Relaxing Effects of Breathing Pseudotsuga menziesii and Lavandula angustifolia Essential Oils on Psychophysiological Status in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Essential Oils
2.3. Indoor Horticultural Activity
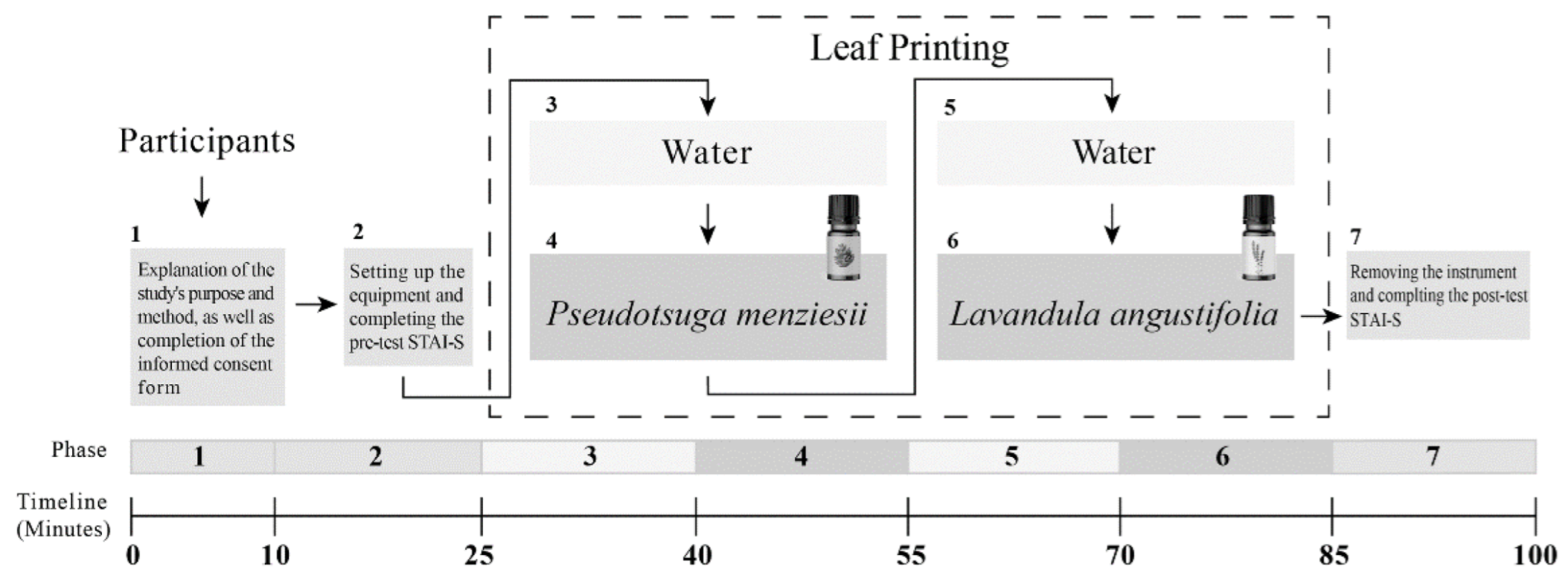
2.4. Experiment Procedure
2.5. Physiological Recordings and Analyses
2.6. Emotional Evaluation
2.7. Statistics
3. Results
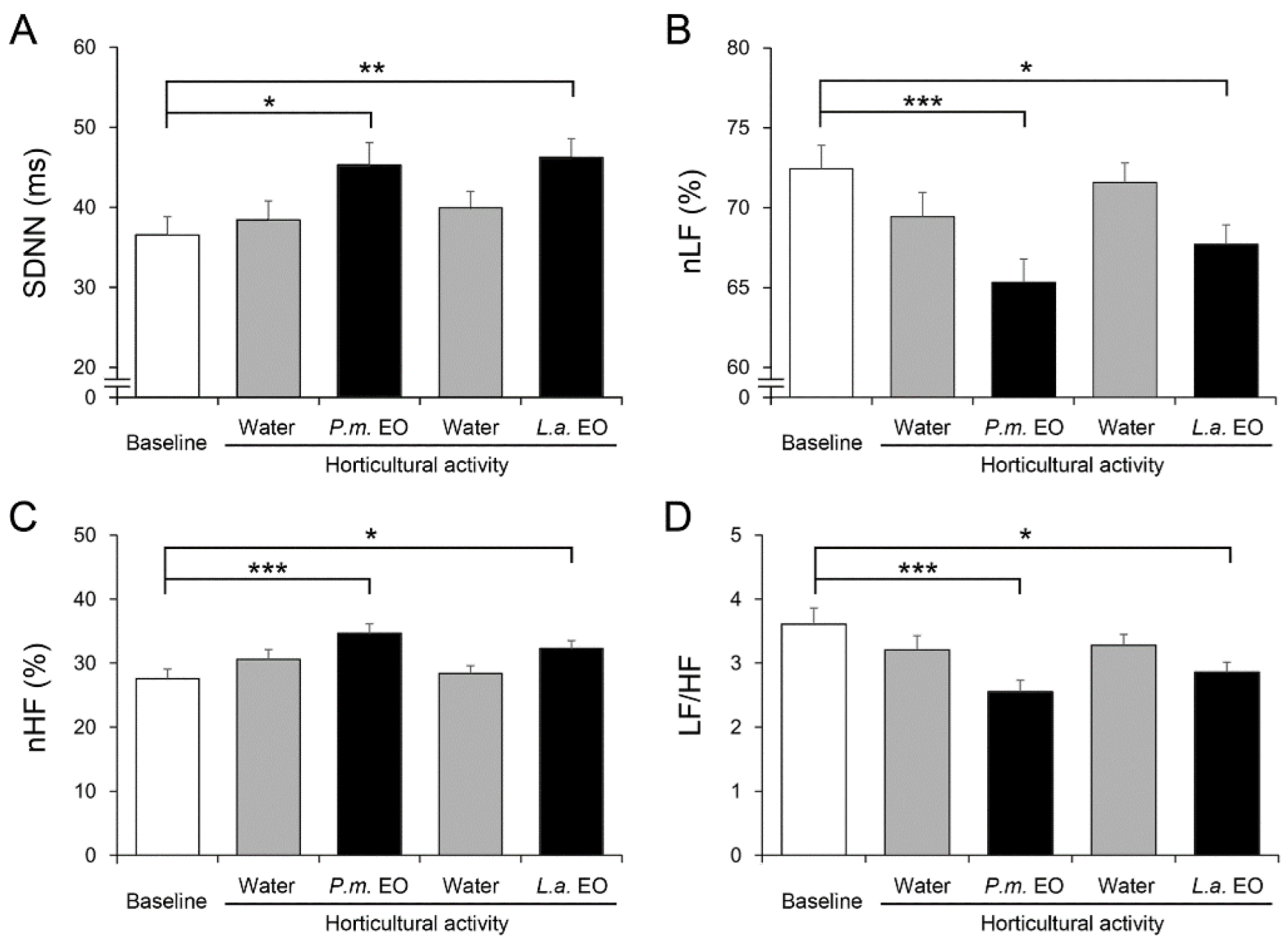
3.1. Physiological Recordings and Analyses
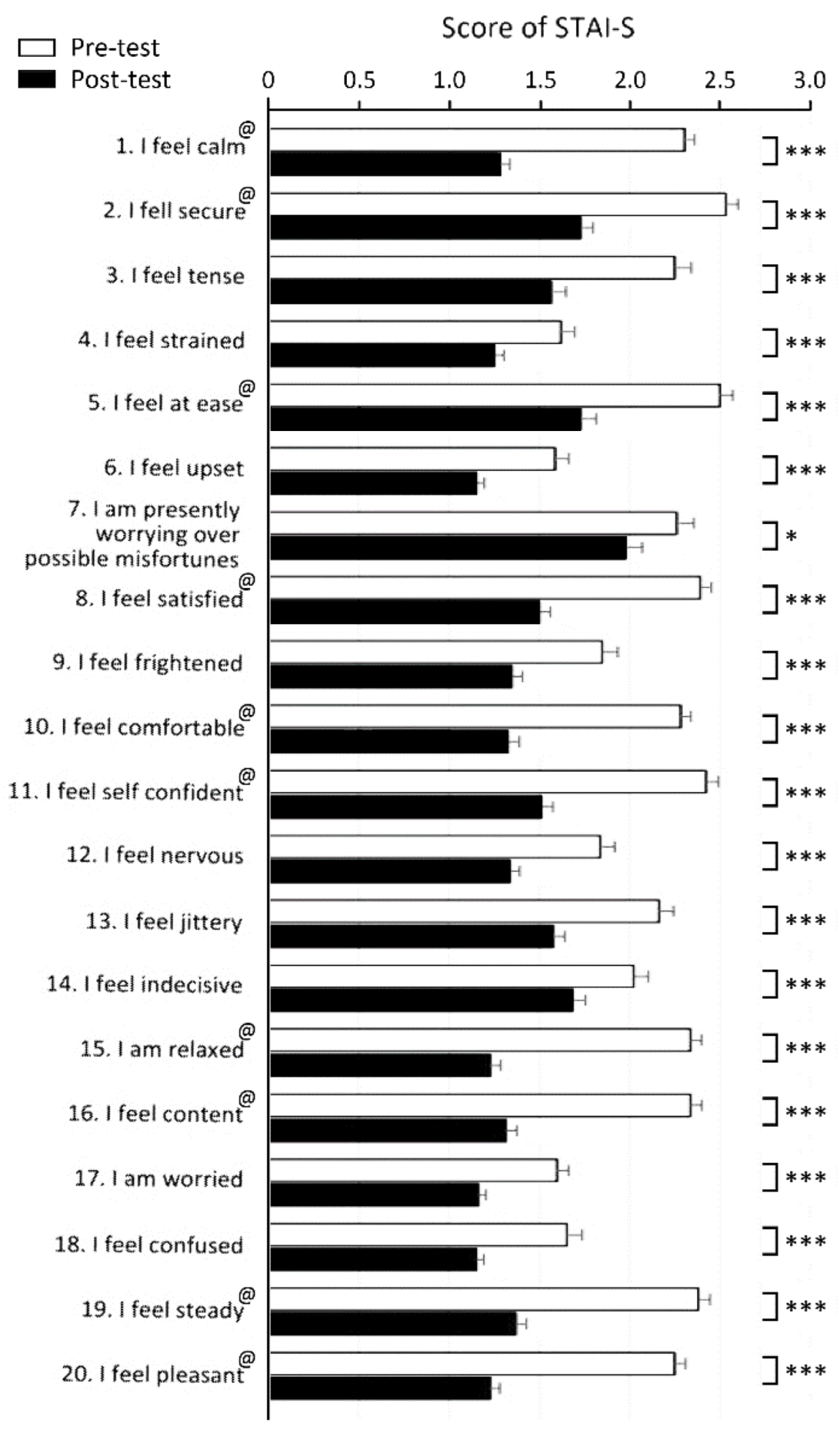
3.2. Emotional Evaluation
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, M.R.; Gillespie, B.W.; Chen, S.Y. Urban nature experiences reduce stress in the context of daily life based on salivary biomarkers. Front. Psychol. 2019, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- White, M.P.; Alcock, I.; Grellier, J.; Wheeler, B.W.; Hartig, T.; Warber, S.L.; Bone, A.; Depledge, M.H.; Fleming, L.E. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci. Rep. 2019, 9, 7730. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Lin, C.M.; Tsai, M.J.; Tsai, Y.C.; Chen, C.Y. Effects of short forest bathing program on autonomic nervous system activity and mood states in middle-aged and elderly individuals. Int. J. Environ. Res. Public Health 2017, 14, 897. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.C.; Cheng, W.C.; Hou, P.C.; Chang, Y.S. Effects of types of horticultural activity on the physical and mental state of elderly individuals. Int. J. Environ. Res. Public Health 2020, 17, 5225. [Google Scholar] [CrossRef]
- Ulrich, R.S.; Simons, R.F.; Losito, B.D.; Fiorito, E.; Miles, M.A.; Zelson, M. Stress recovery during exposure to natural and urban environments. J. Environ. Psychol. 1991, 11, 201–230. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20 (Suppl. S2), 3–8. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.J.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef]
- Gnatta, J.R.; Kurebayashi, L.F.; Turrini, R.N.; Silva, M.J. Aromatherapy and nursing: Historical and theoretical conception. Rev. Esc. Enferm. USP 2016, 50, 127–133. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [PubMed]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L.; et al. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Krähmer, A.; Herwig, N.; Hadian, J.; Schulz, H.; Meiners, T. Metabolomics approaches for analyzing effects of geographic and environmental factors on the variation of root essential oils of Ferula assa-foetida L. J. Agric. Food Chem. 2020, 68, 9940–9952. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Krähmer, A.; Herwig, N.; Schulz, H.; Hadian, J.; Meiners, T. Variation of secondary metabolite profile of Zataria multiflora Boiss. populations linked to geographic, climatic, and edaphic factors. Front. Plant Sci. 2020, 11, 969. [Google Scholar] [CrossRef]
