Associations between Prenatal Exposure to Phthalates and Features of the Metabolic Syndrome in Males from Childhood into Adulthood
Abstract
1. Introduction
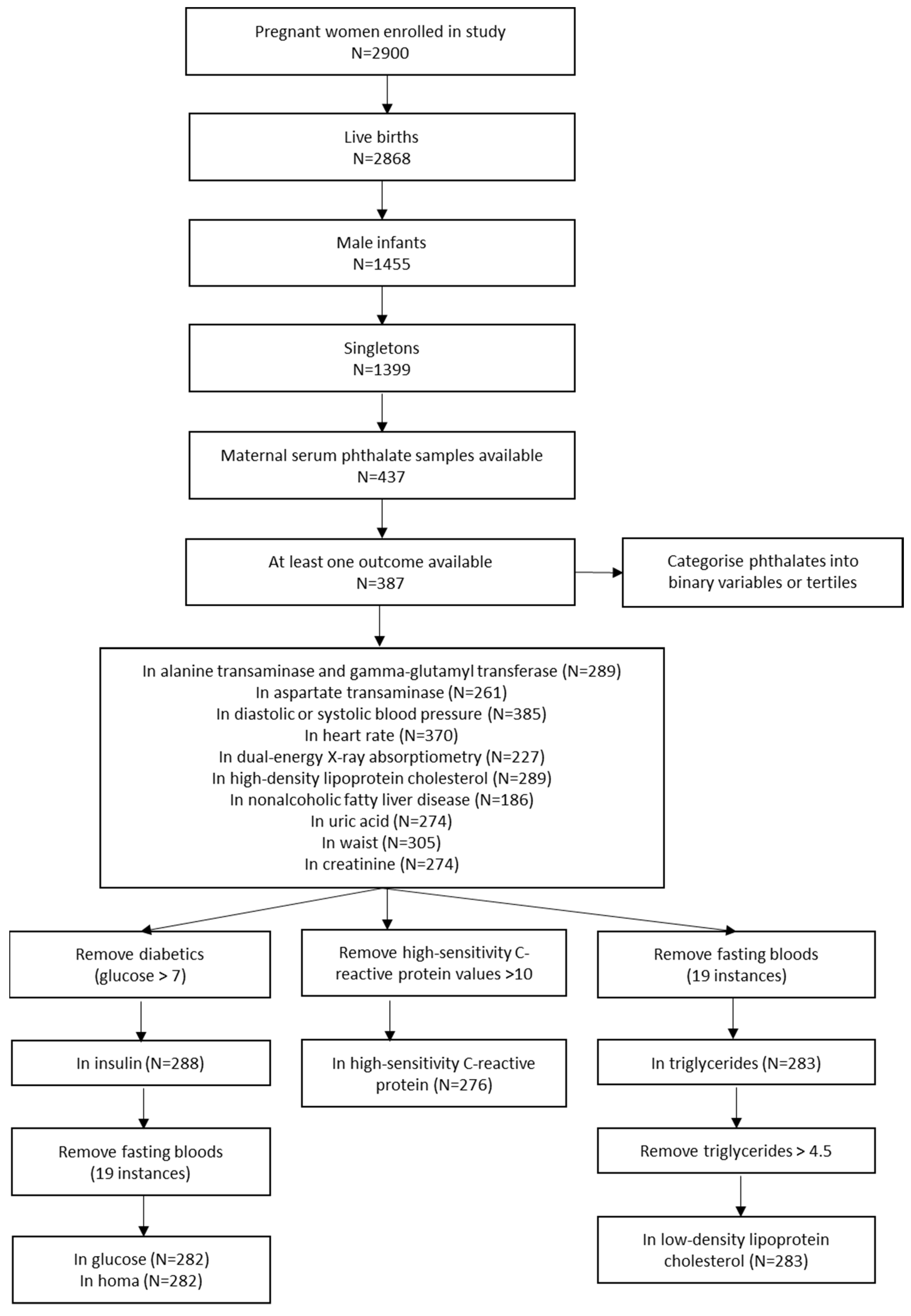
2. Materials and Methods
2.1. Management of Stored Maternal Blood Samples
2.2. Phthalate Measurements and Categorisation
2.3. Metabolic Assessments
2.3.1. Hepatic Ultrasound
2.3.2. Blood Pressure and Anthropometric Measurements
2.3.3. Cardiometabolic Assessment
2.3.4. Dual Energy X-ray Absorptiometry
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Systolic Blood Pressure
3.2. Diastolic Blood Pressure
3.3. Heart Rate
3.4. Waist Circumference
3.5. Alanine Transaminase (ALT)
3.6. Gamma-Glutamyl Transferase (GGT)
3.7. Aspartate Transaminase (AST)
3.8. High Density Lipoprotein Cholesterol (HDL-C)
3.9. Low Density Lipoprotein Cholesterol (LDL-C)
3.10. Uric Acid
3.11. High Sensitivity C-Reactive Protein (hsCRP)
3.12. Insulin
3.13. Glucose
3.14. Homeostatic Model Assessment (HOMA)
3.15. Creatinine
3.16. Triglycerides (TG)
3.17. Dual Energy X-ray Absorptiometry (DEXA)-Fat Mass
3.18. Dual Energy X-ray Absorptiometry (DEXA)-Lean Mass
3.19. Hepatic Ultrasound-Non-Alcoholic Fatty Liver Disease (NAFLD)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef]
- Faienza, M.F.; Wang, D.Q.; Fruhbeck, G.; Garruti, G.; Portincasa, P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern. Emerg. Med. 2016, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Janjua, N.R.; Frederiksen, H.; Skakkebaek, N.E.; Wulf, H.C.; Andersson, A.M. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int. J. Androl. 2008, 31, 118–130. [Google Scholar] [CrossRef]
- Hsu, N.Y.; Lee, C.C.; Wang, J.Y.; Li, Y.C.; Chang, H.W.; Chen, C.Y.; Bornehag, C.G.; Wu, P.C.; Sundell, J.; Su, H.J. Predicted risk of childhood allergy, asthma, and reported symptoms using measured phthalate exposure in dust and urine. Indoor Air 2012, 22, 186–199. [Google Scholar] [CrossRef]
- Adibi, J.J.; Whyatt, R.M.; Hauser, R.; Bhat, H.K.; Davis, B.J.; Calafat, A.M.; Hoepner, L.A.; Perera, F.P.; Tang, D.; Williams, P.L. Transcriptional biomarkers of steroidogenesis and trophoblast differentiation in the placenta in relation to prenatal phthalate exposure. Environ. Health Perspect. 2010, 118, 291–296. [Google Scholar] [CrossRef]
