Effect of Gestational Pesticide Exposure on the Child’s Respiratory System: A Narrative Review
Abstract
1. Introduction
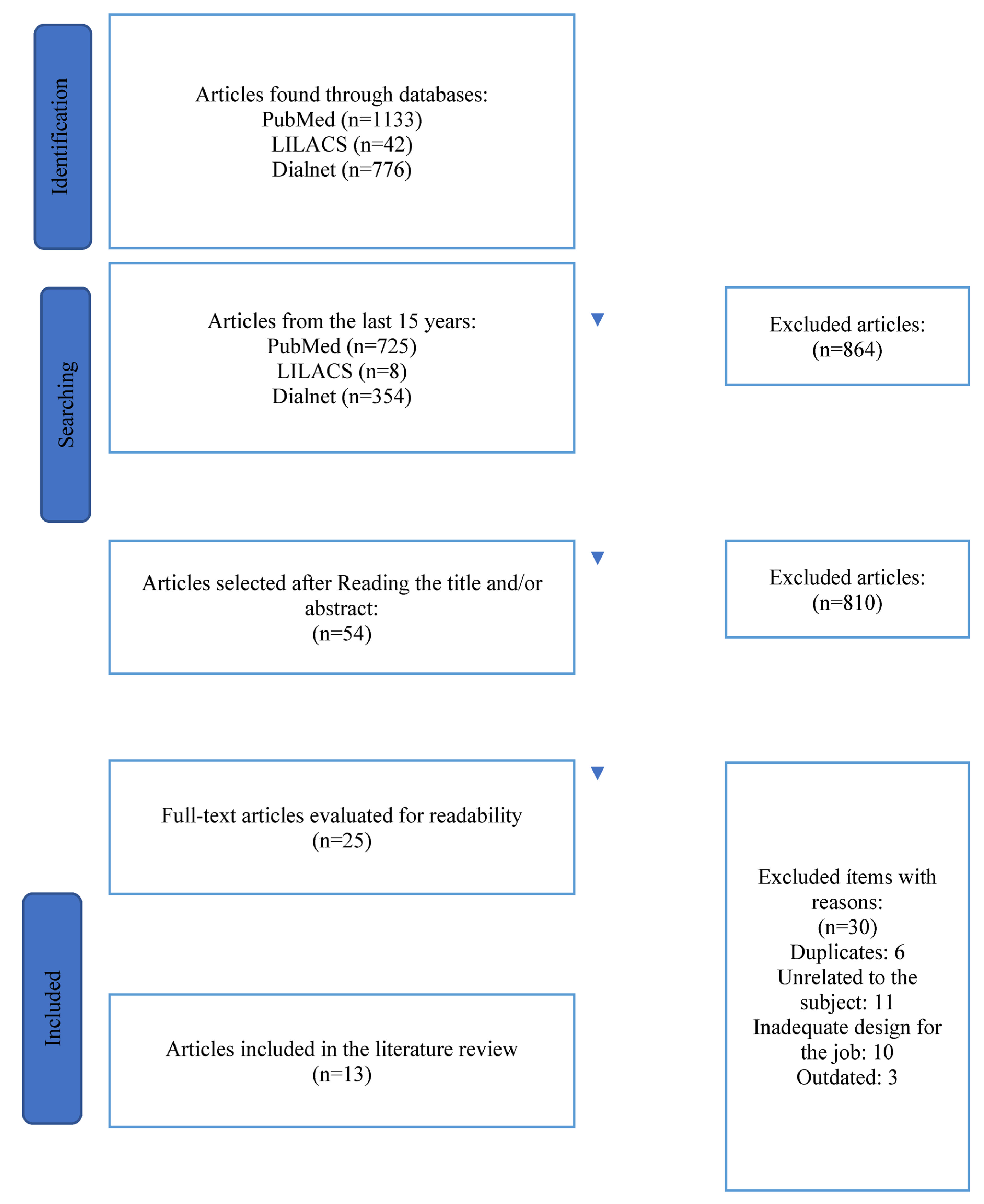
2. Materials and Methods
2.1. Type of Research
2.2. Study Selection Criteria
Inclusion Criteria
2.3. Procedure for Collecting Information: Search Strategy and Selection of Studies
3. Results
3.1. Effects of Pesticides on the Respiratory Tract
3.2. Association between Pesticide Exposure and Risk of Congenital Airway Abnormalities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- De Castilhos Ghisi, N.; Zuanazzi, N.R.; Fabrin, T.; Oliveira, E.C. Glyphosate and its toxicology: A scientometric review. Sci. Total Environ. 2020, 733, 139359. [Google Scholar] [CrossRef] [PubMed]
- Ibarluzea, J.; Alvarez-Pedrerol, M.; Guxens, M.; Marina, L.S.; Basterrechea, M.; Lertxundi, A.; Etxeandia, A.; Goñi, F.; Vioque, J.; Ballester, F.; et al. Sociodemographic, reproductive and dietary predictors of organochlorine compounds levels in pregnant women in Spain. Chemosphere 2011, 82, 114–120. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EU Pesticides Database. 2020. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN (accessed on 7 July 2022).