- Kuroda, K.; Inoue, N.; Ito, Y.; Kubota, K.; Sugimoto, A.; Kakuda, T.; Fushiki, T. Sedative effects of the jasmine tea odor and (R)-(−)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur. J. Appl. Physiol. 2005, 95, 107–114. [Google Scholar] [CrossRef]
- Keville, K.; Green, M. Aromatherapy: A Complete Guide to the Healing Art; Crossing Press: Berkeley, CA, USA, 2012. [Google Scholar]
- Sugawara, Y.; Sugimoto, C.; Minabe, S.; Iura, Y.; Okazaki, M.; Nakagawa, N.; Seto, M.; Maruyama, S.; Hirano, M.; Kitayama, I. Use of human senses as sensors. Sensors 2009, 9, 3184–3204. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Graham, J.E.; Malarkey, W.B.; Porter, K.; Lemeshow, S.; Glaser, R. Olfactory influences on mood and autonomic, endocrine, and immune function. Psychoneuroendocrinology 2008, 33, 328–339. [Google Scholar] [CrossRef]
- Angelucci, F.L.; Silva, V.V.; Dal Pizzol, C.; Spir, L.G.; Praes, C.E.; Maibach, H. Physiological effect of olfactory stimuli inhalation in humans: An overview. Int. J. Cosmet. Sci. 2014, 36, 117–123. [Google Scholar] [CrossRef]
- Hautala, A.J.; Karjalainen, J.; Kiviniemi, A.M.; Kinnunen, H.; Mäkikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Physical activity and heart rate variability measured simultaneously during waking hours. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H874–H880. [Google Scholar] [CrossRef]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J., III; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Hjortskov, N.; Rissén, D.; Blangsted, A.K.; Fallentin, N.; Lundberg, U.; Søgaard, K. The effect of mental stress on heart rate variability and blood pressure during computer work. Eur. J. Appl. Physiol. 2004, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Tashiro, M.; Wu, D.; Yambe, T.; Wang, Q.; Sasaki, T.; Kumagai, K.; Luo, Y.; Nitta, S.; Itoh, M. Autonomic nervous function and localization of cerebral activity during lavender aromatic immersion. Technol. Health Care 2007, 15, 69–78. [Google Scholar] [CrossRef]
- Park, S.A.; Song, C.; Oh, Y.A.; Miyazaki, Y.; Son, K.C. Comparison of physiological and psychological relaxation using measurements of heart rate variability, prefrontal cortex activity, and subjective indexes after completing tasks with and without foliage plants. Int. J. Environ. Res. Public Health 2017, 14, 1087. [Google Scholar] [CrossRef]
