The Effects of White Noise on Attentional Performance and On-Task Behaviors in Preschoolers with ADHD
Abstract
1. Introduction
2. Materials and Methods
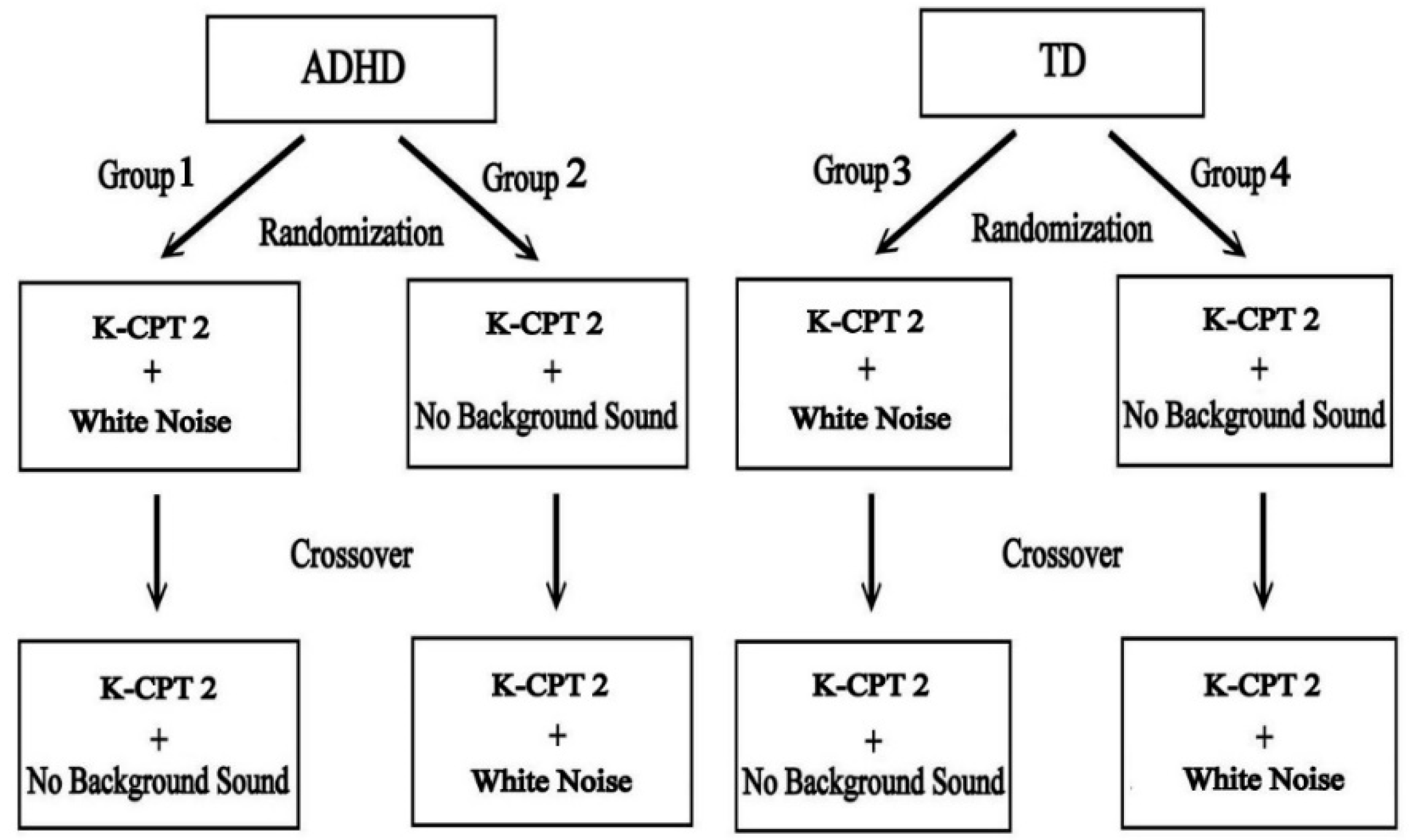
2.1. Research Design
2.2. Participants
2.3. Setting
2.4. Material and Instrumentation
2.4.1. White Noise
2.4.2. The Conners Kiddie Continuous Performance Test–Second Edition (K-CPT 2)
- (1)
- Detectability: An indicator of inattention represents the ability to differentiate non-targets from targets;
- (2)
- Omissions: An indicator of inattention represents the results of the failure to respond to targets;
- (3)
- Commissions: An indicator of impulsivity represents the degree of response to non-targets;
- (4)
- Perseverations: An indicator of impulsivity represents those that are made in less than 100 milliseconds following the presentation of a stimulus;
- (5)
- Hit Reaction Time (HRT): An indicator of inattention or impulsivity represents the response speed of correct responses for the whole administration. An atypically slow HRT (higher T-scores) may indicate inattentiveness; alternatively, a speedy HRT (lower T-scores) may indicate impulsivity;
- (6)
- HRT Standard Deviation (HRT SD): An indicator of inattention represents the measure of response speed consistency during the entire administration. A high HRT SD indicates a more inconsistent response speed.