- Mose, T.; Mortensen, G.K.; Hedegaard, M.; Knudsen, L.E. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod. Toxicol. 2007, 23, 83–91. [Google Scholar] [CrossRef]
- Huang, P.C.; Kuo, P.L.; Chou, Y.Y.; Lin, S.J.; Lee, C.C. Association between prenatal exposure to phthalates and the health of newborns. Environ. Int. 2009, 35, 14–20. [Google Scholar] [CrossRef]
- Wittassek, M.; Angerer, J.; Kolossa-Gehring, M.; Schafer, S.D.; Klockenbusch, W.; Dobler, L.; Gunsel, A.K.; Muller, A.; Wiesmuller, G.A. Fetal exposure to phthalates—A pilot study. Int. J. Hyg. Environ. Health 2009, 212, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019; US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Henriksen, L.S.; Mathiesen, B.K.; Assens, M.; Krause, M.; Skakkebaek, N.E.; Juul, A.; Andersson, A.M.; Hart, R.J.; Newnham, J.P.; Keelan, J.A.; et al. Use of stored serum in the study of time trends and geographical differences in exposure of pregnant women to phthalates. Environ. Res. 2020, 184, 109231. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 225–249. [Google Scholar] [CrossRef]
- Biemann, R.; Blüher, M.; Isermann, B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101546. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Qin, Y.; Chen, M.; Li, X.; Wang, R.; Huang, Z.; Xu, Q.; Yu, M.; Zhang, Y.; Han, X.; et al. Prenatal low-dose DEHP exposure induces metabolic adaptation and obesity: Role of hepatic thiamine metabolism. J. Hazard. Mater. 2020, 385, 121534. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, C.; Tao, F.-B. Association between prenatal phthalate exposure and gestational metabolic syndrome parameters: A systematic review of epidemiological studies. Environ. Sci. Pollut. Res. Int. 2021, 28, 20921–20938. [Google Scholar] [CrossRef]
- Golestanzadeh, M.; Riahi, R.; Kelishadi, R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 35670–35686. [Google Scholar] [CrossRef]
- Kupsco, A.; Wu, H.; Calafat, A.M.; Kioumourtzoglou, M.-A.; Cantoral, A.; Tamayo-Ortiz, M.; Pantic, I.; Pizano-Zárate, M.L.; Oken, E.; Braun, J.M.; et al. Prenatal maternal phthalate exposures and trajectories of childhood adiposity from four to twelve years. Environ. Res. 2022, 204, 112111. [Google Scholar] [CrossRef]