- Shibasaki, H. Cortical activities associated with voluntary movements and involuntary movements. Clin. Neurophysiol. 2012, 123, 229–243. [Google Scholar] [CrossRef]
- Brauchli, P.; Rüegg, P.B.; Etzweiler, F.; Zeier, H. Electrocortical and autonomic alteration by administration of a pleasant and an unpleasant odor. Chem. Senses 1995, 20, 505–515. [Google Scholar] [CrossRef]
- Diego, M.A.; Jones, N.A.; Field, T.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; McAdam, V.; Galamaga, R.; Galamaga, M. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int. J. Neurosci. 1998, 96, 217–224. [Google Scholar] [CrossRef]
- Nishifuji, S.; Sato, M.; Maino, D.; Tanaka, S. Effect of acoustic stimuli and mental task on alpha, beta and gamma rhythms in brain wave. In Proceedings of the SICE Annual Conference 2010, Taipei, Taiwan, 18–21 August 2010; pp. 1548–1554. [Google Scholar]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724–751. [Google Scholar] [CrossRef]
- Tešević, V.; Milosavljević, S.M.; Vajs, V.; Đorđević, I.; Soković, M.; Lavadinović, V.; Novaković, M. Chemical composition and antifungal activity of the essential oil of Douglas fir (Pseudosuga menziesii Mirb. Franco) from Serbia. J. Serb. Chem. Soc. 2009, 74, 1035–1040. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Stojanović-Radić, Z.; Cvetković, V.J.; Jovanović, S.Č.; Dimitrijević, M.; Ickovski, J.D.; Jovanović, N.; Mihajilov-Krstev, T.; Stojanović, G.S. Pseudotsuga menziesii (Pinaceae): Volatile profiles, antimicrobial activity and toxicological evaluation of its essential oil. Chem. Biodivers. 2021, 18, e2100424. [Google Scholar] [CrossRef]
- Buchbauer, G.; Jirovetz, L.; Jäger, W.; Dietrich, H.; Plank, C. Aromatherapy: Evidence for sedative effects of the essential oil of lavender after inhalation. Z. Naturforsch. C J. Biosci. 1991, 46, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M.; Hart, S. Studies on the mode of action of the essential oil of Lavender (Lavandula angustifolia P. Miller). Phytother. Res. 1999, 13, 540–542. [Google Scholar] [CrossRef]
- Umezu, T.; Nagano, K.; Ito, H.; Kosakai, K.; Sakaniwa, M.; Morita, M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006, 85, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Annett, J.M.; Doherty, B.; Leslie, J.C. Anxiolytic effects of lavender oil inhalation on open-field behaviour in rats. Phytomedicine 2007, 14, 613–620. [Google Scholar] [CrossRef]