2.4.3. Test Session Observation: Restricted Academic Situation Scale (RASS)
2.5. Procedures
2.6. Statistical Analyses
3. Results
3.1. Descriptive Statistics
3.2. ADHD and TD Children’s Performance in “No Background Sound” Condition
3.3. ADHD and TD Children’s Performance in “White Noise” Condition
3.4. ADHD Children’s Performance in “White Noise” Condition and TD Children’s Performance in “No Background Sound” Condition
3.5. Comparison of ADHD Preschoolers’ Performance between Two Background Sound Conditions
3.6. Comparison of TD Preschoolers’ Performance between Two Background Sound Conditions
4. Discussion
4.1. The Intrinsic and Extrinsic Symptoms of Preschoolers with ADHD
4.2. The Effects of White Noise on Preschoolers with ADHD
4.3. The Effects of White Noise on TD Preschoolers
4.4. Limitations and Future Research
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatry Association. Attention-Deficit/Hyperactivity Disorder. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatry Association: Arlington, TX, USA, 2013; pp. 59–65. [Google Scholar]
- Knopf, A. Parent survey finds more than 5% of children have ADHD, with preschool prevalence increasing. Brown Univ. Child Adolesc. Behav. Lett. 2018, 34, 3–4. [Google Scholar] [CrossRef]
- Döpfner, M.; Ise, E.; Breuer, D.; Rademacher, C.; Metternich-Kaizman, T.W.; Schürmann, S. Long-term course after adaptive multimodal treatment for children with ADHD: An 8-year follow-up. J. Atten. Disord. 2020, 24, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Bellato, A.; Arora, I.; Hollis, C.; Groom, M.J. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci. Biobehav. Rev. 2020, 108, 182–206. [Google Scholar] [CrossRef]
- Bocharov, A.V.; Savostyanov, A.N.; Slobodskaya, H.R.; Tamozhnikov, S.S.; Levin, E.A.; Saprigyn, A.E.; Proshina, E.A.; Astakhova, T.N.; Merkulova, E.A.; Knyazev, G.G. Associations of hyperactivity and inattention scores with theta and beta oscillatory dynamics of EEG in stop-signal task in healthy children 7–10 years old. Biology 2021, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Coll-Martín, T.; Carretero-Dios, H.; Lupiáñez, J. Attentional networks, vigilance, and distraction as a function of attention-deficit/hyperactivity disorder symptoms in an adult community sample. Br. J. Psychol. 2021, 112, 1053–1079. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; Johnstone, S.J.; McCarthy, R.; Selikowitz, M. EEG development in attention deficit hyperactivity disorder: From child to adult. Clin. Neurophysiol. 2019, 130, 1256–1262. [Google Scholar] [CrossRef]
- Cortese, S.; Kelly, C.; Chabernaud, C.; Proal, E.; Di Martino, A.; Milham, M.P.; Castellanos, F.X. Toward systems neuroscience of ADHD: A meta-analysis of 55 fMRI studies. Am. J. Psychiatry. 2012, 169, 1038–1055. [Google Scholar] [CrossRef]
- Hart, H.; Radua, J.; Nakao, T.; Mataix-Cols, D.; Rubia, K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: Exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 2013, 70, 185–198. [Google Scholar] [CrossRef]
- Sergeant, J. The cognitive-energetic model: An empirical approach to attention-deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 2000, 24, 7–12. [Google Scholar] [CrossRef]