- Sapbamrer, R.; Hongsibsong, S. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: A systematic review. Environ. Sci. Pollut. Res. Int. 2019, 26, 18267–18290. [Google Scholar] [CrossRef] [PubMed]
- Maitre, L.; Robinson, O.; Martinez, D.; Toledano, M.B.; Ibarluzea, J.; Marina, L.S.; Sunyer, J.; Villanueva, C.M.; Keun, H.C.; Vrijheid, M.; et al. Urine metabolic signatures of multiple environmental pollutans in pregnant women: Am exposome approach. Environ. Sci. Technol. 2018, 52, 13469–13580. [Google Scholar] [CrossRef]
- Savy, C.Y.; Fitchett, A.E.; Blain, P.G.; Morris, C.M.; Judge, S.J. Gene expression analysis reveals chronic low level exposure to the pesticide diazinon affects psychological disorders gene sets in the adult rat. Toxicology 2018, 393 (Suppl. C), 90–101. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Seidler, F.J. Oxidative stress from diverse developmental neurotoxicants: Antioxidants protect against lipid peroxidation without preventing cell loss. Neurotoxicol. Teracol. 2010, 32, 124–131. [Google Scholar] [CrossRef]
- Androutsopoulus, V.P.; Hernandez, A.F.; Liesivuori, J.; Tsatsakis, A.M. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology 2013, 307 (Suppl. C), 89–94. [Google Scholar] [CrossRef]
- Zamponi, V.; La Salvia, A.; Tarsitano, M.G.; Mikovic, N.; Rinzivillo, M.; Panzuto, F.; Giannetta, E.; Faggiano, A.; Mazzilli, R. Effect of Neuroendocrine Neoplasm Treatment on Human Reproductive Health and Sexual Function. J. Clin. Med. 2022, 11, 3983. [Google Scholar] [CrossRef]
- Minnetti, M.; Sada, V.; Feola, T.; Giannetta, E.; Pozza, C.; Gianfrilli, D.; Isidori, A.M.; Cozzolino, A. Selenium Supplementation in Pregnant Women with Autoimmune Thyroiditis: A Practical Approach. Nutrients 2022, 14, 2234. [Google Scholar] [CrossRef]
- Mirone, M.; Giannetta, E.; Isidori, A.M. Selenium and reproductive function. A systematic review. J. Endocrinol. Investig. 2013, 36 (Suppl. 10), 28–36. [Google Scholar]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 66, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Wolff, M.S.; Calafat, A.M.; Ye, X.; Bausell, R.; Meadows, M.; Stone, J.; Slama, R.; Engel, S.M. Prenatal exposure to environmental phenols: Concentrations in amniotic fluid and variability in urinary concentrations during pregnancy. Environ. Health Perspect. 2013, 121, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Bradman, A.; Barr, D.B.; Claus Henn, B.G.; Drumheller, T.; Curry, C.; Eskenazi, B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environ. Health Perspect. 2003, 111, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Ji, L.; Hu, Y.; Zhang, J.; Wang, C.; Ding, G.; Chen, L.; Kamijima, M.; Ueyama, J.; et al. Prenatal and postnatal exposure to organophosphate pesticides and childhood neurodevelopment in Shandong, China. Environ. Int. 2017, 108, 119–126. [Google Scholar] [CrossRef]
- Ratanachina, J.; De Matteis, S.; Cullinan, P.; Burney, P. Pesticide exposure and lung function: A systematic review and meta-analysis. Occup. Med. 2020, 70, 14–23. [Google Scholar] [CrossRef]
- Elonheimo, H.M.; Mattila, T.; Andersen, H.R.; Bocca, B.; Ruggieri, F.; Haverinen, E.; Tolonen, H. Environmental substances associated with chronic obstructive pulmonary disease—A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 3945. [Google Scholar] [CrossRef]
- Karmaus, W.; Kuehr, J.; Kruse, H. Infections and atopic disorders in childhood and organochlorine exposure. Arch. Environ. Health 2001, 56, 485–492. [Google Scholar] [CrossRef]
- Salameh, P.R.; Baldi, I.; Brochard, P.; Raherison, C.; Abi Saleh, B.; Salamon, R. Respiratory symptoms in children and exposure to pesticides. Eur. Respir. J. 2003, 22, 507–512. [Google Scholar] [CrossRef]