- Kritsidima, M.; Newton, T.; Asimakopoulou, K. The effects of lavender scent on dental patient anxiety levels: A cluster randomised-controlled trial. Community Dent. Oral Epidemiol. 2010, 38, 83–87. [Google Scholar] [CrossRef]
- Sugawara, Y.; Shigetho, A.; Yoneda, M.; Tuchiya, T.; Matumura, T.; Hirano, M. Relationship between mood change, odour and its physiological effects in humans while inhaling the fragrances of essential oils as well as linalool and its enantiomers. Molecules 2013, 18, 3312–3338. [Google Scholar] [CrossRef]
- Karan, N.B. Influence of lavender oil inhalation on vital signs and anxiety: A randomized clinical trial. Physiol. Behav. 2019, 211, 112676. [Google Scholar] [CrossRef]
- Rhind, J.P. Aromatherapeutic Blending: Essential Oils in Synergy; Singing Dragon: London, UK, 2015. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.L.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Marteau, T.M.; Bekker, H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef]
- Julian, L.J. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63 (Suppl. S11), S467–S472. [Google Scholar] [CrossRef]
- Kristal-Boneh, E.; Raifel, M.; Froom, P.; Ribak, J. Heart rate variability in health and disease. Scand. J. Work Environ. Health 1995, 21, 85–95. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Horsten, M.; Ericson, M.; Perski, A.; Wamala, S.P.; Schenck-Gustafsson, K.; Orth-Gomér, K. Psychosocial factors and heart rate variability in healthy women. Psychosom. Med. 1999, 61, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.M.; Woo, J.M. Determinants for heart rate variability in a normal Korean population. J. Korean Med. Sci. 2011, 26, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Chen, L.Y.; Nazarian, S.; Soliman, E.Z. Reference ranges for short-term heart rate variability measures in individuals free of cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Electrocardiol. 2016, 49, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Matsuoka, K. Causal coherence analysis of heart rate variability and systolic blood pressure variability under mental arithmetic task load. Biol. Psychol. 2005, 69, 217–227. [Google Scholar] [CrossRef]
