Abstract
The study aimed to detect the content of mandipropamid enantiomers in unprocessed and processed tomato, cucumber, Chinese cabbage, and cowpea samples and assess the health risks to Chinese consumers. Data showed that washing and soaking with an acidic solution reduced the mandipropamid residue from vegetable samples by 54.1–82.2%. The pickling process resulted in a 6.2–65.2% loss of mandipropamid from cucumber, Chinese cabbage, and cowpea samples. Peeling and juicing were the best removing techniques for mandipropamid residues in tomato and cucumber (removal rate (RR) value > 91%), and cooking for 5 min could effectively reduce the levels of mandipropamid in Chinese cabbage and cowpea (RR values of 81.4–99.7%). The values of processing factor for the processed vegetable samples are all less than one. No significant enantioselectivity of mandipropamid was found in the vegetables during processing. Health risk data showed that samples of four types of mandipropamid-contaminated vegetables were safe for consumption after processing.
1. Introduction
Food safety is a growing area of global concern due to its direct impact on human health and the environmental ecosystems. While agricultural crops provide the nutrients humans need, they are vulnerable to the threats caused from fungi, bacteria, viruses, insects, and weeds. Pesticides are promoted and applied in agricultural culture and production to control threats and improve the safety of agricultural crops. However, pesticide residues have been found in almost all agricultural crops (both raw and processed), which might cause human adverse reactions and diseases, such as nausea, headaches, and cardiovascular disease [1]. The safety of vegetables, a major component of agricultural crops, is also a major concern for Chinese consumers. In China, vegetables provide various vitamins, minerals, and dietary fibres and are indispensable constituents of the daily family diet [2]. Currently, pesticides are widely used during the growing and production stages of vegetables to ensure good quality and sufficient yield. Some pesticides are absorbed by vegetable tissues and transported through the metabolic system within the vegetable. Some pesticides only attach to the surface of vegetables and protect the vegetable from the fungi and insects of direct contact [3]; however, the use of pesticides has contributed to the potential contamination threat to vegetables and their growing environs.
The most common pathway of pesticide ingestion in humans is through food ingestion, where most foods are consumed after a range of culinary and processing treatments. Several studies have demonstrated that food processing techniques may reduce or increase pesticide concentrations in both raw and processed agricultural crops. Household processing techniques, including washing [4], pickling [5], peeling [6], boiling [7], drying [8], and juicing [9], are the most common approaches for changing (eliminating or concentrating) the residues of pesticides in agricultural products. In accordance with the food processing data presented in the FAO/WHO Joint Meeting on Pesticide Residues (JMPRs) report, differences in pesticide residues occur in crops and their products [10]. In addition, some researchers illustrated that the effect of the processing method on reducing pesticide residues might be influenced by the physicochemical properties of pesticides, such as solubility, volatility, octanol/water distribution coefficient (Kow), and mode of action [11]. For instance, washing and peeling can effectively remove the residues of chlorpyriphos and ethylparathion in tomato, probably due to the log Kow values of 4.7 and 3.8, respectively, which inhibited their transport in plant tissue and located on the outer epidermis of crops [9]. When boiling and steaming were used in processing rice products, volatilization and thermal degradation caused the reduction in pesticide residues in the range of 20.7–100% [12].
Mandipropamid is a chiral systemic fungicide with a broad bactericidal spectrum and can effectively prevent most foliar oomycete pathogens. The market sales of mandipropamid have been growing in the last half decade [13]. As applications increase, potential contamination from mandipropamid residues in the ecosystem became more apparent. A few studies have reported the dissipation and residue distribution of mandipropamid in vegetables and environmental matrices at the enantiomer level [11,14,15], whereas a lack of household processing techniques and data exists. On the basis of our previous research [16], this work evaluated the efficiency of several household processing approaches on removing the residues of mandipropamid enantiomers from four kinds of vegetable samples (tomato, cucumber, Chinese cabbage, and cowpea), calculated and analysed the processing factor (PF) values, and assessed the health risk of mandipropamid for different groups of Chinese consumers.
2. Materials and Methods
2.1. Chemicals and Reagents
The analytical standard of racemic mandipropamid (purity, 98.6%) was purchased from J&K Scientific Ltd. (Beijing, China), and the formulation of suspension concentrate (SC) (23.4%) was obtained from Syngenta Nantong Crop Protection Co., Ltd. (Nantong, China). Chromatographic grade acetonitrile (ACN), methanol (MeOH), ammonium formate (AF) and formic acid (FA) were bought from Thermo Fisher Scientific (Waltham, USA). Analytical grade anhydrous sodium acetate (NaAc), magnesium sulfate (MgSO4), sodium chloride (NaCl), sodium carbonate (Na2CO3), and acetic acid (AA) were purchased by Tianjin Zhiyuan Reagent Co., Ltd. (Tianjin, China). Primary secondary amine (PSA) and octadecylsilane (C18) were obtained from the Bonna-Agela Company (Tianjin, China). Distilled water and 0.22 µm nylon syringe filters were bought from Watson Group Ltd. (Dongguan, China) and PeakSharp Technologies (Beijing) Co., Ltd. (Beijing, China), respectively.