- Glynn, A.; Thuvander, A.; Aune, M.; Johannisson, A.; Darnerud, P.O.; Ronquist, G.; Cnattingius, S. Inmune cell counts and riks of respiratory infections among infants exposed pre and postnatally to organochlorine compounds: A prospective study. Environ. Health 2008, 7, 62. [Google Scholar] [CrossRef]
- Weselak, M.; Arbuckle, T.E.; Wigle, D.T.; Krewski, D. In utero pesticide exposure and childhood morbidity. Environ Res 2007, 103, 79–86. [Google Scholar] [CrossRef]
- Hong, Y.; Xu, X.; Lian, F.; Chen, R. Environmental risk factors for nonsyndromic cleft lip and/or cleft palate in Xinjiang Province, China: A multiethnic study. Cleft Palate Craniof J 2021, 58, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Olivas-Calderón, E.; Recio-Vega, R.; Gandolfi, A.J.; Lantz, R.C.; González-Cortes, T.; Gonzalez-De Alba, C.; Froines, J.R.; Espinosa-Fematt, J.A. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol. Appl. Pharmacol. 2015, 287, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, M.; Phipatanakul, W. Year in review: Pediatric allergy and asthma, excluding food allergy. Ann. Allergy Asthma Immunol. 2015, 114, 175–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raanan, R.; Harley, K.G.; Balmes, J.R.; Bradman, A.; Lipsett, M. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ. Health Perspect. 2015, 123, 179–185. [Google Scholar] [CrossRef]
- Sunyer, J.; Torrent, M.; Muñoz-Ortiz, L.; Ribas-Fitó, N.; Carrizo, D.; Grimalt, J.; Antó, J.M.; Cullinan, P. Prenatal dichlorodiphenyldichloroethylene (DDE) and asthma in children. Environ. Health Perspect. 2005, 113, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.; Torrent, M.; Garcia-Esteban, R.; Ribas-Fitó, N.; Carrizo, D.; Romieu, I.; Antó, J.M.; Grimalt, J.O. Early exposure to dichlorodiphenyldichloroethylene, breastfeeding and asthma at age six. Clin. Exp. Allergy 2006, 36, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Mamane, A.; Raherison, C.; Tessier, J.F.; Baldi, I.; Bouvier, G. Environmental exposure to pesticides and respiratory health. Eur. Respir Rev. 2015, 24, 462–473. [Google Scholar] [CrossRef]
- Gascon, M.; Sunyer, J.; Casas, M.; Martínez, D.; Ballester, F.; Basterechea, M.; Bonde, J.P.; Chatzi, L.; Chevrier, C.; Eggesbø, M.; et al. Prenatal exposure to DEE and PCB 153 and respiratory health in early childhood: A meta-analysis. Epidemiology 2014, 25, 544–553. [Google Scholar] [CrossRef]
- Gilden, R.; Friedmann, E.; Holmes, K.; Yolton, K.; Xu, Y.; Lanphear, B.; Chen, A.; Braun, J.; Spanier, A. Gestational pesticide exposure and child respiratory health. Int. J. Environ. Res. Public Health 2020, 17, 7165. [Google Scholar] [CrossRef]
- Romitti, P.A.; Herring, A.M.; Dennis, L.K.; Wong-Gibbons, D.L. Meta-analysis: Pesticides and orofacial clefts. Cleft Palate-Craniofac. J. Off. Publ. Am. Cleft. Palate-Craniofac. Assoc. 2007, 44, 358–365. [Google Scholar] [CrossRef]
- Yang, W.; Carmichael, S.L.; Roberts, E.M.; Kegley, S.E.; Padula, A.M.; English, P.B.; Shaw, G.M. Residential agricultural pesticide exposures and risk of neural tube defects and orofacial clefts among offspring in the San Joaquin Valley of California. Am. J. Epidemiol. 2014, 179, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Tian, S.; Jiao, X.; Mi, N.; Zhang, B.; Song, T.; An, L.; Zheng, X.; Zhuang, D. Association of Parental Environmental Exposures and Supplementation Intake with Risk of Nonsyndromic Orofacial Clefts: A Case-Control Study in Heilongjiang Province, China. Nutrients 2015, 7, 7172–7184. [Google Scholar] [CrossRef] [PubMed]
