Comparative Mask Protection against Inhaling Wildfire Smoke, Allergenic Bioaerosols, and Infectious Particles
Abstract
1. Introduction
2. Materials and Methods
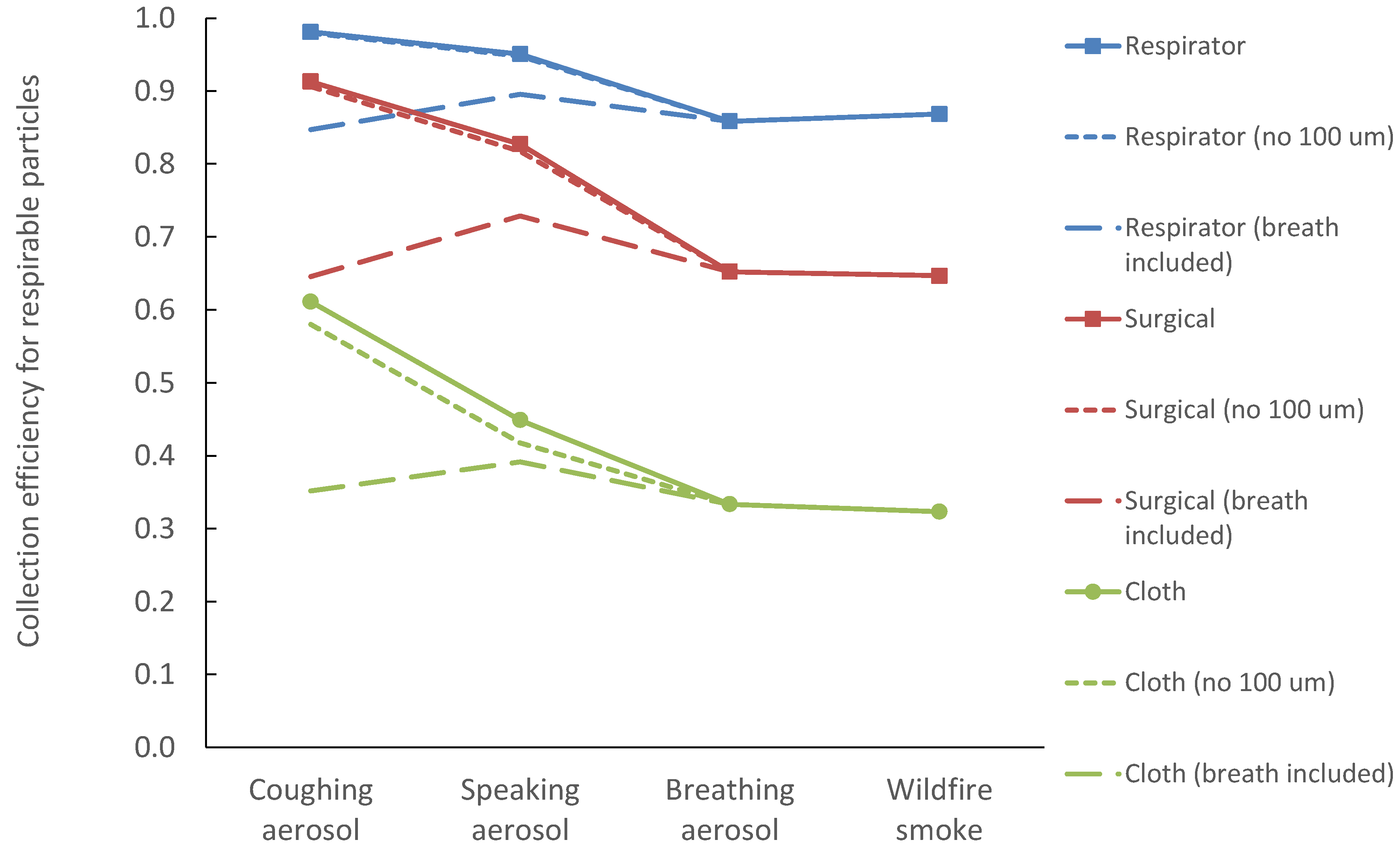
3. Results
4. Discussion
4.1. Impact of Particle Size Types on Mask Protection
4.2. Comparison to Experimental Data
4.3. Uncertainties and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kodros, J.K.; O’Dell, K.; Samet, J.M.; L’Orange, C.; Pierce, J.R.; Volckens, J. Quantifying the Health Benefits of Face Masks and Respirators to Mitigate Exposure to Severe Air Pollution. GeoHealth 2021, 5, e2021GH000482. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Current Issues in the Assessment of Respiratory Protective Devices for Occupational and Non-Occupational Uses: Proceedings of a Workshop; Nicholson, A., Yost, O., Giammaria, C., Eds.; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar]
- CDPH. Get the Most out of Masking. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/Get-the-Most-out-of-Masking.aspx (accessed on 8 July 2022).