2.2. Instrumentation and Sample Pretreatment
Liquid chromatography with tandem mass spectrometry (LC–MS/MS) was applied for the determination of mandipropamid enantiomers. Chromatographic separation was performed on a Daicel Chiralcel OZ-3R column (150 × 2.1 mm i.d., particle size 3 µm, Daicel Chiral Technologies Co., Ltd., Shanghai, China) installed on a Shimadzu LC-20AD XR LC system (Shimadzu Corporation, Kyoto, Japan), and quantitative analysis was conducted on an AB Sciex API 4000 Q-Trap quadrupole MS system (Applied Biosystems, Foster City, CA, USA). The extraction solution for vegetable samples was ACN with 1% AA, the extraction method was vortex extraction, and the purification sorbent was a different combination of PSA, C18, and MgSO4, while the brine samples were filtered through gravity filter paper and a 0.22 μm nylon syringe filter and then directly injected into the instrument. The detailed liquid chromatographic conditions, mass spectrometric parameters, and pre-treatment procedure were the same as those in our previous study [16].
2.3. Household Processing Techniques
In the household processing experiment, all four kinds of vegetable samples were gathered on the 1st day after the last application in the field trials, which were carried out in 2021 with the application of 23.4% mandipropamid SC at a spray dosage of 140.4 g a.i./hm2 (the recommended high dosage) three times with an interval of 7 d. After different processing procedures, the vegetable samples were chopped into small pieces with an average size of approximately 1 cm3 and then homogenized before pre-treatment. Figure 1 demonstrates the scheme of household processing techniques for four types of vegetable samples. Three replicates were performed in each treatment. The detailed steps of the different processes are listed in the following subsections.
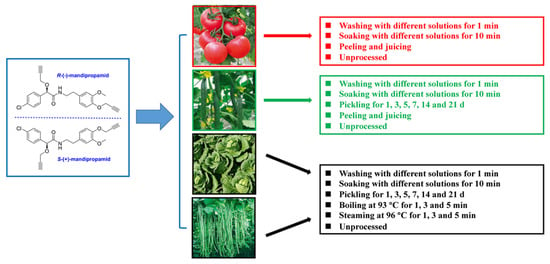
Figure 1.
Scheme for assigning processing to four types of mandipropamid-treated vegetable samples.
2.3.1. Washing and Soaking
Four kinds of non-toxic solutions, including 10% (v/v) acidic solution (AA solution), 10% (w/v) solution (NaCl solution), 10% alkaline (w/v) solution (Na2CO3 solution), and tap water, were chosen to assess the effect of washing on the residues mandipropamid enantiomers in tomato, cucumber, Chinese cabbage, and cowpea. Mandipropamid-contaminated vegetable samples collected in the field trials in August 2021, which were directly transferred to the lab and prepared as lab samples, were washed in different solutions for 1 min [17,18]. In this study, four types of contaminated vegetable samples were soaked for 10 min in the above four solutions [19]. The processed samples were extracted and detected using the analysis method described in Section 2.2.
2.3.2. Pickling
The contaminated cucumber samples were cut into small pieces with an average size of approximately 1 cm3, and the contaminated Chinese cabbage and cowpea samples were drained as the raw material of sauerkraut [20,21,22,23]. The pretreated vegetable samples were pickled in 10% NaCl solution in a 5 L fermentation tank stored in the dark at 25 °C. The pickled vegetable and brine samples were collected at 7 time points (0, 1, 3, 5, 7, 14, and 21 d), and all the samples were stored at −20 °C for further analysis.
2.3.3. Peeling and Juicing
The mandipropamid-contaminated cucumber or tomato samples were peeled with a knife of 1 mm approximate thickness at room temperature [24,25]. The peel sample and a partial pulp sample were subsequently detected. Another part of the pulp sample was homogenized to obtain the juice and puree samples.
2.3.4. Cooking
Part of the mandipropamid-contaminated Chinese cabbage or cowpea samples were boiled in 93 °C water for 1, 3, and 5 min, and another part was steamed over boiling water (96 °C) for 1, 3, and 5 min [26,27]. The samples were then immediately analyzed as a whole for remaining mandipropamid enantiomers.