- Heggeseth, B.C.; Holland, N.; Eskenazi, B.; Kogut, K.; Harley, K.G. Heterogeneity in childhood body mass trajectories in relation to prenatal phthalate exposure. Environ. Res. 2019, 175, 22–33. [Google Scholar] [CrossRef]
- Harley, K.G.; Berger, K.; Rauch, S.; Kogut, K.; Claus Henn, B.; Calafat, A.M.; Huen, K.; Eskenazi, B.; Holland, N. Association of prenatal urinary phthalate metabolite concentrations and childhood BMI and obesity. Pediatr. Res. 2017, 82, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Peterson, K.E.; Meeker, J.D.; Sanchez, B.N.; Zhang, Z.; Cantoral, A.; Solano, M.; Tellez-Rojo, M.M. Exposure to Bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr. Obes. 2018, 13, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Burgio, E.; Lopomo, A.; Migliore, L. Obesity and diabetes: From genetics to epigenetics. Mol. Biol. Rep. 2015, 42, 799–818. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Liu, H.-l.; Fu, H.; Niu, Q.-s.; Wu, H.-b.; Huang, F. Prenatal exposure to phthalates with preterm birth and gestational age: A systematic review and meta-analysis. Chemosphere 2021, 282, 130991. [Google Scholar] [CrossRef] [PubMed]
- Newnham, J.P.; Evans, S.J.; Michael, C.A.; Stanley, F.J.; Landau, L.I. Effects of frequent ultrasound during pregnancy: A randomised controlled trial. Lancet 1993, 342, 887–891. [Google Scholar] [CrossRef]
- Hart, R.; Doherty, D.A.; Frederiksen, H.; Keelan, J.A.; Hickey, M.; Sloboda, D.; Pennell, C.E.; Newnham, J.P.; Skakkebaek, N.E.; Main, K.M. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: A pilot study. Reproduction 2014, 147, 379–390. [Google Scholar] [CrossRef]
- Hart, R.J.; Frederiksen, H.; Doherty, D.A.; Keelan, J.A.; Skakkebaek, N.E.; Minaee, N.S.; McLachlan, R.; Newnham, J.P.; Dickinson, J.E.; Pennell, C.E.; et al. The Possible Impact of Antenatal Exposure to Ubiquitous Phthalates Upon Male Reproductive Function at 20 Years of Age. Front. Endocrinol. (Lausanne) 2018, 9, 288. [Google Scholar] [CrossRef]
- Ayonrinde, O.T.; Olynyk, J.K.; Beilin, L.J.; Mori, T.A.; Pennell, C.E.; de Klerk, N.; Oddy, W.H.; Shipman, P.; Adams, L.A. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology 2011, 53, 800–809. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Growth Charts Center for Disease Control and Prevention 2000. Available online: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed on 10 March 2000).