- Olejarczyk, E.; Bogucki, P.; Sobieszek, A. The EEG split alpha peak: Phenomenological origins and methodological aspects of detection and evaluation. Front. Neurosci. 2017, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Tsai, S.Y.; Juan, C.H.; Muggleton, N.G.; Liang, W.K. Low delta and high alpha power are associated with better conflict control and working memory in high mindfulness, low anxiety individuals. Soc. Cogn. Affect. Neurosci. 2019, 14, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Vakili, A.M.; Sho’ouri, N. Beta wave activity analysis of EEG during mental painting reflects influence of artistic expertise. In Proceedings of the 2019 26th National and 4th International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 27–28 November 2019; pp. 225–229. [Google Scholar]
- Chandharakool, S.; Koomhin, P.; Sinlapasorn, J.; Suanjan, S.; Phungsai, J.; Suttipromma, N.; Songsamoe, S.; Matan, N.; Sattayakhom, A. Effects of tangerine essential oil on brain waves, moods, and sleep onset latency. Molecules 2020, 25, 4865. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Savostyanov, A.N.; Levin, E.A. Uncertainty, anxiety, and brain oscillations. Neurosci. Lett. 2005, 387, 121–125. [Google Scholar] [CrossRef]
- Oathes, D.J.; Ray, W.J.; Yamasaki, A.S.; Borkovec, T.D.; Castonguay, L.G.; Newman, M.G.; Nitschke, J. Worry, generalized anxiety disorder, and emotion: Evidence from the EEG gamma band. Biol. Psychol. 2008, 79, 165–170. [Google Scholar] [CrossRef]
- Cho, H.; Sowndhararajan, K.; Jung, J.W.; Jhoo, J.W.; Kim, S. Fragrance chemicals in the essential oil of Mentha arvensis reduce levels of mental stress. J. Life Sci. 2013, 23, 933–940. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Miran, M.S.; Li, S.; Jiang, M. Ultrasound-guided second trimester fetal electroencephalography in two pregnant volunteers: A technical note. J. Vasc. Interv. Neurol. 2016, 9, 60–65. [Google Scholar] [PubMed]
- Leung, L.S.; Luo, T. Cholinergic modulation of general anesthesia. Curr. Neuropharmacol. 2021, 19, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrväinen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid. Based Complement. Alternat. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef]
- Mcsweeney, J.; Rainham, D.; Johnson, S.A.; Sherry, S.B.; Singleton, J. Indoor nature exposure (INE): A health-promotion framework. Health Promot. Int. 2015, 30, 126–139. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Savostyanov, A.N.; Levin, E.A. Alpha oscillations as a correlate of trait anxiety. Int. J. Psychophysiol. 2004, 53, 147–160. [Google Scholar] [CrossRef]
- Puzi, N.M.; Jailani, R.; Norhazman, H.; Zaini, N.M. Alpha and Beta brainwave characteristics to binaural beat treatment. In Proceedings of the 2013 IEEE 9th International Colloquium on Signal Processing and its Applications, Kuala Lumpur, Malaysia, 8–10 March 2013; pp. 344–348. [Google Scholar]