2.3.5. Data Calculation
The removal rate (RR, %) is one parameter used to assess the effect of processing techniques on the change in pesticide residues in processed products [28]. The values are calculated by the equation RR = (C1− − C2)/C1 × 100%, where C1 and C2 is the residue concentration (µg/kg) in unprocessed and processed products, respectively. RR values of <100% indicate a decrease in pesticide residues, while RR values of >100% indicate an increase in pesticide residues in processed products. PF value, the ratio of C2 and C1, is also used to measure the effectiveness of a processing approach [2]. As the PF values < 1, the process technique (washing or boiling) can reduce pesticide residues in vegetables [25]. A PF value of 1 indicates that the method has no effect on pesticide residues. However, when PF values are more than 1, the concentrations of pesticides in vegetables increase after certain treatments, such as juicing [29]. Enantiomeric fraction (EF) values are calculated by the equation EF = CR/(CR + CS), where CR and CS are the residue levels of R- and S-enantiomer, respectively, and applied to evaluate the potential enantioselectivity of mandipropamid in vegetable samples after different processing treatments. As 0 < EF < 0.5, a preferential removal efficiency was conducted for R-enantiomer. An EF value of 0.5 indicates no enantioselectivity. Moreover, the S-enantiomer is preferentially removed for EF values in the range of 0.5–1 [21,30].
2.4. Health Risk Estimation
Hazard quotient (HQ) values, including long-term (HQChronic) and short-term (HQAcute), are used to assess the health exposure risk of mandipropamid in vegetable samples after different processing procedures [29]. The JMPR evaluation report demonstrates that the acute reference dose (ARfD) value of mandipropamid is unnecessary and that the acute risk can be negligible to humans. Therefore, the long-term risk should be assessed for mandipropamid and the HQChronic is calculated as the ratio of international estimated daily intake (IEDI) and acceptable daily intake (ADI). The IEDI value is obtained from the equation IEDI = (FI × RL)/b.w., where FI is the food intake data, RL is the residue level of mandipropamid in processed food samples, and b.w. is the body weight. The ADI value for mandipropamid is 0.2 mg/kg b.w. [14].
2.5. Statistical Analysis
All the processing experiments were performed three times. Excel 2010 (Microsoft Corporation, Washington, DC, USA) was applied to the data analysis. Through Duncan’s multiple range test, the data were statistically evaluated by one-way analysis of variance (ANOVA, p = 0.05) with SPSS ver. 24.0 statistical software (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Method Validation
The analytical method of mandipropamid enantiomers in vegetable samples was quantified by the approach of calibration curves and validated by limit of detection (LOD), limit of quantitation (LOQ), and recovery and relative standard deviation (RSD). Based on the reported determination approach [16], the LODs and LOQs of two mandipropamid enantiomers in the whole part, skin, pulp, puree, and juice of different vegetable samples were 0.7 and 2.5 μg/kg, respectively. In Table S1 (Supporting Information), average recoveries of R-enantiomer and S-enantiomer in different parts were 82.6–99.6% for tomato, 88.7–100.8% for cucumber, 96.0–101.0% for Chinese cabbage, and 89.8–98.8% for cowpea, with RSDs of 0.2–13.1% in the spiked level range of 2.5–500 μg/kg.
3.2. Effect of Household Processing Techniques
3.2.1. Washing and Soaking
The data on the effects of washing and soaking on the residues of mandipropamid enantiomers and racemate in four types of vegetable samples are shown in Table 1, Table 2, Table 3 and Table 4. For example, after washing in tap water, the R-enantiomer, S-enantiomer, and rac-mandipropamid in cucumber samples had RR values of 23.6, 23.9, and 23.8%, respectively. The RR values were approximately 47% higher in the cucumber samples washed in NaCl solution and Na2CO3 solution, and the corresponding RR values were more than 2.2 times higher in the cucumber samples washed in AA solution. Additionally, the PF values of washing in AA solution were significantly lower than those in the other three solutions (p < 0.001, Table 1 and Table 2). For cowpea, the RR values of the three analytes in samples washed in AA solution were significantly higher by approximately three times than those in samples washed in tap water (p < 0.001, Table 3 and Table 4). In Table 1 and Table 2, the RR values of the three analytes in tomato and cucumber samples soaked in 10% AA solution (75.3–82.2%), 10% Na2CO3 solution (71.5–77.0%), 10% NaCl solution (69.9–73.1%), and tap water (57.8–60.1%) were significantly higher than those in samples washed in the four solutions (p < 0.001). The data in Table 3 and Table 4 showed that after soaking, the RR values of R-enantiomer (56.2–76.9%), S-enantiomer (57.2–76.8%), and rac-mandipropamid (56.7–76.8%) were significantly higher than those after washing, and the PF values for soaking (0.231–0.438) were significantly lower than those for washing (0.444–0.820) (p < 0.001).

Table 1.
Residue level, removal rate (RR) and processing factor (PF) values of mandipropamid in unprocessed, washing, and soaking tomato samples.

Table 2.
Residue level, RR, and PF values of mandipropamid in unprocessed, washing and soaking cucumber samples.

Table 3.
Residue level, RR, and PF values of mandipropamid in unprocessed, washing, and soaking Chinese cabbage samples.

Table 4.
Residue level, RR, and PF values of mandipropamid in unprocessed, washing, and soaking cowpea samples.