- Huang, R.C.; Mori, T.A.; Burke, V.; Newnham, J.; Stanley, F.J.; Landau, L.I.; Kendall, G.E.; Oddy, W.H.; Beilin, L.J. Synergy between adiposity, insulin resistance, metabolic risk factors, and inflammation in adolescents. Diabetes Care 2009, 32, 695–701. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Lew, M.J. Three inferential questions, two types of p-value. Am. Stat. Online Discuss. 2016, 70, 1–2. [Google Scholar]
- Valvi, D.; Casas, M.; Romaguera, D.; Monfort, N.; Ventura, R.; Martinez, D.; Sunyer, J.; Vrijheid, M. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ. Health Perspect. 2015, 123, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Berman, Y.E.; Doherty, D.A.; Main, K.M.; Frederiksen, H.; Keelan, J.A.; Newnham, J.P.; Hart, R.J. The influence of prenatal exposure to phthalates on subsequent male growth and body composition in adolescence. Environ. Res. 2021, 195, 110313. [Google Scholar] [CrossRef] [PubMed]
- Berman, Y.E.; Doherty, D.A.; Main, K.M.; Frederiksen, H.; Hickey, M.; Keelan, J.A.; Newnham, J.P.; Hart, R.J. Associations between Prenatal Exposure to Phthalates and Timing of Menarche and Growth and Adiposity into Adulthood: A Twenty-Years Birth Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4725. [Google Scholar] [CrossRef]
- Hou, J.W.; Lin, C.L.; Tsai, Y.A.; Chang, C.H.; Liao, K.W.; Yu, C.J.; Yang, W.; Lee, M.J.; Huang, P.C.; Sun, C.W.; et al. The effects of phthalate and nonylphenol exposure on body size and secondary sexual characteristics during puberty. Int. J. Hyg. Environ. Health 2015, 218, 603–615. [Google Scholar] [CrossRef]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Caudill, S.P.; Brock, J.W.; Needham, L.L.; Calafat, A.M. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Health Perspect. 2004, 112, 331–338. [Google Scholar] [CrossRef]
- Olsen, L.; Lampa, E.; Birkholz, D.A.; Lind, L.; Lind, P.M. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf. 2012, 75, 242–248. [Google Scholar] [CrossRef]
- Frederiksen, H.; Jorgensen, N.; Andersson, A.M. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J. Anal. Toxicol. 2010, 34, 400–410. [Google Scholar] [CrossRef]
- Mouritsen, A.; Frederiksen, H.; Sorensen, K.; Aksglaede, L.; Hagen, C.; Skakkebaek, N.E.; Main, K.M.; Andersson, A.M.; Juul, A. Urinary phthalates from 168 girls and boys measured twice a year during a 5-year period: Associations with adrenal androgen levels and puberty. J. Clin. Endocrinol. Metab. 2013, 98, 3755–3764. [Google Scholar] [CrossRef]
| Phthalate Diester | Human Serum Metabolite | ||
|---|---|---|---|
| Di-ethyl phthalate | DEP | Mono-ethyl phthalate | MEP |
| Di-iso-butyl phthalate | DiBP | Mono-iso-butyl phthalate | MiBP |
| Di-n-butyl phthalate | DnBP | Mono-n-butyl phthalate | MnBP |
| Mono-(3-hydroxybutyl) phthalate | MHBP a | ||
| Butylbenzyl phthalate | BBzP | Mono-benzyl phthalate | MBzP |
| Di-(2-ethyl-hexyl) phthalate | DEHP | Mono-(2-ethyl-hexyl) phthalate | MEHP |
| Mono-(2-ethyl-5-carboxypentyl) phthalate | MECPP a | ||
| Mono-(2-carboxymethyl-hexyl) phthalate | MCMHP a | ||