- McDonnell, M.D.; Abbott, D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput Biol. 2009, 5, e1000348. [Google Scholar] [CrossRef]
- Söderlund, G.B.W.; Sikström, S.; Smart, A. Listen to the noise: Noise is beneficial for cognitive performance in ADHD. J. Child Psychol. Psychiatry 2007, 48, 840–847. [Google Scholar] [CrossRef]
- Madjar, N.; Gazoli, R.; Manor, I.; Shoval, G. Contrasting effects of music on reading comprehension in preadolescents with and without ADHD. Psychiatry Res. 2020, 291, 113207. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.P.; Leung, P.W.L.; Tang, C.S.K. A vigilance study of ADHD and control children: Event rate and extra-task stimulation. J. Dev. Phys. Disabil. 2000, 12, 187–201. [Google Scholar] [CrossRef]
- Salamati, A.; Hosseini, S.A.; Haghgou, H. Effectiveness of vestibular stimulation on visual attention in children with attention deficit hyperactivity disorder. Arch. Rehabil. 2014, 15, 18–25. Available online: https://rehabilitationj.uswr.ac.ir/article-1-1333-en.htm (accessed on 12 November 2020).
- Rothbart, M.K. Temperament and development. In Temperament in Childhood; Kohnstamm, G., Bates, J., Rothbart, M.K., Eds.; Wiley: Chichester, UK, 1989; pp. 187–248. [Google Scholar]
- Geissler, J.; Romanos, M.; Hegerl, U.; Hensch, T. Hyperactivity and sensation seeking as autoregulatory attempts to stabilize brain arousal in ADHD and mania? Atten. Defic. Hyperact. Disord. 2014, 6, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Zentall, S.S.; Zentall, T.R. Optimal stimulation: A model of disordered activity and performance in normal and deviant children. Psychol. Bull. 1983, 94, 446–471. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, W.A.; Brumback, R.A. Primary disorder of vigilance: A novel explanation of inattentiveness, daydreaming, boredom, restlessness, and sleepiness. J. Pediatr. 1990, 116, 720–725. [Google Scholar] [CrossRef]
- Hegerl, U.; Hensch, T. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev. 2014, 44, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Moss, F.; Ward, L.M.; Sannita, W.G. Stochastic resonance and sensory information processing: A tutorial and review of application. Clin. Neurophysiol. 2004, 115, 267–281. [Google Scholar] [CrossRef]
- Baijot, S.; Slama, H.; Söderlund, G.; Dan, B.; Deltenre, P.; Colin, C.; Deconinck, N. Neuropsychological and neurophysiological benefits from white noise in children with and without ADHD. Behav. Brain Funct. 2016, 12, 11. [Google Scholar] [CrossRef]
- Batho, L.P.; Martinussen, R.; Wiener, J. The effects of different types of environmental noise on academic performance and perceived task difficulty in adolescents with ADHD. J. Atten. Disord. 2020, 24, 1181–1191. [Google Scholar] [CrossRef]
- Chen, I.-C.; Chan, H.-Y.; Lin, K.-C.; Huang, Y.-T.; Tsai, P.-L.; Huang, Y.-M. Listening to White Noise Improved Verbal Working Memory in Children with Attention-Deficit/Hyperactivity Disorder: A Pilot Study. Int. J. Environ. Res. Public Health. 2022, 19, 7283. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Johnson, C.; Bradley-Johnson, S. White noise to decrease problem behaviors in the classroom for a child with attention deficit hyperactivity disorder (ADHD). Child Fam. Behav. Ther. 2015, 37, 38–50. [Google Scholar] [CrossRef]