- Bergmann, K.C.; Kugler, S.; Zuberbier, T.; Becker, S. Face Masks Suitable for Preventing COVID-19 and Pollen Allergy. A study in the Exposure Chamber. Allergo J. Int. 2021, 30, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Erdem, M.; Kara, C.O.; Alptürk, U.; Topuz, B. The Effect of Face Mask Usage on the Allergic Rhinitis Symptoms in Patients with Pollen Allergy During the COVID-19 Pandemic. Am. J. Otolaryngol. 2022, 43, 103206. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. Frameworks for Protecting Workers and the Public from Inhalation Hazards; Samet, J., Downey, A., Yost, O.C., Eds.; The National Academies Press: Washington, DC, USA, 2022. [Google Scholar]
- OSHA. US Department of Labor. Respiratory Protection. Available online: https://www.osha.gov/respiratory-protection/standards (accessed on 8 July 2022).
- US Department of Labor. Protecting Workers: Guidance on Mitigating and Preventing the Spread of COVID-19 in the Workplace. Available online: https://www.osha.gov/coronavirus/safework (accessed on 8 July 2022).
- Cal/OSHA. Department of Industrial Relations. Cal/Osha and Statewide Industry Guidance on COVID-19. Available online: https://www.dir.ca.gov/dosh/coronavirus/Guidance-by-Industry.html (accessed on 8 July 2022).
- 3M Personal Safety Division. 3M. Comparison of Ffp2, Kn95, and N95 Filtering Facepiece Respirator Classes. Available online: https://multimedia.3m.com/mws/media/1791500O/comparison-ffp2-kn95-n95-filtering-facepiece-respirator-classes-tb.pdf (accessed on 20 May 2022).
- Konda, A.; Prakash, G.A.; Moss, A.; Schmoldt, M.; Grant, G.D.; Guha, S. Response to Letters to the Editor on Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks: Revised and Expanded Results. ACS Nano 2020, 14, 10764–10770. [Google Scholar] [CrossRef]
- Cappa, C.; William, D.; Barreda, R.S.; Bouvier, N.M.; Levintal, E.; Wexler, A.S.; Roman, S.A. A Highly Efficient Cloth Facemask Design. Aerosol Sci. Technol. 2022, 56, 12–28. [Google Scholar] [CrossRef]
- O’Kelly, E.; Arora, A.; Pirog, S.; Ward, J.; Clarkson, P.J. Comparing the Fit of N95, Kn95, Surgical, and Cloth Face Masks and Assessing the Accuracy of Fit Checking. PLoS ONE 2021, 16, e0245688. [Google Scholar] [CrossRef]
- Pan, J.; Harb, C.; Leng, W.; Marr, L.C. Inward and Outward Effectiveness of Cloth Masks, a Surgical Mask, and a Face Shield. Aerosol Sci. Technol. 2021, 55, 718–733. [Google Scholar] [CrossRef]
- Oestenstad, R.; Bartolucci, A.A. Factors Affecting the Location and Shape of Face Seal Leak Sites on Half-Mask Respirators. J. Occup. Environ. Hyg. 2021, 7, 332–341. [Google Scholar] [CrossRef]
- Regli, A.; Sommerfield, A.; von Ungern-Sternberg, B.S. The Role of Fit Testing N95/Ffp2/Ffp3 Masks: A Narrative Review. Anaesthesia 2021, 76, 91–100. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Abbasnezhad, N.; Seydani, M.Z.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An Overview of Filtration Efficiency through the Masks: Mechanisms of the Aerosols Penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- F2100-21, ASTM; Standard Specification for Performance of Materials Used in Medical Face Masks. ASTM International: West Conshohocken, PA, USA, 2021.
- F3502-21, ASTM; Standard Specification for Barrier Face Coverings. ASTM International: West Conshohocken, PA, USA, 2021.