- Suhl, J.; Romitti, P.A.; Rocheleau, C.; Cao, Y.; Burns, T.L.; Conway, K.; Bell, E.M.; Stewart, P.; Langlois, P. National Birth Defects Prevention Study. Parental occupational pesticide exposure and nonsyndromic orofacial clefts. J. Occup. Environ. Hyg. 2018, 15, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Cantonwine, D.E.; Anzalota Del Toro, L.V.; Calafat, A.M.; Valentin-Blasini, L.; Davis, M.D.; Baker, S.E.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ. Health 2014, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Thulstrup, A.M.; Bonde, J.P. Maternal occupational exposure and risk of specific birth defects. Occup. Med. 2006, 56, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.M.; Hoppin, J.A.; Córdoba, L.; Cano, J.C.; Soto-Martínez, M.; Eskenazi, B.; Lindh, C.H.; van Wendel de Joode, B. Prenatal pesticide exposure and respiratory health outcomes in the first year of life: Results from the infants’ Environmental Health (ISA) study. Int. J. Hyg. Environ. Health 2020, 225, 113474. [Google Scholar] [CrossRef]
- Figà-Talamanca, I. Occupational risk factors and reproductive health of women. Occup. Med. 2006, 56, 521–531. [Google Scholar] [CrossRef]
- Collotta, M.; Bertazzi, P.A.; Bollati, V. Epigenetics and pesticides. Toxicology 2013, 307, 35–41. [Google Scholar] [CrossRef]
- Hernández, A.F.; Lacasaña, M.; Gil, F.; Rodríguez-Barranco, M.; Pla, A.; López-Guarnido, O. Evaluation of pesticide-induced oxidative stress from a gene-environment interaction perspective. Toxicology 2013, 307, 95–102. [Google Scholar] [CrossRef]
- Marsit, C.J.; Eddy, K.; Kelsey, K.T. MicroRNA responses to cellular stress. Cancer Res. 2006, 66, 10843–10848. [Google Scholar] [CrossRef]
- Russo, M.; Humes, S.T.; Figueroa, A.M.; Tagmount, A.; Zhang, P.; Loguinov, A.; Lednicky, J.A.; Sabo-Attwood, T.; Vulpe, C.D.; Liu, B. Organochlorine pesticide dieldrin suppress cellular interferon-related antiviral gene expression. Toxicol. Sci. 2021, 182, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Giambò, F.; Leone, G.M.; Gattuso, G.; Rizzo, R.; Cosentino, A.; Cinà, D.; Teodoro, M.; Costa, C.; Tsatsakis, A.; Fenga, C.; et al. Genetic and epigenetic alterations induced by pesticide exposure: Integrated analysis of gene expression, microRNA expression, and DNA methylation datasets. Int. J. Environ. Res. Public Health 2021, 18, 8697. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Objectives | Methodology | Sample Size | Rural Area/ City/Country | Tissue Sample Source | Evaluated Exposure (Active Matter) | Exposure Measurement Method | Results |
|---|---|---|---|---|---|---|---|---|
| Sunyer et al. (2005) [26] | To identify any association between prenatal DDE and other organochlorine compounds and atopy and asthma during infancy. | Longitudinal study | 468 children | Menorca is one of the Balearic Islands in the northwest Mediterranean Sea. | In total, 405 (84%) had organochlorine compounds in cord serum measured. Blood was drawn at 4 years of age in 360 children, 306 of whom had IgEs and peripheral white blood cells measured. Asthma was defined based on wheezing at 4 years of age, persistent wheezing, or doctor-diagnosed asthma. | Specific IgE against house dust mite (Der p1), cat (Fel d1), and grass was measured using the CAP method, with levels > 0.34 kU/L being considered positive. | Prenatal DEE and other organochlorines were measured in cord serum by GC with electron capture detection and GC coupled to chemical ionization negative-ion mass spectrometry. | Prenatal exposure to DDE may contribute to development of asthma. |