- Pickens, T.A.; Khan, S.P.; Berlau, D.J. White noise as a possible therapeutic option for children with ADHD. Complement Ther. Med. 2019, 42, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, G.B.W.; Jobs, E.N. Differences in speech recognition between children with attention deficits and typically developed children disappear when exposed to 65 dB of auditory noise. Front. Psychol. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Pammer, K. The impact of concurrent noise on visual search in children with ADHD. J. Atten. Disord. 2018, 22, 1344–1353. [Google Scholar] [CrossRef]
- Angwin, A.J.; Wilson, W.J.; Ripollés, P.; Rodriguez-Fornells, A.; Arnott, W.L.; Barry, R.J.; Cheng, B.B.Y.; Garden, K.; Copland, D.A. White noise facilitates new-word learning from context. Brain Lang 2019, 199, 1. [Google Scholar] [CrossRef]
- Daud, S.N.; Sudirman, R. Evaluating the effect of mozart music and white noise on electroencephalography pattern toward visual memory. Adv. Sci. Technol. Eng. Syst. J. 2017, 2, 1372–1380. [Google Scholar] [CrossRef]
- Ohbayashi, W.; Kakigi, R.; Nakata, H. Effects of white noise on event-related potentials in somatosensory Go/No-go paradigms. Neuroreport 2017, 28, 788–792. [Google Scholar] [CrossRef]
- Woods, J.R.; Williams, J.G.; Tavel, M. The two period crossover design in medical research. Ann. Intern. Med. 1989, 110, 560–566. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition Technical Manual and Interpretive Manual; Psychological Corporation: San Antonio, TX, USA, 2012. [Google Scholar]
- Reynolds, C.; Kamphaus, R. Behavior Assessment System for Children, 3rd ed.; Pearson Education: San Antonio, TX, USA, 2015. [Google Scholar]
- Helps, S.K.; Bamford, S.; Sonuga-Barke, E.J.; Söderlund, G.B. Different effects of adding white noise on cognitive performance of sub-, normal and super-attentive school children. PLoS ONE 2014, 9, e112768. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, G.B.W.; Jakob, Å.J.; Rothén, B.; Ellen, T.H.; Magnusson, A.; Fälth, L. Sensory white noise improves reading skills and memory recall in children with reading disability. Brain Behav. 2021, 11, e02114. [Google Scholar] [CrossRef] [PubMed]
- Manjarrez, E.; Mendez, I.; Martinez, L.; Flores, A.; Mirasso, C.R. Effects of auditory noise on the psychophysical detection of visual signals: Cross-modal stochastic resonance. Neurosci. Lett. 2007, 415, 231–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- National Institute on Deafness and Other Communication Disorders. Available online: http://www.nidcd.nih.gov/health/hearing/pages/noise.aspx (accessed on 1 September 2021).
- Rosvold, H.E.; Mirsky, A.F.; Sarason, I.; Bransome, E.D.; Beck, L.H. A continuous performance test of brain damage. J. Consult. Psychol. 1956, 20, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Conners, C.K. Conners Kiddie Continuous Performance Test, 2nd ed.; Multi-Health Systems Inc.: Toronto, ON, Canada, 2015. [Google Scholar]