- Shakya, K.M.; Noyes, A.; Kallin, R.; Peltier, R.E. Evaluating the Efficacy of Cloth Facemasks in Reducing Particulate Matter Exposure. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 352–357. [Google Scholar] [CrossRef]
- Asadi, S.; Christopher, D.; Barreda, C.S.; Wexler, A.S.; Bouvier, N.M.; William, D. Ristenpart. Efficacy of Masks and Face Coverings in Controlling Outward Aerosol Particle Emission from Expiratory Activities. Sci. Rep. 2020, 10, 15665. [Google Scholar] [CrossRef] [PubMed]
- Drewnick, F.; Pikmann, J.; Fachinger, F.; Moormann, L.; Sprang, F.; Borrmann, S. Aerosol Filtration Efficiency of Household Materials for Homemade Face Masks: Influence of Material Properties, Particle Size, Particle Electrical Charge, Face Velocity, and Leaks. Aerosol Sci. Technol. 2020, 55, 63–79. [Google Scholar] [CrossRef]
- Pöhlker, M.; Krüger, O.O.; Förster, J.-D.; Berkemeier, T.; Elbert, W.; Fröhlich-Nowoisky, J.; Pöschl, U.; Pöhlker, C.; Bagheri, G.; Bodenschatz, E. Respiratory Aerosols and Droplets in the Transmission of Infectious Diseases. arXiv 2021, arXiv:2103.01188. [Google Scholar]
- Rahid, T.; Sharmeen, S.; Biswas, S. Effectiveness of N95 Masks against SARS-CoV-2: Performance Efficiency, Concerns, and Future Directions. ACS Chem. Health Saf. 2022, 29, 135–164. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology; John Wiley and Sons: New York, NY, USA, 1982. [Google Scholar]
- Wagner, J.; Sparks, T.L.; Miller, S.; Chen, W.; Macher, J.M.; Waldman, J.M. Modeling the Impacts of Physical Distancing and Other Exposure Determinants on Aerosol Transmission. J. Occup. Environ. Hyg. 2021, 18, 495–509. [Google Scholar] [CrossRef]
- Wagner, J.; Macher, J. Automated Spore Measurements Using Microscopy, Image Analysis, and Peak Recognition of near-Monodisperse Aerosols. Aerosol Sci. Technol. 2012, 46, 862–873. [Google Scholar] [CrossRef]
- Sparks, T.; Wagner, J. Composition of Particulate Matter During a Wildfire Smoke Episode in an Urban Area. Aerosol Sci. Technol. 2021, 55, 734–747. [Google Scholar] [CrossRef]
- Adachi, K.; Dibb, J.E.; Scheuer, E.; Katich, J.M.; Schwarz, J.P.; Perring, A.E.; Mediavilla, B.; Guo, H.; Campuzano-Jost, P.; Jimenez, J.L. Fine Ash-Bearing Particles as a Major Aerosol Component in Biomass Burning Smoke. J. Geophys. Res. Atmos. 2022, 127, e2021JD035657. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Allan, J.D.; Coe, H.; Taylor, J.W.; Duck, T.J.; Pierce, J.R. Aged Boreal Biomass-Burning Aerosol Size Distributions from Bortas 2011. Atmos. Chem. Phys. 2015, 15, 1633–1646. [Google Scholar] [CrossRef]