| Sunyer et al. (2006) [27] | To investigate the association of DEE with childhood asthma measured up to age 6 and the effect of DEE on the protective effect of breastfeeding on asthma. | Longitudinal study | 462 children | Menorca is one of the Balearic Islands in the northwest Mediterranean Sea. | Organochlorine compounds were measured in cord serum of 402 (84%) children. Blood was drawn in 360 children at 4 years of age, 285 of whom had organochlorine compounds measured. | A weal of 2 mm or greater in the presence of a positive histamine control and a negative uncoated control constituted a positive skin test. A positive skin test to at least one allergen (Der p 1, Der f 1, cat, dog, grass pollen, mixed tree, mixed graminae, parietaria) was considered indicative of atopy. | DDE and DDT were measured in cord serum by GC with electron capture detection and GC coupled to chemical ionization negative-ion mass spectrometry | DDE does not modify the protective effect of breastfeeding on asthma. Asthma and persistent wheezing were associated with DDE at birth but not with DDE at 4 years. |
| Romitti et al. (2007) [31] | The risk of orofacial clefts associated with pesticide exposure was examined by performing a meta-analysis of studies published between 1966 and 2005. | Systematic review | 19 studies | Many of the studies identified as suitable for analysis used a retrospective design with different sample sizes, levels of exposure assessment, and phenotypic assessment. Maternal exposure is associated with an increased risk of clefting. | ||||
| Lewis et al. (2014) [35] | To describe distributions, evaluate subjects within temporal variability, and identify predictors of urinary DEET concentrations in pregnant women in Puerto Rico. | Cohort study | 54 pregnant women | Puerto Rico | Urinary concentrations | At three separate time points (20 ± 2 weeks, 24 ± 2 weeks, and 28 ± 2 weeks of gestation). | Measured urinary concentrations of the insect-repellent DEET and two of its metabolites, four pyrethroid insecticide metabolites, and two chlorophenoxy herbicides. | These distributions were calculated and compared with those of women of reproductive age in the US population. Pesticide use in a group of pregnant women was shown to be associated with exposure to several pesticides |
| Yang et al. (2014) [32] | Use population-based data on specific birth defects. | Cases and controls | 73 cases with anencephaly, 123 with spina bifida, 277 with CLP, and 117 with cleft palate only, in addition to 785 controls. | San Joaquin Valley, California. | Exposure estimates were based on residential proximity to agricultural pesticide applications during early pregnancy. | Chemical groups included petroleum derivatives for anencephaly, hydroxybenzonitrile herbicides for spina bifida, and 2,6-dinitroaniline herbicides and dithiocarbamates-methyl isothiocyanate for CLP. The specific chemicals included 2,4-D dimethylamine salt, methomyl, imidacloprid, and α-(para-nonylphenyl)-ω-hydroxypoly(oxyethylene) phosphate ester for anencephaly; the herbicide bromoxynil octanoate for spina bifida; and trifluralin and maneb for CLP. | A total of 38% of the subjects were exposed to 52 chemical groups and 257 specific chemicals. | Results have been inconsistent. Some associations have been observed between gestational pesticide exposure and specific birth defect phenotypes, but the data are insufficient to draw clear results. |
| Mamane et al. (2015) [28] | To test the respiratory effects of environmental exposure to pesticides on children and adults. | Literature review | 20 studies | Studies have identified that prenatal exposition to pesticides increases the risk of asthma and wheezing in young children. | ||||