| Di-octyl phthalate | DOP | Mono-3-carboxypropyl phthalate | MCPP a |
| Di-iso-nonyl phthalate | DiNP | Mono-iso-nonyl phthalate | MiNP |
| Mono-carboxy-iso-octyl phthalate | MCiOP a | ||
| Di-iso-decyl phthalate | DiDP | Mono-iso-decyl phthalate | MiDP |
| Sums of phthalate metabolites | |||
| ΣMBP(i + n) | Sum of MiBP and MnBP in ng/mL | ||
| ∑DEHPmetab | Molar sum of MEHP, MCMHP, and MECPP expressed as DEHP in ng/mL | ||
| ∑DiNPmetab | Molar sum of MiNP and MCIOP expressed as DiNP in ng/mL | ||
| ∑DEHPmetab + ∑DiNPmetab | Molar sum of MEHP, MCMHP, MECPP, MiNP, MCiOP expressed as MEHP in ng/mL | ||
| ∑low MW phth.metab | Molar sum of MEP, MiBP, MnBP, and MHBP expressed as MEP in ng/mL | ||
| ∑high MW phth.metab | Molar sum of MBzP, MEHP, MCMHP, MECPP, MCPP, MiNP, MCiOP, and MiDP expressed as MEHP in ng/mL | ||
| ∑all phth.metab | Molar sum of MEP, MiBP, MnBP, MHBP, MBzP, MEHP, MCMHP, MECPP, MCPP, MiNP, MCiOP, and MiDP expressed as MEHP in ng/mL | ||
| Not Included in Study | Included in Study | ||||
|---|---|---|---|---|---|
| (Maternal Serum Phthalate Levels or Outcome Data Unavailable) n = 1012 | (Maternal Serum Phthalate Levels and Outcome Data Available) n = 387 | ||||
| N | Median (IQR) or N(%) | N | Median (IQR) or N(%) | p | |
| Maternal characteristics in pregnancy | |||||
| Maternal age at delivery (years) | 1009 | 27.9 (23.5, 31.8) | 386 | 28.9 (24.3, 32.5) | 0.084 |
| Smoked during pregnancy | 1012 | 270 (26.7) | 387 | 79 (20.4) | 0.015 |
| Maternal height (m) | 1012 | 1.6 (1.6, 1.7) | 387 | 1.6 (1.6, 1.7) | 0.038 |
| Maternal BMI category | 1012 | 387 | 0.629 | ||
| Underweight | 114 (11.3) | 42 (10.9) | |||
| Normal | 710 (70.3) | 282 (72.9) | |||
| Overweight | 118 (11.7) | 43 (11.1) | |||
| Obese | 70 (6.9) | 20 (5.2) | |||
| Maternal education since leaving school | 1012 | 387 | 0.969 | ||
| <High school | 331 (32.7) | 124 (32.0) | |||
| High school | 201 (19.9) | 77 (19.9) | |||
| Trade | 384 (37.9) | 146 (37.7) | |||
| University | 96 (9.5) | 40 (10.3) | |||
| Family Income at birth | 957 | 371 | 0.609 | ||
| <$7000 | 90 (9.4) | 31 (8.36) | |||
| $7000–$11,999 | 88 (9.2) | 32 (8.63) | |||
| $12,000–$23,999 | 246 (25.7) | 86 (23.2) | |||
| $24,000–$35,999 | 237 (24.8) | 91 (24.5) | |||
| >$36,000 | 296 (30.9) | 131 (35.3) | |||
| Breastfeeding of Gen2 participant | 827 | 359 | 0.3318 | ||
| Never breastfed | 87 (10.5) | 28 (7.8) | |||
| breastfed for <6 months | 308 (37.2) | 141 (39.3) | |||
| breastfed for >6 months | 432 (52.2) | 190 (52.9) | |||
| Participants characteristics at birth | |||||
| Gestational age at birth | 1012 | 387 | |||
| <34 weeks | 32 (3.2) | 7 (1.8) | |||
| 34–36 weeks | 69 (6.8) | 18 (4.7) | |||
| 37+ weeks | 911 (90.0) | 362 (93.5) | |||
| Birthweight (kg) | 1012 | 3.4 (3.1, 3.8) | 387 | 3.5 (3.1, 3.8) | 0.738 |
| Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | 3 Years | 5 Years | 8 Years | 10 Years | 14 Years | 17 Years | 20 Years | 22 Years | 27 Years |
| Systolic blood pressure | MCiOP | MCiOP | MCiOP | MCiOP | MCiOP | ||||