- Nicas, M.; Nazaroff, W.W.; Hubbard, A. Toward Understanding the Risk of Secondary Airborne Infection: Emission of Respirable Pathogens. J. Occup. Environ. Hyg. 2005, 52, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, W.E.; Swett, K.; Leng, I.; Peters, T.R. Exposure to Influenza Virus Aerosols During Routine Patient Care. J. Infect. Dis. 2013, 207, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic Analysis of SARS-CoV-2 in Two Wuhan Hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Santarpi, J.; Herrera, V.L.; Rivera, D.N.; Ratnesar-Shumate, S.; Reid, S.P.; Denton, P.W.; Martens, J.W.S.; Fang, Y.; Conoan, N.; Callahan, M.V.; et al. The Infectious Nature of Patient-Generated SARS-CoV-2 Aerosol. Medrxiv 2020, 32, 706–711. [Google Scholar]
- Hawks, S.A.; Prussin, A.J., II; Kuchinsky, S.C.; Pan, J.; Marr, L.C.; Duggal, N.K. Infectious SARS-CoV-2 Is Emitted in Aerosol Particles. mBio 2021, 12, e0252721. [Google Scholar] [CrossRef]
- Bake, B.; Larsson, P.; Ljungkvist, G.; Ljungström, E.; Olin, A.C. Exhaled Particles and Small Airways. Respir. Res. 2019, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Nazaroff, W. Indoor Aerosol Science Aspects of SARS-CoV-2 Transmission. Indoor Air 2022, 32, e12970. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, F.; Aylor, D.E. Settling Speed of Clusters of Spores. Phytopathology 1984, 74, 969–972. [Google Scholar] [CrossRef]
- Bandh, S.; Kamili, A.N.; Ganai, B.A. Identification of Some Aspergillus Species Isolated from Dal Lake, Kashmir by Traditional Approach of Morphological Observation and Culture. Afr. J. Microbiol. Res. 2012, 6, 5824–5827. [Google Scholar]
- Laws, R.L.; Jain, S.; Cooksey, G.S.; Mohle-Boetani, J.; McNary, J.; Wilken, J.; Harrison, R.; Leistikow, B.; Vugia, D.J.; Windham, G.C.; et al. Coccidioidomycosis outbreak among inmate wildland firefighters: California, 2017. Am. J. Ind. Med. 2021, 64, 266–273. [Google Scholar] [CrossRef]
- Kobziar, L.; Thompson, G. Wildfire smoke, a potential infectious agent. Science 2020, 370, 1408–1410. [Google Scholar] [CrossRef]
- Sondermeyer, C.; Gail, L.; Nguyen, A.; Vugia, D.; Jain, S. Regional Analysis of Coccidioidomycosis Incidence—California, 2000-2018. Morb. Mortal. Wkly. Rep. 2020, 69, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Heaney, A.; Head, J.R.; Broen, K.; Click, K.; Taylor, J.; Balmes, J.R.; Zelner, J.; Remais, J.V. Coccidioidomycosis and COVID-19 Co-Infection, United States, 2020. Emerg. Infect. Dis. 2021, 27, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Fears, A.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, C.S.; Plante, K.S.; Mirchandani, D.; Plante, J.; Aguilar, P.V.; Fernandez, D.; et al. Comparative Dynamic Aerosol Efficiencies of Three Emergent Coronaviruses and the Unusual Persistence of SARS-CoV-2 in Aerosol Suspensions. medRxiv 2020, 26, 2168–2171. [Google Scholar]