3.2.2. Pickling
Figure 2 shows the concentration, RR, and PF values of mandipropamid in samples of pickled cucumber, Chinese cabbage, and cowpea at different intervals. Residues of mandipropamid were significantly reduced in the pickled cucumber samples, with a RR value of 17.8–64.5% during the 21-day pickling period, followed by the pickled cowpea samples, with a RR value of 8.1–51.3%. In pickled Chinese cabbage samples, the mandipropamid enantiomers and racemate showed relatively low removal rates. The PF data (Figure 2) are also demonstrated the different removal efficiencies for mandipropamid in different vegetable samples after pickling. For example, the PF values of pickling decreased significantly from 0.796 to 0.356, from 0.883 to 0.486, and from 0.935 to 0.658 for the R-enantiomer in cucumber, cowpea, and Chinese cabbage samples, respectively (p < 0.001). Moreover, some mandipropamid residues were transferred from vegetables into brine (pickling solution). In Table S2 (Supporting Information), the concentrations of mandipropamid enantiomers and racemate in brine samples increased from the first day to the seventh day and then decreased in the following 14 days.
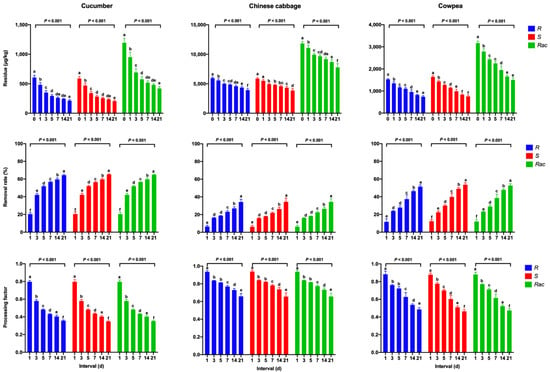
Figure 2.
Residues, removal rates (RRs) of mandipropamid enantiomers, and racemate and processing factors (PFs) for different intervals after pickling in cucumber, Chinese cabbage, and cowpea samples. Error bars represent the standard deviation (SD) of triplicates. Each lowercase letter indicates significant differences between the seven sampling intervals (p ≤ 0.05, Duncan’s multiple range test).
3.2.3. Peeling and Juicing
As shown in Table 5, the percentages of mandipropamid residues were <10% in tomato pulp samples and approximately 1% in cucumber pulp samples after peeling. More than 90% of mandipropamid is found in vegetable skins. After peeling, the tomato and cucumber pulp samples are cut into quarters and separated into puree and juice. The RR values of mandipropamid residues in puree and juice samples are 97.1–97.9% for tomato and 99.4–99.5% for cucumber. The corresponding PF values are in the range of 0.005–0.029.

Table 5.
Residue level, RR, and PF values of enantiomeric and racemic mandipropamid in unprocessed and different parts of tomato and cucumber samples.
3.2.4. Cooking
The effect of the cooking process on the mandipropamid residues in Chinese cabbage and cowpea samples was investigated for two treatments: boiling and steaming. After boiling for 1, 3, and 5 min, the RR values of mandipropamid significantly increased from 44.6 to 99.0% in Chinese cabbage, with PF values of 0.009–0.554, and from 65.1 to 99.7% in cowpea, with PF values of 0.003–0.349 (p < 0.001, Figure 3). After steaming, the concentrations of R-enantiomer significantly decreased from 5969 to 1108 µg/kg in Chinese cabbage, with decreasing PF values from 0.793 to 0.186 after steaming for 5 min (p < 0.001, Figure 3). For cowpea, the difference in removal efficiency between boiling and steaming was not significant. The results show that these two cooking treatments also effectively removes mandipropamid residues from Chinese cabbage and cowpea.
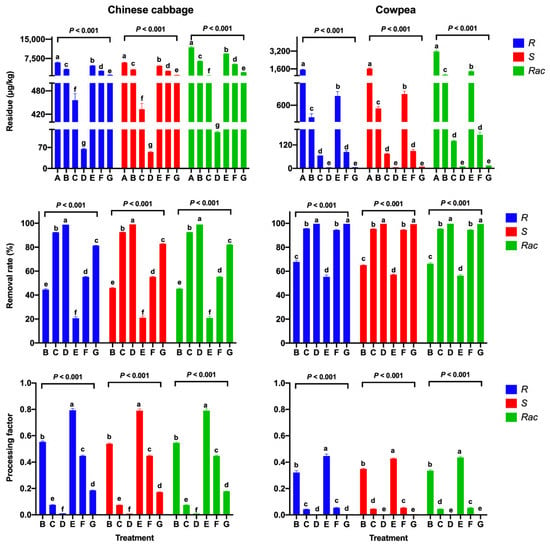
Figure 3.
Residues and RRs of mandipropamid enantiomers and racemate and PFs for different cooking steps in Chinese cabbage and cowpea samples (A: unprocessed; B: boiled for 1 min; C: boiled for 3 min; D: boiled for 5 min; E: steam for 1 min; F: steam for 3 min; G: steam for 5 min). Error bars represent the SD of triplicates. Each lowercase letter indicates significant differences between the seven sampling intervals (p ≤ 0.05, Duncan’s multiple range test).