| Gascon et al. (2014) [29] | To examine the association between prenatal exposure to DDE and 153 PCB and children’s respiratory health in European birth cohorts. | Cohort study | 4608 mothers and children | 7 European countries. | Studied the association between prenatal exposure to DDE and PCB 153 and children’s respiratory health in European birth cohorts. | Modeling occurrences of the outcomes on the estimates of cord-serum concentrations of PCB 153 and DDE as continuous variables (per doubling exposure) and as cohort-specific tertiles. | The prenatal DDE exposure increases the risk of respiratory health symptoms in children below 18 months. | |
| Olivas-Calderón et al. (2015) [23] | To demonstrate that arsenic exposure during early childhood or in utero in children was associated with impaired lung function. | Cross-sectional study | 275 healthy children. | Located in the north-central part of Mexico. | Cross-sectional study in a cohort of children associating lung inflammatory biomarkers and lung function with urinary. | Arsenic urinary levels. | Inflammation biomarkers were measured in sputum by ELISA, and the lung function was evaluated by spirometry. | Arsenic exposure negatively affects during the early stages and significantly increases the frequency of an abnormal spirometric pattern in children. Fifty-eight percent of the children studied were found to have a restrictive spirometry pattern. |
| Hauptman et al. (2015) [24] | To discuss the epidemiology and evidence of known or proposed mechanisms of environmental, chemical, infectious, perinatal, and infectious exposures that relate to the development of pediatric allergy and asthma | Systematic review | The new evidence explores the effects of high- and low-molecular-weight phthalates, such as pesticides, dichlorophenols, and broad-spectrum antimicrobials, and their impact on the development of asthma. | |||||
| Raanan et al. (2015) [25] | To investigate the relationship between childhood exposure to organochlorine pesticides and respiratory outcomes. | Cohort study | 359 mothers and children. | Chamascos, Mexico. | Urine. | DAP metabolites of OP pesticides, specifically DE and DM metabolites. | Twice during pregnancy (mean = 13 and 26 weeks gestation) and from children five times during childhood (0.5–5 years). | Prenatal concentrations of organophosphate pesticides present respiratory symptoms compatible with asthma in infancy. |
| Hao et al. (2015) [33] | To test the possible association of possible parental environmental exposures and maternal supplement intake with the risk of nonsyndromic orofacial clefting | Retrospective study | 499 cases and 480 controls. | Heilongjiang Province. | Extracted information on case and control mothers from interviewer-administered questionnaires. The interview was administered by a trained interviewer and addressed exposures from one month before conception through the end of the first trimester. | Women were asked whether they used multivitamins, folic acid supplements, or cod liver oil during the one-month preconceptional period or first trimester. | Mothers were defined as being exposed to organic solvents when reporting contact with industrial cleaning products (degreasers), paints, printing inks, or glues in their jobs. They were considered exposed to heavy metals (cadmium, cobalt, or lead) exposure if their jobs involved production of pigments or batteries, galvanization, or recycling of electric tools. Mothers were considered exposed to pesticides if they were engaged in agriculture during the periconceptional period. | The results showed that maternal history of fever and common cold without fever, paternal smoking and alcohol consumption, maternal exposure to organic solvents, heavy metals or pesticides, and multivitamin use during the preconception period were associated with cleft lip or no cleft palate and cleft palate only. |