- Santarpia, J.; Herrera, V.L.; Rivera, D.N.; Ratnesar-Shumate, S.; Reid, S.P.; Ackerman, D.N.; Denton, P.W.; Jacob, W.; Martens, S.; Fang, Y.; et al. The Size and Culturability of Patient-Generated SARS-CoV-2 Aerosol. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 706–711. [Google Scholar] [CrossRef]
- Ruzer, L.S.; Harley, N.H. Aerosols Handbook: Measurement, Dosimetry, and Health Effects, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Schijven, J.; Vermeulen, L.C.; Swart, A.; Meijer, A.; Duizer, E.; de Roda, A.M.H. Quantitative Microbial Risk Assessment for Airborne Transmission of SARS-CoV-2 Via Breathing, Speaking, Singing, Coughing, and Sneezing. Environ. Health Perspect. 2021, 129, 47002. [Google Scholar] [CrossRef]
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and Respirable Particle Definitions for Human Health Risk Assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef]
- Fennelly, K. Particle Sizes of Infectious Aerosols: Implications for Infection Control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Wainwright, C.E.; France, M.W.; O’Rourke, P.; Anuj, S.; Kidd, T.J.; Nissen, M.D.; Sloots, T.P.; Coulter, C.; Ristovski, Z.; Hargreaves, M.; et al. Cough-Generated Aerosols of Pseudomonas aeruginosa and Other Gram-Negative Bacteria from Patients with Cystic Fibrosis. Thorax 2009, 64, 926–931. [Google Scholar] [CrossRef]
- Asadi, S.; Wexler, S.; Cappa, C.D.; Barreda, S.; Bouvier, N.M.; Ristenpart,, D.W. Aerosol Emission and Superemission during Human Speech Increase with Voice Loudness. Sci. Rep. 2019, 9, 2348. [Google Scholar]
- Alsved, M.; Nygren, D.; Thuresson, S.; Fraenkel, C.; Medstrand, P.; Löndahl, J. Size distribution of exhaled aerosol particles containing SARS-CoV-2 RNA. Infect. Dis. 2022, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Miller, M.D.; Balmes, J.R. Health Effects of Wildfire Smoke in Children and Public Health Tools: A Narrative Review. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shibata, T.; Wilson, J.; Maidin, A. Challenges in evaluating PM concentration levels, commuting exposure, and mask efficacy in reducing PM exposure in growing, urban communities in a developing country. Sci. Total Environ. 2016, 543, 416–424. [Google Scholar] [CrossRef]
- Duncan, S.; Bodurtha, P.; Naqvi, S. The Protective Performance of Reusable Cloth Face Masks, Disposable Procedure Masks, Kn95 Masks and N95 Respirators: Filtration and Total Inward Leakage. PLoS ONE 2021, 16, e0258191. [Google Scholar] [CrossRef] [PubMed]
- Leith, D.; L’Orange, C.; Volckens, J. Quantitative Protection Factors for Common Masks and Face Coverings. Environ. Sci. Technol. 2021, 55, 3136–3143. [Google Scholar] [CrossRef]