3.2.5. Potential Enantioselectivity
After washing and soaking in different solutions, the difference between the R- and S-enantiomer residues in the four vegetables remained nearly constant, with EF values close to 0.5 (Figure 4). No significant enantioselectivity was also found in the pickled vegetable samples. Although after peeling or cooking, a few EF values were changed considerably, such as EF values increased to 0.565 in tomato skin samples and to 0.541 in cucumber skin samples or EF values decreased to 0.447 after steaming for 5 min and to 0.382 after boiling for 5 min in cowpea samples (Figure 4), the reduction in mandipropamid enantiomers was not different in most cases.
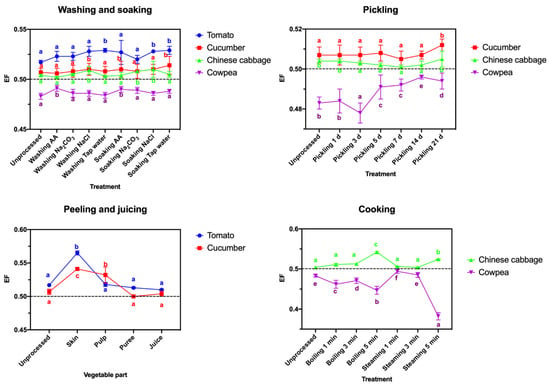
Figure 4.
EF values of mandipropamid in four types of vegetable samples after different processing treatments. Each lowercase letter indicates significant differences between the different treatments (p ≤ 0.05, Duncan’s multiple range test).
3.3. Health Risk
The HQ values were calculated and used to assess the health risks of mandipropamid in processed vegetable samples for different groups of Chinese consumers. For the chronic risk assessment, HQchronic values were calculated and are shown in Table 6, Table 7, Table 8 and Table 9. After different processing treatments, the HQchronic values of rac-mandipropamid were <2% for the tomato samples, <8% for the cucumber samples, <40% for the Chinese cabbage samples, and <11% for the cowpea samples.

Table 6.
Hazard quotient (HQ) of rac-mandipropamid in unprocessed (UP) and processing tomato samples for different groups of Chinese consumers.

Table 7.
Hazard quotient (HQ) of rac-mandipropamid in unprocessed (UP) and processing cucumber samples for different groups of Chinese consumers.

Table 8.
Hazard quotient (HQ) of rac-mandipropamid in unprocessed (UP) and processing Chinese cabbage samples for different groups of Chinese consumers.

Table 9.
Hazard quotient (HQ) of rac-mandipropamid in unprocessed (UP) and processing cowpea samples for different groups of Chinese consumers.
4. Discussion
Based on the method validation data (Table S1, Supporting Information), the developed extraction and detection method can be employed in the following processing experiments. Due to log Kow of 3.829, most mandipropamid residues remained in the surface of vegetables [9] and washing can effectively remove those, which is consistent with our obtained results that washing in different aqueous solutions (including acidic, basic, neutral solutions, and tap water) can remove most mandipropamid residues from tomato, cucumber, Chinese cabbage, and cowpea samples. Among the four solutions, tap water had the lowest reduction efficiency, NaCl solution and Na2CO3 solution were the next lowest, and AA solution was the most effective in reducing mandipropamid residues from all four types of vegetable samples. Similar results have been reported for other pesticides in a number of previous studies. For example, Kin and Huat found that for cucumber and strawberry samples, the removal efficiencies of washing in 10% AA solution (RR values of 44–70%) were more excellent than those in 10% Na2CO3 solution (RR values of 30–50%), 10% NaCl solution (RR values of 23–40%), and tap water (RR values of 10–20%) for eight pesticides [18]. Soliman reported that washing in AA solution had the best effect in reducing pesticide residues from potato samples [31]. Compared with washing (PF values of 0.413–0.764), soaking increased the reduction amount of mandipropamid enantiomers in tomato and cucumber samples with a lower PF value of 0.178–0.422, which illustrated that soaking had a better removing efficiency. Similarly, soaking was also found to be more effective than washing in removing mandipropamid residues from Chinese cabbage and cowpea samples. In the investigation of the reduction effect of soaking for pesticide residues in potato samples, Zohair found higher RR values (>75%) compared with the corresponding data of washing [19]. The possible reason is that soaking provides a relatively long-term contact of the aqueous solution with the vegetable sample. It was shown that washing and soaking effectively removed the mandipropamid residue from the four vegetables.