- Choi, N.Y.; Wu, Y.T.; Park, S.A. Effects of olfactory stimulation with aroma oils on psychophysiological responses of female adults. Int. J. Environ. Res. Public Health 2022, 19, 5196. [Google Scholar] [CrossRef]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation aromatherapy via brain-targeted nasal delivery: Natural volatiles or essential oils on mood disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Chien, L.W.; Cheng, S.L.; Liu, C.F. The effect of lavender aromatherapy on autonomic nervous system in midlife women with insomnia. Evid. Based Complement. Alternat. Med. 2012, 2012, 740813. [Google Scholar] [CrossRef]
- Sayorwan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J. Med. Assoc. Thai. 2012, 95, 598–606. [Google Scholar] [PubMed]
- Jafari-Koulaee, A.; Elyasi, F.; Taraghi, Z.; Sadat Ilali, E.; Moosazadeh, M. A systematic review of the effects of aromatherapy with lavender essential oil on depression. Cent. Asian J. Glob. Health 2020, 9, e442. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Sillani, G.; Schuwald, A.; Friedland, K. Pharmacological basis of the anxiolytic and antidepressant properties of Silexan®, an essential oil from the flowers of lavender. Neurochem. Int. 2021, 143, 104899. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Kashiwadani, H.; Kanmura, Y.; Kuwaki, T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: Discovering novel therapeutics in the flavours and fragrances of cannabis. Front. Psychiatry 2021, 12, 583211. [Google Scholar] [CrossRef]
- Malloggi, E.; Menicucci, D.; Cesari, V.; Frumento, S.; Gemignani, A.; Bertoli, A. Lavender aromatherapy: A systematic review from essential oil quality and administration methods to cognitive enhancing effects. Appl. Psychol. Health Well Being 2022, 14, 663–690. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.S.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-Pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Guzmán-Gutiérrez, S.L.; Gómez-Cansino, R.; García-Zebadúa, J.C.; Jiménez-Pérez, N.C.; Reyes-Chilpa, R. Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily inhalation of α-pinene in mice: Effects on behavior and organ accumulation. Phytother. Res. 2014, 28, 1284–1287. [Google Scholar] [CrossRef]
- Kasuya, H.; Okada, N.; Kubohara, M.; Satou, T.; Masuo, Y.; Koike, K. Expression of BDNF and TH mRNA in the brain following inhaled administration of α-pinene. Phytother. Res. 2015, 29, 43–47. [Google Scholar] [CrossRef]
