahim, Z.; van Loosdrecht, M.C.; Ahmad, A.; Nawahwi, M.Z. The effect of hydraulic retention time on granular sludge biomass in treating textile wastewater. Water Res. 2011, 45, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.Y.; Wang, X.H.; Song, C.; Wang, S.G.; Fan, M.H.; Li, X.M. Aerobic granulation for methylene blue biodegradation in a sequencing batch reactor. Desalination 2011, 276, 233–238. [Google Scholar] [CrossRef]
- Franca, R.D.; Vieira, A.; Mata, A.M.; Carvalho, G.S.; Pinheiro, H.M.; Lourenco, N.D. Effect of an azo dye on the performance of an aerobic granular sludge sequencing batch reactor treating a simulated textile wastewater. Water Res. 2015, 85, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sarvajith, M.; Reddy, G.K.K.; Nancharaiah, Y.V. Textile dye biodecolourization and ammonium removal over nitrite in aerobic granular sludge sequencing batch reactors. J. Hazard. Mater. 2018, 342, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Lotito, A.M.; Fratino, U.; Mancini, A.; Bergna, G.; Di Iaconi, C. Effective aerobic granular sludge treatment of a real dyeing textile wastewater. Int. Biodeterior. Biodegrad. 2012, 69, 62–68. [Google Scholar] [CrossRef]
- Lotito, A.M.; De Sanctis, M.; Di Iaconi, C.; Bergna, G. Textile wastewater treatment: Aerobic granular sludge vs activated sludge systems. Water Res. 2014, 54, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.; Amin, M.F.M.; Yahya, A.; Aris, A.; Muda, K. Characteristics of developed granules containing selected decolourising bacteria for the degradation of textile wastewater. Water Sci. Technol. 2010, 61, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, B.; Fallah, N.; Bonakdarpour, B.; Elyasi, S. The development of aerobic granules from slaughterhouse wastewater in treating real dyeing wastewater by Sequencing Batch Reactor (SBR). J. Environ. Chem. Eng. 2018, 6, 5536–5543. [Google Scholar] [CrossRef]
- Kolekar, Y.M.; Nemade, H.N.; Markad, V.L.; Adav, S.S.; Patole, M.S.; Kodam, K.M. Decolorization and biodegradation of azo dye, reactive blue 59 by aerobic granules. Bioresour. Technol. 2012, 104, 818–822. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Zou, J.T.; Yu, F.F.; Chen, J.; Mannina, G.; Li, Y.M. Influence of ferric iron dosing on aerobic granular sludge: Granule formation, nutrient removal and microbial community. J. Chem. Technol. Biotechnol. 2021, 96, 1277–1284. [Google Scholar] [CrossRef]
- Zou, J.T.; Pan, J.Y.; Wu, S.Y.; Qian, M.J.; He, Z.F.; Wang, B.B.; Li, J. Rapid control of activated sludge bulking and simultaneous acceleration of aerobic granulation by adding intact aerobic granular sludge. Sci. Total Environ. 2019, 674, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Good, I.J. The Population Frequencies of Species and the Estimation of Population Parameters. Biometrika 1953, 40, 237–264. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Gao, D.; Liu, L.; Liang, H.; Wu, W.M. Comparison of four enhancement strategies for aerobic granulation in sequencing batch reactors. J. Hazard. Mater. 2011, 186, 320–327. [Google Scholar] [CrossRef]
- de Kreuk, M.K.; Kishida, N.; van Loosdrecht, M.C.M. Aerobic granular sludge–state of the art. Water Sci. Technol. 2007, 55, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Tay, J.H. Characteristics and stability of aerobic granules cultivated with different starvation time. Appl. Microbiol. Biotechnol. 2007, 75, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.L.; Moreira, I.S.; Ribeiro, A.R.; Santos, L.H.M.L.M.; Delerue-Matos, C.; Tiritan, M.E.; Castro, P.M.L. Treatment of a simulated wastewater amended with a chiral pharmaceuticals mixture by an aerobic granular sludge sequencing batch reactor. Int. Biodeterior. 2016, 115, 277–285. [Google Scholar] [CrossRef]
- Zheng, X.L.; Sun, P.D.; Lou, J.Q.; Fang, Z.G.; Guo, M.X.; Song, Y.Q.; Tang, X.D.; Jiang, T. The long-term effect of nitrite on the granule-based enhanced biological phosphorus removal system and the reversibility. Bioresour. Technol. 2013, 132, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.S.; Moghaddam, M.R.A. Aerobic Granular Sludge for Dye Biodegradation in a Sequencing Batch Reactor with Anaerobic/Aerobic Cycles. CLEAN–Soil Air Water 2016, 44, 438–443. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Liu, Y.; Tay, J.H. Relationship between size and mass transfer resistance in aerobic granules. Lett. Appl. Microbiol. 2005, 40, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. (Water Pollut. Control. Fed.) 1976, 48, 835–852. [Google Scholar] [PubMed]
- Beun, J.J.; van Loosdrecht, M.C.M.; Heijnen, J.J. Aerobic granulation in a sequencing batch airlift reactor. Water Res. 2002, 36, 702–712. [Google Scholar] [CrossRef]