In the pickling trial, the concentrations of mandipropamid were slightly reduced in cucumber, Chinese cabbage, and cowpea samples over the pickling time. Microbiological degradation may be the main pathway for removing pesticide residues from vegetables in the pickling process [21]. Different reduction efficiencies were obtained for the analyte in different vegetables, which might be related to the characteristics of the vegetables [20,22], such as specific bacteria generated after pickling different vegetables [5]. Based on the chemical properties of pesticides and environmental conditions, peeling and juicing are two efficient approaches for removing pesticide residues from vegetable products [32]. The results showed that the peeling technique had a significant effect in removing mandipropamid residues from tomato and cucumber samples, with the skins accumulating significant amounts of mandipropamid. The present data are in agreement with some previous studies, where the RR value of peeling was 63% for chlorpyrifos residues in tomato sample with a PF value of 0.09 [33], and the RR value for dichlorvos residues in cucumber sample was 57% after peeling [34]. The reason was possibly related to the mode of action of the analyte: before the systemic interaction, the contact of mandipropamid and vegetable occurred and almost remained on the surface of the skin [3]. Although no significant difference was observed between the RR and PF values in juice and puree, the lower water solubility of mandipropamid (4.2 mg/L) made it difficult to be transported into tomato or cucumber juice [24]. Boiling was also an effective processing technique for reducing the mandipropamid residue in Chinese cabbage and cowpea. A similar process was found in the study of Kontou et al. [26] and Rowayshed et al. [35], where boiling removed more than 70% of maneb residues from tomato samples and removed >90% of carbamate pesticide residues from eggplant, respectively. Compared with boiling, steaming may be a less effective treatment for reducing pesticide residues in vegetables, as only high temperatures and water vapour can cause pesticide loss. However, after steaming, the residues of mandipropamid in Chinese cabbage and cowpea decreased to a great extent, which possibly contributed to the character of vegetables [3]. Thus, the above results demonstrated that significant reductions in mandipropamid were found in tomato and cucumber samples after peeling and juicing, and in Chinese cabbage and cowpea samples after cooking, and that the processing techniques have a significant removal effect on the mandipropamid residue in vegetables.
Based on the EF values (Figure 4), the distribution of mandipropamid in tomato, cucumber, Chinese cabbage and cowpea was not significantly enantioselective after the above four process treatments, indicating that household processing may not induce enantioselectivity of mandipropamid residues in vegetables. The maximum residue limit (MRL) value is used to evaluate the safety of pesticide residues in vegetables [16]. The MRL values of mandipropamid were 300, 200, and 25,000 µg/kg for tomato, cucumber, and Chinese cabbage in China, respectively [36], and 1000 µg/kg for beans with pods in the European Union [37]. According to the concentration data of rac-mandipropamind in the processed vegetable samples, the tomato sample was safe after washing, soaking, peeling, and juicing; the cucumber sample was safe after peeling and juicing; the Chinese cabbage sample was safe after all kinds of processing; and the cowpea sample was safe after cooking (>1 min). In addition, all the HQchronic values were less than 100%, indicating that the health risks of the four mandipropamid-contaminated vegetable samples are acceptable for Chinese consumers after processing. The residue and health risk assessment results illustrated the impressive removal efficiency of the processing method for mandipropamid residues in tomato, cucumber, Chinese cabbage, and cowpea samples.
5. Conclusions
In this study, the effects of different processing techniques, including washing, soaking, pickling, peeling, juicing, boiling, and steaming, on mandipropamid residues were investigated in four types of vegetables. The results showed that the concentration of mandipropamid decreased after the above processing procedure. The addition of 10% AA, 10% Na2CO3, and 10% NaCl significantly increased the removal efficiency of mandipropamid compared with tap water washing and soaking. The PF values (0.178–0.459) demonstrated that washing and soaking with the acidic solution reduced the mandipropamid residue more than the other three solutions. The decrease in PF from 0.938 to 0.348 illustrated that pickling is a moderate processing technique for reducing mandipropamid residues from vegetable samples. Peeling and juicing were able to effectively remove the mandipropamid residue from the tomato and cucumber samples. For Chinese cabbage and cowpea, the concentrations of mandipropamid were reduced by >81% after boiling and steaming for 5 min. After these post-processing treatments, there was no significant enantioselectivity of mandipropamid in the processed vegetable samples. HQ values < 100% demonstrated that the health risks of mandipropamid in four types of processed vegetables is negligible for Chinese consumers. The results not only provide information about the impact of mandipropamid on food safety, but also help screen contaminated agricultural products for processing methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph192315543/s1, Table S1: Recoveries and relative standard deviations (RSDs) of mandipropamid enantiomers in different parts of tested vegetable samples, Table S2: Residues of mandipropamid enantiomers and racemate in brine at different intervals after pickling cucumber, Chinese cabbage, and cowpea samples.