- Barkley, R.A. Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment; The Guilford Press: New York, NY, USA, 1990. [Google Scholar]
- Minder, F.; Zuberer, A.; Brandeis, D.; Drechsler, R. A review of the clinical utility of systematic behavioral observations in attention deficit hyperactivity disorder (ADHD). Child Psychiatry Hum. Dev. 2018, 49, 572–606. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A.; McMurray, M.B.; Edelbrock, C.S.; Robbins, K. The response of aggressive and nonaggressive ADHD children to two doses of methylphenidate. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 873–881. [Google Scholar] [CrossRef]
- Tawney, J.W.; Gast, D.L. Single Subject Research in Special Education; Merrill: New York, NY, USA, 1984. [Google Scholar]
- Bakeman, R.; Gottman, J.M. Applying observational methods: A systematic view. In Handbook of Infant Development, 2nd ed.; Osofsky, J.D., Ed.; Wiley: New York, NY, USA, 1987; pp. 818–854. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Stibbe, T.; Huang, J.; Paucke, M.; Ulke, C.; Strauss, M. Gender differences in adult ADHD: Cognitive function assessed by the test of attentional performance. PLoS ONE 2020, 15, e0240810. [Google Scholar] [CrossRef]
- Vallesi, A.; Tronelli, V.; Lomi, F.; Pezzetta, R. Age differences in sustained attention tasks: A meta-analysis. Psychon. Bull. Rev. 2021, 28, 1755–1775. [Google Scholar] [CrossRef]
- O’Neill, S.; Rajendran, K.; Mahbubani, S.M.; Halperin, J.M. Preschool predictors of ADHD symptoms and impairment during childhood and adolescence. Curr. Psychiatry Rep. 2017, 19, 95. [Google Scholar] [CrossRef]
- Döpfner, M.; Hautmann, C.; Görtz-Dorten, A.; Klasen, F.; Ravens-Sieberer, U.; BELLA Study Group. Long-term course of ADHD symptoms from childhood to early adulthood in a community sample. Eur. Child Adolesc. Psychiatry 2015, 24, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Francx, W.; Oldehinkel, M.; Oosterlaan, J.; Heslenfeld, D.J.; Hartman, C.A.; Hoekstra, P.J.; Franke, B.; Beckmann, C.F.; Buitelaar, J.K.; Mennes, M. The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex 2015, 73, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Wichstrøm, L.; Berg-Nielsen, T.S.; Angold, A.; Egger, H.L.; Solheim, E.; Sveen, T.H. Prevalence of psychiatric disorders in preschoolers. J. Child Psychol. Psychiatry 2012, 53, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Adamo, N.; Hodsoll, J.; Asherson, P.; Buitelaar, J.K.; Kuntsi, J. Ex-Gaussian, frequency and reward analyses reveal specificity of reaction time fluctuations to ADHD and not autism traits. J. Abnorm. Child Psychol. 2019, 47, 557–567. [Google Scholar] [CrossRef]
- Lin, H.Y.; Hsieh, H.C.; Lee, P.; Hong, F.Y.; Chang, W.D.; Liu, K.C. Auditory and visual attention performance in children with ADHD: The attentional deficiency of ADHD is modality specific. J. Atten. Disord. 2017, 21, 856–864. [Google Scholar] [CrossRef]
- Salum, G.A.; Sato, J.R.; Manfro, A.G.; Pan, P.M.; Gadelha, A.; do Rosário, M.C.; Polanczyk, G.V.; Castellanos, F.X.; Sonuga-Barke, E.; Rohde, L.A. Reaction time variability and attention-deficit/hyperactivity disorder: Is increased reaction time variability specific to attention-deficit/hyperactivity disorder? Testing predictions from the default-mode interference hypothesis. Atten. Defic. Hyperact. Disord. 2019, 11, 47–58. [Google Scholar] [CrossRef]