- Li, Y.M.; Zou, J.T.; Zhang, L.L.; Sun, J. Aerobic granular sludge for simultaneous accumulation of mineral phosphorus and removal of nitrogen via nitrite in wastewater. Bioresour. Technol. 2014, 154, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, Q.; Chen, J.; Zhang, B.; Liu, C.; Huangfu, X. Distinct granulation pathways of aerobic granular sludge under poly aluminum chloride enhancement. Sci. Total Environ. 2022, 807, 150829. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Wang, L.F.; Xiang, F.; Zhao, L.; Qiao, Z. Activated Sludge Microbial Community and Treatment Performance of Wastewater Treatment Plants in Industrial and Municipal Zones. Int. J. Environ. Res. Public Health 2020, 17, 436. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Liao, G.; Wu, C.; Dai, C.; Bin, L.; Gao, X.; Zhao, Y.; Li, P.; Huang, S.; Fu, F.; et al. Rapid granulation of aerobic granular sludge and maintaining its stability by combining the effects of multi-ionic matrix and bio-carrier in a continuous-flow membrane bioreactor. Sci. Total Environ. 2022, 813, 152644. [Google Scholar]
- Zhao, L.J.; Su, C.Y.; Liu, W.H.; Qin, R.H.; Tang, L.Q.; Deng, X.; Wu, S.M.; Chen, M.L. Exposure to polyamide 66 microplastic leads to effects performance and microbial community structure of aerobic granular sludge. Ecotoxicol. Environ. Saf. 2020, 190, 110070. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Wei, D.; Xu, W.Y.; Feng, R.; Du, B.; Wei, Q. Nitrogen removal in a combined aerobic granular sludge and solid-phase biological denitrification system: System evaluation and community structure. Bioresour. Technol. 2019, 288, 121504. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Pang, H.; He, J.; Nan, J.; Wang, M.; Li, L. Start-up of aerobic granular biofilm at low temperature: Performance and microbial community dynamics. Sci. Total Environ. 2020, 698, 134311. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, K.J.; Tian, C.; Yuan, Q. Aerobic granular sludge treating low-strength municipal wastewater: Efficient carbon, nitrogen and phosphorus removal with hydrolysis-acidification pretreatment. Sci. Total Environ. 2021, 792, 148297. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.M.S.; Amorim, C.L.; Costa, J.; Mesquita, D.P.; Ferreira, E.C.; Castro, P.M.L. Long-term stability of a non-adapted aerobic granular sludge process treating fish canning wastewater associated to EPS producers in the core microbiome. Sci. Total Environ. 2021, 756, 144007. [Google Scholar] [CrossRef] [PubMed]
- Kouki, S.; Saidi, N.; M’Hiri, F.; Nasr, H.; Cherif, H.; Ouzari, H.; Hassen, A. Isolation and characterization of facultative mixotrophic ammonia-oxidizing bacteria from constructed wetlands. J. Environ. Sci. 2011, 23, 1699–1708. [Google Scholar] [CrossRef]
| Parameters | Seed CAS | Seed AGS |
|---|---|---|
| SVI30 (mL/g) | 50.3 | 83.0 |
| SVI5 (mL/g) | 88.1 | 96.5 |
| MLSS (mg/L) | 5102 | 2988 |
| MLVSS (mg/L) | 3024 | 2636 |
| Mean particle size (μm) | 48.6 | 1050 |
| Polysaccharides (PS) in EPS (mg/(gVSS)) | 10.2 ± 0.4 | 14.1 ± 0.9 |
| Proteins (PN) in EPS (mg/(gVSS)) | 29.8 ± 0.5 | 85.9 ± 5.6 |
| Samples | OUTs | Shannon | Simpson | Coverage (%) |
|---|---|---|---|---|
| seed CAS | 828 | 6.852 | 0.932 | 99.66 |
| R1-40d | 397 | 6.218 | 0.968 | 99.74 |
| R1-72d | 387 | 4.828 | 0.859 | 99.56 |
| seed AGS | 397 | 5.447 | 0.920 | 99.76 |
| R2-22d | 343 | 5.523 | 0.950 | 99.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, J.; Yang, J.; He, H.; Wang, X.; Mei, R.; Cai, L.; Li, J. Effect of Seed Sludge Type on Aerobic Granulation, Pollutant Removal and Microbial Community in a Sequencing Batch Reactor Treating Real Textile Wastewater. Int. J. Environ. Res. Public Health 2022, 19, 10940. https://doi.org/10.3390/ijerph191710940
Zou J, Yang J, He H, Wang X, Mei R, Cai L, Li J. Effect of Seed Sludge Type on Aerobic Granulation, Pollutant Removal and Microbial Community in a Sequencing Batch Reactor Treating Real Textile Wastewater. International Journal of Environmental Research and Public Health. 2022; 19(17):10940. https://doi.org/10.3390/ijerph191710940
Chicago/Turabian StyleZou, Jinte, Jiaqi Yang, Hangtian He, Xiaofei Wang, Rongwu Mei, Lei Cai, and Jun Li. 2022. "Effect of Seed Sludge Type on Aerobic Granulation, Pollutant Removal and Microbial Community in a Sequencing Batch Reactor Treating Real Textile Wastewater" International Journal of Environmental Research and Public Health 19, no. 17: 10940. https://doi.org/10.3390/ijerph191710940
APA StyleZou, J., Yang, J., He, H., Wang, X., Mei, R., Cai, L., & Li, J. (2022). Effect of Seed Sludge Type on Aerobic Granulation, Pollutant Removal and Microbial Community in a Sequencing Batch Reactor Treating Real Textile Wastewater. International Journal of Environmental Research and Public Health, 19(17), 10940. https://doi.org/10.3390/ijerph191710940