Author Contributions
Conceptualization, S.M.; methodology, S.M.; software, S.M.; data curation, S.M. and L.D.; writing—original draft preparation, S.M.; validation, L.D. and Y.Y.; formal analysis, L.D.; investigation, Y.Y. and D.C.; resources, K.Z.; writing—review and editing, K.Z.; visualization, K.Z.; supervision, K.Z.; project administration, K.Z.; funding acquisition, K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32260686, 32060629), Guizhou Provincial Science and Technology Projects (QKHJC-ZK[2022]ZD022), and the Program of Introducing Talents of Discipline to Universities of China (111 Program, D20023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sandoval-Insausti, H.; Chiu, Y.H.; Dong, H.L.; Wang, S.; Chavarro, J.E. Intake of fruits and vegetables by pesticide residue status in relation to cancer risk. Environ Int. 2021, 156, 106744. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, A.; Hanot, V.; Bragard, C.; Bedoret, T.; van Loco, J. Effect of household and industrial processing on the levels of pesticide residues and degradation products in melons. Food Addit. Contam. A 2012, 29, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Słowik-Borowiec, M.; Szpyrka, E. Selected food processing techniques as a factor for pesticide residue removal in apple fruit. Environ. Sci. Pollut. Res. 2020, 27, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Polat, B.; Tiryaki, O. Assessing washing methods for reduction of pesticide residues in Capia pepper with LC-MS/MS. J. Environ. Sci. Health B. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Bian, Y.L.; Guo, G.; Liu, F.M.; Chen, X.C.; Wang, Z.Y.; Hou, T.Y. Meptyldinocap and azoxystrobin residue behaviors in different ecosystems under open field conditions and distribution on processed cucumber. J. Agric. Food Chem. 2020, 100, 648–655. [Google Scholar] [CrossRef]
- Keikotlhaile, B.M.; Spanoghe, P.; Steurbaut, W. Effects of food processing on pesticide residues in fruits and vegetables: A meta-analysis approach. Food. Chem. Toxicol. 2010, 48, 1–6. [Google Scholar] [CrossRef]
- Liu, N.; Dong, F.S.; Liu, X.G.; Xu, J.; Li, Y.B.; Han, Y.T.; Zhu, Y.L.; Cheng, Y.P.; Chen, Z.L.; Tao, Y.; et al. Effect of household canning on the distribution and reduction of thiophanate-methyl and its metabolite carbendazim residues in tomato. Food Control. 2014, 43, 115–120. [Google Scholar] [CrossRef]
- Kong, Z.Q.; Quan, R.; Fan, B.; Liao, Y.H.; Chen, J.Y.; Li, M.M.; Dai, X.F. Stereoselective behaviors of the fungicide triadimefon and its metabolite triadimenol during malt storage and beer brewing. J. Hazard. Mater. 2020, 400, 123238. [Google Scholar] [CrossRef]
- Jankowska, M.; Kaczynski, P.; Hrynko, I.; Lozowicka, B. Dissipation of six fungicides in greenhouse-grown tomatoes with processing and health risk. Environ. Sci. Pollut. Res. 2016, 23, 11885–11900. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). Updating the Principles and Methods of Risk Assessment: MRLs for Pesticides and Veterinary Drugs; FAO: Rome, Italy, 2006. [Google Scholar]
- Hendawi, M.Y.; Romeh, A.A.; Mekky, T.M. Effect of food processing on residue of imidacloprid in strawberry fruits. J. Agric. Sci. Techol. 2018, 15, 951–959. [Google Scholar] [CrossRef]
- Shakoori, A.; Yazdanpanah, H.; Kobarfard, F.; Shojaee, M.H.; Salamzadeh, J. The effects of house cooking process on residue concentrations of 41 multi-class pesticides in rice. Iran J. Pharm. Res. 2018, 17, 571–584. [Google Scholar]
- Han, J.H.; Chen, Y.; Liu, Z.Y.; Chen, D.; Zhang, K.K.; Hu, D.Y. Enantioselective environmental behavior of the chiral fungicide mandipropamid in four types of Chinese soil. Soil Sci. Soc. Am. J. 2021, 85, 574–590. [Google Scholar] [CrossRef]
- JMPR (Joint FAO/WHO Meeting on Pesticide Residues). JMPR Evaluation: Mandipropamid. Available online: http://www.fao.org/fileadmin/templates/agphome/documents/PestsPesticides/JMPR/Evaluation08/Mandipropamid.pdf (accessed on 10 February 2022).