| Gilden et al. (2020) [30] | To examine the association of gestational urinary OP and 3PBA concentrations with child wheeze, forced expiratory volume in one second at ages 4 and 5 years, and wheeze trajectory patterns through age 8 years. | Prospective pregnancy and birth cohort study | Total of 468 pregnant women were enrolled between March 2003 and January 2006, and 390 mothers delivered live-born singletons who were followed from birth to age 8 years. | The greater Cincinnati, Ohio, USA metropolitan area. | Mothers provided urine samples twice during pregnancy, at 16 and 26 weeks gestation. | Inclusion criteria during enrollment of pregnant women included: 16 ± 3 weeks gestation, ≥18 years old, living in a home built before 1978 (this was to focus the cohort on potential lead exposure), no history of HIV infection, and not taking medications for seizure or thyroid disorders. | The HOME Study was designed to assess the relationship between low-level environmental chemical exposures and many aspects of child health, including child respiratory outcomes. | Gestational OP and 3PBA metabolites were linked to childhood respiratory symptoms in participants with maternal and genetic susceptibility. |
| Suhl et al. (2018) [34] | To examine the relationship between parental occupational pesticide exposures and nonsyndromic orofacial clefts in their offspring. | Cases and controls | Examined risk factors for over 30 major structural birth defects among deliveries from October 1997 through December 2011. Approximately 100 controls per year per site were recruited. | Arkansas, California, Georgia, Iowa, Massachusetts, North Carolina, New Jersey, New York, Texas, and Utah. | Live births were followed for 1 year to confirm diagnosis. | Nonsyndromic OFCs. | Parental occupational exposures to insecticides, herbicides, and fungicides, alone or in combinations, during maternal (1 month before through 3 months after conception) and paternal (3 months before through 3 months after conception) critical exposure periods between orofacial cleft cases and unaffected controls. | This study observed associations mostly close to unity between maternal occupational pesticide exposure and orofacial clefts. Associations for paternal occupational pesticide exposure were mostly close to or below unity for cleft lip ± cleft palate and mostly positive for cleft palate. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura-Miranda, M.I.; Fernández-Medina, I.M.; Guillén-Romera, E.; Ortíz-Amo, R.; Ruíz-Fernández, M.D. Effect of Gestational Pesticide Exposure on the Child’s Respiratory System: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15418. https://doi.org/10.3390/ijerph192215418
Ventura-Miranda MI, Fernández-Medina IM, Guillén-Romera E, Ortíz-Amo R, Ruíz-Fernández MD. Effect of Gestational Pesticide Exposure on the Child’s Respiratory System: A Narrative Review. International Journal of Environmental Research and Public Health. 2022; 19(22):15418. https://doi.org/10.3390/ijerph192215418
Chicago/Turabian StyleVentura-Miranda, María Isabel, Isabel María Fernández-Medina, Eulalia Guillén-Romera, Rocío Ortíz-Amo, and María Dolores Ruíz-Fernández. 2022. "Effect of Gestational Pesticide Exposure on the Child’s Respiratory System: A Narrative Review" International Journal of Environmental Research and Public Health 19, no. 22: 15418. https://doi.org/10.3390/ijerph192215418
APA StyleVentura-Miranda, M. I., Fernández-Medina, I. M., Guillén-Romera, E., Ortíz-Amo, R., & Ruíz-Fernández, M. D. (2022). Effect of Gestational Pesticide Exposure on the Child’s Respiratory System: A Narrative Review. International Journal of Environmental Research and Public Health, 19(22), 15418. https://doi.org/10.3390/ijerph192215418