- Kasuya, H.; Iida, S.; Ono, K.; Satou, T.; Koike, K. Intracerebral distribution of α-pinene and the anxiolytic-like effect in mice following inhaled administration of essential oil from Chamaecyparis obtuse. Nat. Prod. Commun. 2015, 10, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Gutiérrez, S.L.; Bonilla-Jaime, H.; Gómez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, S.A. Horticultural therapy program for middle-aged women’s depression, anxiety, and self-identify. Complement. Ther. Med. 2018, 39, 154–159. [Google Scholar] [CrossRef]
- Fu, S.R.; Lee, M.F.; Ou, S.J. Effects of reminiscing about nostalgic smells on the physiological and psychological responses of older people in long-term care facilities. Ageing Soc. 2020, 40, 501–511. [Google Scholar] [CrossRef]
- Rashidi Fakari, F.; Tabatabaeichehr, M.; Kamali, H.; Rashidi Fakari, F.; Naseri, M. Effect of inhalation of aroma of geranium essence on anxiety and physiological parameters during first stage of labor in nulliparous women: A randomized clinical trial. J. Caring Sci. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Kim, C.; Song, C. Physiological and psychological relaxation effects of fir essential oil on university students. Int. J. Environ. Res. Public Health 2022, 19, 5063. [Google Scholar] [CrossRef]
- Steinmetz, G.; Pryor, G.T.; Stone, H. Olfactory adaptation and recovery in man as measured by two psychophysical techniques. Percept. Psychophys. 1970, 8, 327–330. [Google Scholar] [CrossRef]
- Masago, R.; Matsuda, T.; Kikuchi, Y.; Miyazaki, Y.; Iwanaga, K.; Harada, H.; Katsuura, T. Effects of inhalation of essential oils on EEG activity and sensory evaluation. J. Physiol. Anthropol. Appl. Human Sci. 2000, 9, 35–42. [Google Scholar] [CrossRef]
- Tabert, M.H.; Steffener, J.; Albers, M.W.; Kern, D.W.; Michael, M.; Tang, H.; Brown, T.R.; Devanand, D.P. Validation and optimization of statistical approaches for modeling odorant-induced fMRI signal changes in olfactory-related brain areas. NeuroImage 2007, 34, 1375–1390. [Google Scholar] [CrossRef]
- Krbot Skorić, M.; Adamec, I.; Jerbić, A.B.; Gabelić, T.; Hajnšek, S.; Habek, M. Electroencephalographic response to different odors in healthy individuals: A promising tool for objective assessment of olfactory disorders. Clin. EEG Neurosci. 2015, 46, 370–376. [Google Scholar] [CrossRef]