- Hartanto, T.A.; Krafft, C.E.; Iosif, A.M.; Schweitzer, J.B. A trial-by-trial analysis reveals more intense physical activity is associated with better cognitive control performance in attention-deficit/hyperactivity disorder. Child Neuropsychol. 2016, 22, 618–626. [Google Scholar] [CrossRef]
- Sarver, D.E.; Rapport, M.D.; Kofler, M.J.; Raiker, J.S.; Friedman, L.M. Hyperactivity in attention-deficit/hyperactivity disorder (ADHD): Impairing deficit or compensatory behavior? J. Abnorm. Child Psychol. 2015, 43, 1219–1232. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Diers, K.; Strunz, L.; Scherbaum, N.; Mette, C. Listening to Mozart improves current mood in adult ADHD—A randomized controlled pilot study. Front. Psychol. 2019, 10, 1104. [Google Scholar] [CrossRef]
| Attribute Category | ADHD (N = 52) | TD (N = 52) | t | p |
|---|---|---|---|---|
| Mean age (SD) | 5.52 (0.43) | 5.40 (0.39) | 1.46 | 0.15 |
| Mean IQ (SD) | 105.94 (9.26) | 108.35 (6.33) | −1.55 | 0.13 |
| Gender, N (%) | ||||
| Male | 35 (67.3%) | 35 (67.3%) | ||
| Female | 17 (32.7%) | 17 (32.7%) | ||
| Education, N (%) | ||||
| Pre-Kindergarten | 11 (21.2%) | 11 (21.2%) | ||
| Kindergarten | 41 (78.8%) | 41 (78.8%) | ||
| ADHD presentation, N (%) | ||||
| Inattentive Presentation | 4 (7.7%) | |||
| Hyperactive–Impulsive Presentation | 27 (51.9%) | |||
| Combined presentation | 21 (40.4%) |
| White Noise ADHD (n = 52)/TD (n = 52) | NBS ADHD (n = 52)/TD (n = 52) | t (WN/NBS) | Cohen’s d Effect Size (WN/NBS) | |
|---|---|---|---|---|
| Attention, M (SD) | ||||
| Detectability | 54.12 (5.66)/54.23 (6.38) | 56.37 (6.83)/52.71 (6.04) | −0.10/2.89 * | 0.02/0.57 |
| Omissions | 54.02 (4.85)/54.29 (7.70) | 56.94 (6.14)/53.21 (5.10) | −0.21/3.37 * | 0.04/0.66 |
| Commissions | 57.27 (6.24)/55.62 (7.03) | 57.63 (6.96)/53.52 (5.76) | 1.27/3.29 * | 0.25/0.64 |
| Perseverations | 55.56 (6.21)/54.90 (8.19) | 56.33 (5.42)/53.12 (5.09) | 0.46/3.11 * | 0.09/0.61 |
| Hit Reaction Time | 55.42 (6.33)/56.98 (7.58) | 56.73 (8.71)/55.63 (4.93) | −1.14/0.79 | 0.22/0.16 |
| HRT SD | 53.71 (5.41)/53.85 (6.65) | 57.79 (8.22)/51.85 (4.35) | −0.11/4.61 ** | 0.02/0.90 |
| On-Task Behavior, M (SD) | ||||
| Vocalizations | 2.31 (1.21)/2.38 (1.26) | 3.13 (1.50)/1.92 (1.14) | −0.32/4.65 ** | 0.06/0.91 |
| Off task | 4.31 (1.28)/4.56 (1.65) | 5.69 (1.98)/4.25 (0.95) | −0.86/4.75 ** | 0.17/0.93 |
| Out of seat | 0.62 (0.77)/0.62 (0.72) | 0.83 (0.81)/0.54 (0.58) | 0.01/2.09 * | 0.01/0.41 |
| Fidgets | 7.63 (1.88)/6.48 (1.75) | 9.87 (2.87)/5.98 (1.52) | 3.24 */8.63 ** | 0.63/1.69 |
| Play with objects | 0.63 (0.77)/0.75 (1.53) | 0.90 (1.19)/0.60 (0.69) | −0.49/1.61 | 0.10/0.31 |
| ADHD (n = 52) (White Noise) | TD (n = 52) (No Background Sound) | t | Cohen’s d Effect Size | |
|---|---|---|---|---|
| Attention, M (SD) | ||||
| Detectability | 54.12 (5.66) | 52.71 (6.04) | 1.22 | 0.24 |
| Omissions | 54.02 (4.85) | 53.21 (5.10) | 0.83 | 0.16 |
| Commissions | 57.27 (6.24) | 53.52 (5.76) | 3.18 * | 0.62 |
| Perseverations | 55.56 (6.21) | 53.12 (5.09) | 1.97 | 0.43 |