- Pierlot, A.P.; Alexander, D.L.J.; Schütz, J.A. Impact of Wearing on Filtration Performance of Electrostatic Filter Face Masks. Int. J. Env. Res. Public Health 2022, 19, 5032. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Blachere, F.M.; Thewlis, R.E.; Vishnu, A.; Davis, K.A.; Cao, G.; Palmer, J.E.; Clark, K.E.; Fisher, M.A.; Khakoo, R.; et al. Measurements of Airborne Influenza Virus in Aerosol Particles from Human Coughs. PLoS ONE 2010, 5, e15100. [Google Scholar] [CrossRef]
- Dinkele, R.; Gessner, S.; McKerry, A.; Leonard, B.; Leukes, J.; Seldon, R.; Warner, D.F.; Wood, R. Aerosolization of Mycobacterium Tuberculosis by Tidal Breathing. Am. J. Respir. Crit. Care Med. 2022, 206, 206–216. [Google Scholar] [CrossRef]
- Milton, D.K.; Fabian, M.P.; Cowling, B.J.; Grantham, M.L.; McDevitt, J.J. Influenza Virus Aerosols in Human Exhaled Breath: Particle Size, Culturability, and Effect of Surgical Masks. PLoS Pathog. 2013, 9, e1003205. [Google Scholar] [CrossRef]



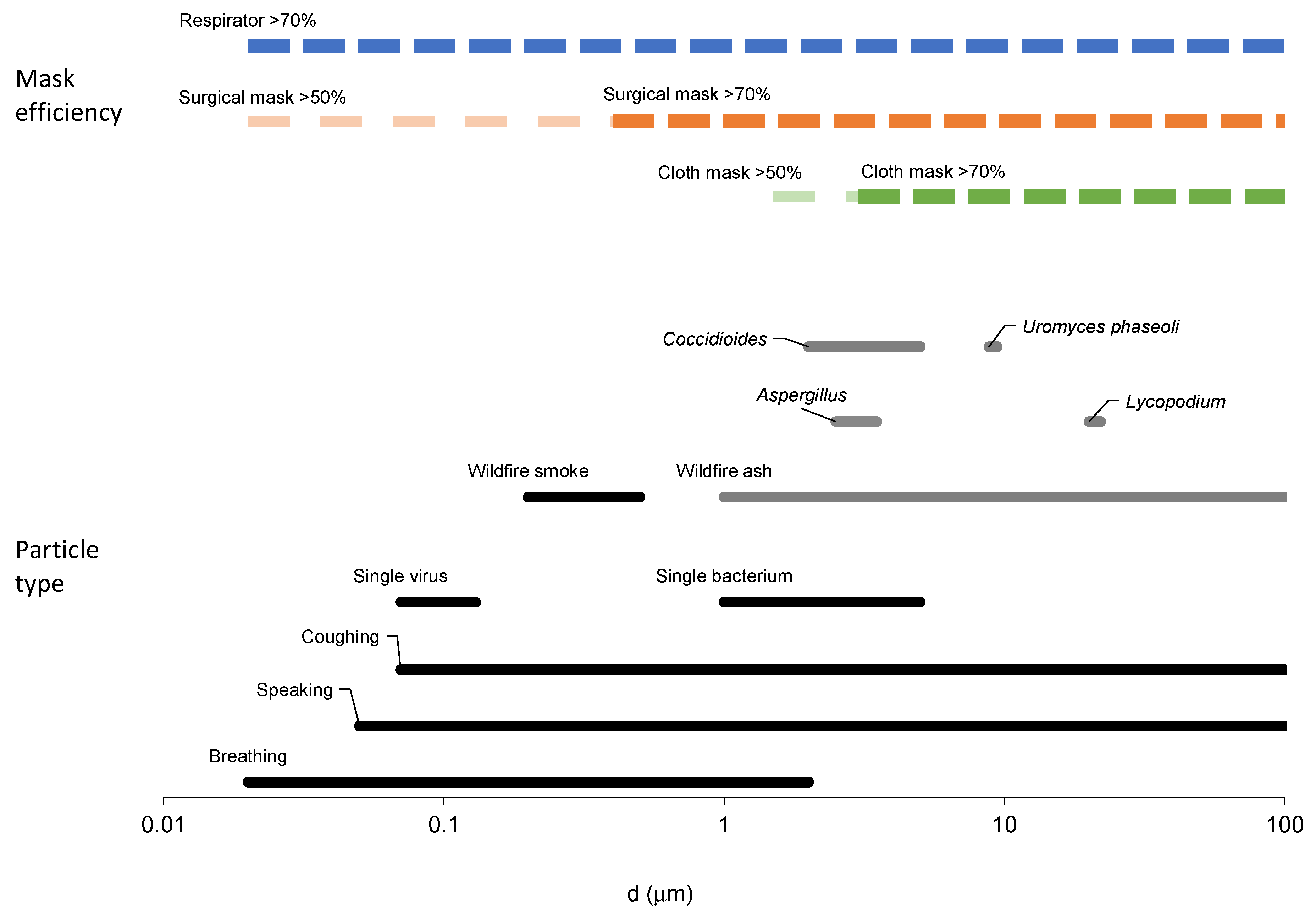
| Particle Type | Reported by Source | Modeled in This Work | |||||
|---|---|---|---|---|---|---|---|
| Range (µm) | A (#/cm3) * | D (µm) * | Sigma * | Source | CMD (µm) * | GSD * | |
| Allergenic, inflammatory, or irritant and plant pathogenic particles | |||||||
| Lycopodium species, Ambrosia elatior | 20–22 | [38] | 21 | 1.1 | |||
| Uromyces phaseoli | 8.8–9.4 | [38] | 9.1 | 1.1 | |||
| Aspergillus (niger, flavus, fumigatus, japonicus, terreus) | 2.5–3.5 | [39] | 3 | 1.1 | |||
| Wildfire smoke and ash | |||||||
| Smoke | 0.2–0.5 | [26] | 0.25 | 1.3 | |||
| Ash | 1–20 | [26] | 5 | 1.7 | |||
| Human-emitted particles | |||||||
| Coughing, bronchiolar mode 1 (B1) | 262 | 0.07 | 0.9 | [23] | Same as source | ||
| Coughing, bronchiolar mode 2 (B2) | 0.37 | 0.3 | 0.9 | [23] | Same as source | ||
| Coughing, laryngeal-tracheal (LT) | 4.3 | 1 | 0.98 | [23] | Same as source | ||
| Coughing, oral mode 1 (O1) | 1.4 | 11 | 0.95 | [23] | Same as source | ||
| Coughing, oral mode 2 (O2) | 0.5 | 128 | 1 | [23] | Same as source | ||
| Speaking B1 | 9.8 | 0.07 | 0.9 | [23] | Same as source | ||
| Speaking B2 | 1.4 | 0.3 | 0.9 | [23] | Same as source | ||
| Speaking LT | 1.7 | 1 | 0.9 | [23] | Same as source | ||
| Speaking O1 | 0.03 | 10 | 0.98 | [23] | Same as source | ||
| Speaking O2 | 0.17 | 96 | 0.97 | [23] | Same as source | ||
| Breathing B1 | 7.1 | 0.07 | 0.9 | [23] | Same as source | ||
| Breathing B2 | 1.1 | 0.3 | 0.9 | [23] | Same as source | ||
| Viral and microbial pathogens | |||||||
| Coccidioides species | 2–5 | [43] | 3 | 1.3 | |||
| SARS-CoV-2 | 0.07–0.13 | [44,45] | Min size = 0.1 µm ** | ||||
| Influenza A, B, C | 0.08–0.12 | [46] | Min size = 0.1 µm ** | ||||
| Mycobacterium tuberculosis | 1–5 | [46] | Min size = 1 µm ** | ||||