- Lee, G.Y.; Lee, C.; Park, G.H.; Jang, J.H. Amelioration of Scopolamine-induced learning and memory impairment by α-pinene in C57BL/6 mice. Evid. Based Complement. Alternat. Med. 2017, 2017, 4926815. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.S.; Koo, B.S.; et al. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila model of Alzheimer’s disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Herz, R.S. Aromatherapy facts and fictions: A scientific analysis of olfactory effects on mood, physiology and behavior. Int. J. Neurosci. 2009, 119, 263–290. [Google Scholar] [CrossRef] [PubMed]
- DeGuzman, P.; Jain, A.; Tabert, M.H.; Parra, L.C. Olfaction modulates inter-subject correlation of neural responses. Front. Neurosci. 2020, 14, 702. [Google Scholar] [CrossRef] [PubMed]

| Pseudotsuga menziesii EO * | Lavandula angustifolia EO ** |
|---|---|
| β-pinene 21.16% | linalool 36.03% |
| α-pinene 14.97% | linalyl acetate 30.67% |
| terpinolene 11.01% | β-ocimene 4.49% |
| δ-3-carene 8.87% | lavandulyl acetate 4.33% |
| sabinene 6.37% | terpinen-4-ol 3.3% |
| γ-terpinene 4.86% | β-farnesene 2.78% |
| limonene 3.15% | β-caryophyllene 2.72% |
| α-terpinene 3.08% | 3-octanone 1.32% |
| myrcene 2.38% | 1-Octen-3-yl acetate 1.24% |
| citronellyl acetate 2.61% | lavandulol 1.01% |
| β-phellandrene 1.76% | |
| camphene 1.61% | |
| α-terpineol 1.41% | |
| para-Cymene 1.08% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-H.; Chen, S.-J.; Lee, C.-L.; Wu, C.-W.; Chang, Y.-S. Relaxing Effects of Breathing Pseudotsuga menziesii and Lavandula angustifolia Essential Oils on Psychophysiological Status in Older Adults. Int. J. Environ. Res. Public Health 2022, 19, 15251. https://doi.org/10.3390/ijerph192215251
Chung Y-H, Chen S-J, Lee C-L, Wu C-W, Chang Y-S. Relaxing Effects of Breathing Pseudotsuga menziesii and Lavandula angustifolia Essential Oils on Psychophysiological Status in Older Adults. International Journal of Environmental Research and Public Health. 2022; 19(22):15251. https://doi.org/10.3390/ijerph192215251
Chicago/Turabian StyleChung, Ya-Hui, Shiu-Jen Chen, Ching-Luug Lee, Chun-Wei Wu, and Yu-Sen Chang. 2022. "Relaxing Effects of Breathing Pseudotsuga menziesii and Lavandula angustifolia Essential Oils on Psychophysiological Status in Older Adults" International Journal of Environmental Research and Public Health 19, no. 22: 15251. https://doi.org/10.3390/ijerph192215251
APA StyleChung, Y.-H., Chen, S.-J., Lee, C.-L., Wu, C.-W., & Chang, Y.-S. (2022). Relaxing Effects of Breathing Pseudotsuga menziesii and Lavandula angustifolia Essential Oils on Psychophysiological Status in Older Adults. International Journal of Environmental Research and Public Health, 19(22), 15251. https://doi.org/10.3390/ijerph192215251