- Xu, G.F.; Jia, X.H.; Zhang, H.P.; Zhang, J.Y.; Nie, J.Y. Enantioselective fate of mandipropamid in grape and during processing of grape wine. Environ. Sci. Pollut. Res. 2020, 27, 40148–40155. [Google Scholar] [CrossRef]
- Li, J.M.; Lan, T.T.; Yang, G.Q.; Mu, S.Y.; Zhang, K.K. Enantioselective evaluation of the chiral fungicide mandipropamid: Dissipation, distribution and potential dietary intake risk in tomato, cucumber, Chinese cabbage and cowpea. Ecotox. Environ. Safe. 2022, 232, 113260. [Google Scholar] [CrossRef]
- Kaushik, G.; Satya, S.; Naik, S.N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Kin, C.M.; Huat, T.G. Headspace solid-phase microextraction for the evaluation of pesticide residue contents in cucumber and strawberry after washing treatment. Food Chem. 2010, 123, 760–764. [Google Scholar] [CrossRef]
- Zohair, A. Behaviour of some organophosphorus and organochlorine pesticides in potatoes during soaking in different solutions. Food. Chem. Toxicol. 2001, 39, 751–755. [Google Scholar] [CrossRef]
- Lu, Y.L.; Yang, Z.H.; Shen, L.Y.; Liu, Z.M.; Zhou, Z.Q.; Diao, J.L. Dissipation behavior of organophosphorus pesticides during the cabbage pickling process: Residue changes with salt and vinegar content of pickling solution. J. Agric. Food Chem. 2013, 61, 2244–2252. [Google Scholar] [CrossRef]
- Zhao, Y.; Ouyang, M.N.; Xiong, Y.B.; Wang, D.D.; Guo, H.M.; Yang, Z.H. The different dissipation behavior of chiral pesticide paclobutrazol in the brine during Chinese cabbage pickling process. Chirality 2019, 31, 230–235. [Google Scholar] [CrossRef]
- Guo, H.M.; Zhao, Y.; Ouyang, M.N.; Yang, Z.H.; Li, J.H. The enantioselective effects and potential risks of paclobutrazol residue during cucumber pickling process. J. Hazard. Mater. 2020, 386, 121882. [Google Scholar] [CrossRef]
- Yang, X.Z.; Hu, W.Z.; Xiu, Z.L.; Jiang, A.L.; Yang, X.Y.; Sarengaowa, J.Y.R.; Guan, Y.G.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.T.; Li, W.M.; Dong, F.S.; Xu, J.; Liu, X.G.; Li, Y.B.; Kong, Z.Q.; Liang, X.Y.; Zheng, Y.Q. The behavior of chlorpyrifos and its metabolite 3, 5, 6-trichloro-2-pyridinol in tomatoes during home canning. Food Control. 2013, 31, 560–565. [Google Scholar] [CrossRef]
- Ramezani, M.K.; Shahriari, D. Dissipation behaviour, processing factors and risk assessment for metalaxyl in greenhouse-grown cucumber. Pest Manag. Sci. 2015, 71, 579–583. [Google Scholar] [CrossRef]
- Kontou, S.; Tsipi, D.; Tzia, C. Stability of the dithiocarbamate pesticide maneb in tomato homogenates during cold storage and thermal processing. Food Addit. Contam. 2004, 21, 1083–1089. [Google Scholar] [CrossRef]
- Huan, Z.B.; Xu, Z.; Jiang, W.; Chen, Z.Q.; Luo, J.H. Effect of Chinese traditional cooking on eight pesticides residue during cowpea processing. Food Chem. 2015, 170, 118–122. [Google Scholar] [CrossRef]
- Noh, H.H.; Kwon, H.Y.; Lee, H.S.; Ro, J.H.; Kyung, K.S.; Moon, B.C. Determination of pyraclostrobin and trifloxystrobin residues in red pepper powder processed from raw red pepper. Food Anal. Methods 2019, 12, 94–99. [Google Scholar] [CrossRef]
- Jankowska, M.; Łozowcka, B. The processing factors of canning and pasteurization for the most frequently occurring fungicides and insecticides in apples and their application into dietary risk assessment. Food Chem. 2022, 371, 131179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Q.Q.; Zhong, Y.R.; Wang, Z.; He, Z.Z.; Zhang, Y.Q.; Wang, M.H. Enantioselective bioactivity, toxicity, and degradation in vegetables and soil of chiral fungicide mandipropamid. J. Agr. Food Chem. 2021, 69, 13416–13424. [Google Scholar] [CrossRef]
- Soliman, K.M. Changes in concentration of pesticide residues in potatoes during washing and home preparation. Food. Chem. Toxicol. 2001, 39, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.; Đurović-Pejčev, R. Food processing as a means for pesticide residue dissipation. Pestic. Phytomed. 2016, 31, 89–105. [Google Scholar] [CrossRef]
- Rani, M.; Saini, S.; Kumari, B. Persistence and effect of processing on chlorpyriphos residues in tomato (Lycopersicon esculantum Mill.). Ecotox. Environ. Safe 2013, 95, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.F.; Certel, M.H.; Göçmen, H. Residue contents of DDVP (Dichlorvos) and diazinon applied on cucumbers grown in greenhouses and their reduction by duration of a pre-harvest interval and post-harvest culinary application. Food Chem. 2006, 98, 127–135. [Google Scholar] [CrossRef]
- Rowayshed, G.; Sharaf, A.M.M.; Mostafa, M.M. Stability of carbamate pesticides residue in some vegetables throughout the household processing and cooking. Middle East J. Appl. Sci. 2013, 3, 205–215. [Google Scholar]
- National Health and Family Planning Commission; Ministry of Agriculture of the People’s Republic of China. National Food Safety Standard–Maximum Residue Limits for Pesticides in Food (GB 2763–2021). Available online: http://www.nhc.gov.cn/sps/s7891/202202/abb7090ad744405fba8244893839206d.shtml (accessed on 10 February 2022).
- EU (European Union). EU Pesticide Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=_pesticide.residue.CurrentMRL&language=_EN (accessed on 10 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).