| Minimum Infectious Size | Collection Efficiency, Etot | |||
|---|---|---|---|---|
| Aerosol | Respirator | Surgical Mask | Cloth Mask | |
| Lycopodium | 1.00 | 1.00 | 1.00 | |
| Uromyces | 1.00 | 1.00 | 1.00 | |
| Aspergillus | 1.00 | 0.98 | 0.86 | |
| Coccidioides | 0.99 | 0.97 | 0.78 | |
| Wildfire ash | 1.00 | 0.98 | 0.92 | |
| Wildfire smoke | 0.90 | 0.68 | 0.33 | |
| Breathing (B1 + B2) | none | 0.84 | 0.65 | 0.39 |
| SARS-CoV-2 | 0.85 | 0.65 | 0.34 | |
| TB | 0.99 | 0.95 | 0.58 | |
| Coughing (B1 + B2 + LT + O) | none | 0.83 | 0.65 | 0.40 |
| SARS-CoV-2 | 0.84 | 0.64 | 0.35 | |
| TB | 1.00 | 0.98 | 0.81 | |
| Coughing (B2 + LT + O) | none | 0.98 | 0.92 | 0.61 |
| SARS-CoV-2 | 0.98 | 0.92 | 0.61 | |
| TB | 1.00 | 0.98 | 0.81 | |
| Coughing (LT) | none | 0.98 | 0.91 | 0.47 |
| SARS-CoV-2 | 0.98 | 0.91 | 0.47 | |
| TB | 0.99 | 0.96 | 0.62 | |
| Coughing (O) | none | 1.00 | 1.00 | 0.99 |
| SARS-CoV-2 | 1.00 | 1.00 | 0.99 | |
| TB | 1.00 | 1.00 | 0.99 | |
| Speaking (B1 + B2 + LT + O) | none | 0.86 | 0.69 | 0.41 |
| SARS-CoV-2 | 0.88 | 0.72 | 0.39 | |
| TB | 0.99 | 0.97 | 0.70 | |
| Speaking (B2 + LT + O) | none | 0.95 | 0.83 | 0.45 |
| SARS-CoV-2 | 0.95 | 0.83 | 0.45 | |
| TB | 0.99 | 0.97 | 0.70 | |
| Speaking (LT) | none | 0.98 | 0.91 | 0.47 |
| SARS-CoV-2 | 0.98 | 0.91 | 0.47 | |
| TB | 0.99 | 0.96 | 0.61 | |
| Speaking (O) | none | 1.00 | 1.00 | 1.00 |
| SARS-CoV-2 | 1.00 | 1.00 | 1.00 | |
| TB | 1.00 | 1.00 | 1.00 | |
| Min | 0.83 | 0.64 | 0.33 | |
| Max | 1.00 | 1.00 | 1.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, J.; Macher, J.M.; Chen, W.; Kumagai, K. Comparative Mask Protection against Inhaling Wildfire Smoke, Allergenic Bioaerosols, and Infectious Particles. Int. J. Environ. Res. Public Health 2022, 19, 15555. https://doi.org/10.3390/ijerph192315555
Wagner J, Macher JM, Chen W, Kumagai K. Comparative Mask Protection against Inhaling Wildfire Smoke, Allergenic Bioaerosols, and Infectious Particles. International Journal of Environmental Research and Public Health. 2022; 19(23):15555. https://doi.org/10.3390/ijerph192315555
Chicago/Turabian StyleWagner, Jeff, Janet M. Macher, Wenhao Chen, and Kazukiyo Kumagai. 2022. "Comparative Mask Protection against Inhaling Wildfire Smoke, Allergenic Bioaerosols, and Infectious Particles" International Journal of Environmental Research and Public Health 19, no. 23: 15555. https://doi.org/10.3390/ijerph192315555
APA StyleWagner, J., Macher, J. M., Chen, W., & Kumagai, K. (2022). Comparative Mask Protection against Inhaling Wildfire Smoke, Allergenic Bioaerosols, and Infectious Particles. International Journal of Environmental Research and Public Health, 19(23), 15555. https://doi.org/10.3390/ijerph192315555