| Hit Reaction Time | 55.42 (6.33) | 55.63 (4.93) | −0.19 | 0.04 |
| HRT SD | 53.71 (5.41) | 51.85 (4.35) | 1.94 | 0.38 |
| On-Task Behavior, M (SD) | ||||
| Vocalizations | 2.31 (1.21) | 1.92 (1.14) | 1.67 | 0.33 |
| Off task | 4.31 (1.28) | 4.25 (0.95) | 0.26 | 0.05 |
| Out of seat | 0.62 (0.77) | 0.54 (0.58) | 0.58 | 0.12 |
| Fidgets | 7.63 (1.88) | 5.98 (1.52) | 4.94 ** | 1.02 |
| Play with objects | 0.63 (0.77) | 0.60 (0.69) | 0.27 | 0.04 |
| ADHD (n = 52) | t | Cohen’s d Effect Size | ||
|---|---|---|---|---|
| White Noise | No Background Sound | |||
| Attention, M (SD) | ||||
| Detectability | 54.12 (5.66) | 56.37 (6.83) | −2.03 * | 0.36 |
| Omissions | 54.02 (4.85) | 56.94 (6.14) | −2.97 * | 0.53 |
| Commissions | 57.27 (6.24) | 57.63 (6.96) | −0.42 | 0.05 |
| Perseverations | 55.56 (6.21) | 56.33 (5.42) | −0.83 | 0.13 |
| Hit Reaction Time | 55.42 (6.33) | 56.73 (8.71) | −1.20 | 0.17 |
| HRT SD | 53.71 (5.41) | 57.79 (8.22) | −3.64 * | 0.59 |
| On-Task Behavior, M (SD) | ||||
| Vocalizations | 2.31 (1.21) | 3.13 (1.50) | −3.35 * | 0.60 |
| Off task | 4.31 (1.28) | 5.69 (1.98) | −4.60 ** | 0.83 |
| Out of seat | 0.62 (0.77) | 0.83 (0.81) | −2.03 * | 0.27 |
| Fidgets | 7.63 (1.88) | 9.87 (2.87) | −5.20 ** | 0.92 |
| Play with objects | 0.63 (0.77) | 0.90 (1.19) | −1.79 | 0.27 |
| TD (n = 52) | t | Cohen’s d Effect Size | ||
|---|---|---|---|---|
| White Noise | No Background Sound | |||
| Attention, M (SD) | ||||
| Detectability | 54.23 (6.38) | 52.71 (6.04) | 1.26 | 0.24 |
| Omissions | 54.29 (7.70) | 53.21 (5.10) | 1.04 | 0.17 |
| Commissions | 55.62 (7.03) | 53.52 (5.76) | 1.88 | 0.33 |
| Perseverations | 54.90 (8.19) | 53.12 (5.09) | 1.53 | 0.26 |
| Hit Reaction Time | 56.98 (7.58) | 55.63 (4.93) | 1.16 | 0.21 |
| HRT SD | 53.85 (6.65) | 51.85 (4.35) | 1.85 | 0.36 |
| On-Task Behavior, M (SD) | ||||
| Vocalizations | 2.38 (1.26) | 1.92 (1.14) | 1.94 | 0.38 |
| Off task | 4.56 (1.65) | 4.25 (0.95) | 1.21 | 0.23 |
| Out of seat | 0.62 (0.72) | 0.54 (0.58) | 0.73 | 0.12 |
| Fidgets | 6.48 (1.75) | 5.98 (1.52) | 1.44 | 0.31 |
| Play with objects | 0.75 (1.53) | 0.60 (0.69) | 0.70 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y. The Effects of White Noise on Attentional Performance and On-Task Behaviors in Preschoolers with ADHD. Int. J. Environ. Res. Public Health 2022, 19, 15391. https://doi.org/10.3390/ijerph192215391
Lin H-Y. The Effects of White Noise on Attentional Performance and On-Task Behaviors in Preschoolers with ADHD. International Journal of Environmental Research and Public Health. 2022; 19(22):15391. https://doi.org/10.3390/ijerph192215391
Chicago/Turabian StyleLin, Hung-Yu. 2022. "The Effects of White Noise on Attentional Performance and On-Task Behaviors in Preschoolers with ADHD" International Journal of Environmental Research and Public Health 19, no. 22: 15391. https://doi.org/10.3390/ijerph192215391
APA StyleLin, H.-Y. (2022). The Effects of White Noise on Attentional Performance and On-Task Behaviors in Preschoolers with ADHD. International Journal of Environmental Research and Public Health, 19(22), 15391. https://doi.org/10.3390/ijerph192215391