| Diastolic blood pressure | High MEP | ||||||||
| Mid MECPP | Mid MECPP | Mid MECPP | Mid MECPP | ||||||
| Heart rate | Mid MiBP | Mid MiBP | Mid MiBP | Mid MiBP | |||||
| Mid MEHP | |||||||||
| MCPP | MCPP | MCPP | |||||||
| Waist circumference | Mid MiBP | ||||||||
| Mid MEHP | Mid MEHP | ||||||||
| Mid ∑DEHP | Mid ∑DEHP | ||||||||
| Fat mass | |||||||||
| Lean mass | High MECPP | ||||||||
| High MCMHP | |||||||||
| MCiOP | |||||||||
| Alanine transaminase | High MEHP | ||||||||
| High MECPP | |||||||||
| Mid MiNP | |||||||||
| MCiOP | |||||||||
| High ∑DEHP | High ∑DEHP | ||||||||
| Mid ∑DiNP | |||||||||
| Gamma-glutamyl transferase | High ∑DiNP | ||||||||
| Aspartate transaminase | High MECPP | ||||||||
| High MiNP | |||||||||
| Non-alcoholic fatty liver disease | Mid MnBP | ||||||||
| High-density lipoprotein cholesterol | MCPP | MCPP | MCPP | ||||||
| High ∑DiNP | |||||||||
| Low-density lipoprotein cholesterol | High MiBP | ||||||||
| Mid MiNP | Mid MiNP | Mid MiNP | |||||||
| High ∑MBP(i + n) | High ∑MBP(i + n) | ||||||||
| High ∑DEHP | |||||||||
| Triglycerides | Mid MnBP | Mid MnBP | Mid MnBP | ||||||
| High MEHP | High MEHP | High MEHP | |||||||
| Mid MiNP | Mid MiNP | ||||||||
| Mid ∑MBP(i + n) | Mid ∑MBP(i + n) | Mid ∑MBP(i + n) | |||||||
| High ∑DEHP | High ∑DEHP | ||||||||
| Mid ∑DINP | |||||||||
| High ∑DEHPDINP | |||||||||
| Uric acid | High MEP | ||||||||
| Mid MiBP | Mid MiBP | ||||||||
| Mid MiNP | |||||||||
| Mid ∑MBP(i + n) | Mid ∑MBP(i + n) | ||||||||
| Mid ∑DINP | |||||||||
| High-sensitivity C-reactive protein | High MiBP | High MiBP | |||||||
| Mid MiNP | Mid MiNP | Mid MiNP | |||||||
| High MiNP | |||||||||
| High ∑DiNP | |||||||||
| Mid ∑allphth | |||||||||
| Insulin | Mid MEHP | Mid MEHP | |||||||
| Homeostatic model assessment | Mid MEHP | Mid MEHP | |||||||
| Glucose | High MiBP | High MiBP | |||||||
| MbZP | |||||||||
| High ∑DEHPDINP | |||||||||
| Creatinine | High MECPP | High MECPP | |||||||
| High MCMHP | High MCMHP | ||||||||
| Mid ∑DEHP | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berman, Y.E.; Doherty, D.A.; Mori, T.A.; Beilin, L.J.; Ayonrinde, O.T.; Adams, L.A.; Huang, R.-C.; Olynyk, J.K.; Keelan, J.A.; Newnham, J.P.; et al. Associations between Prenatal Exposure to Phthalates and Features of the Metabolic Syndrome in Males from Childhood into Adulthood. Int. J. Environ. Res. Public Health 2022, 19, 15244. https://doi.org/10.3390/ijerph192215244
Berman YE, Doherty DA, Mori TA, Beilin LJ, Ayonrinde OT, Adams LA, Huang R-C, Olynyk JK, Keelan JA, Newnham JP, et al. Associations between Prenatal Exposure to Phthalates and Features of the Metabolic Syndrome in Males from Childhood into Adulthood. International Journal of Environmental Research and Public Health. 2022; 19(22):15244. https://doi.org/10.3390/ijerph192215244
Chicago/Turabian StyleBerman, Ye’elah E., Dorota A. Doherty, Trevor A. Mori, Lawrence J. Beilin, Oyekoya T. Ayonrinde, Leon A. Adams, Rae-Chi Huang, John K. Olynyk, Jeffrey A. Keelan, John P. Newnham, and et al. 2022. "Associations between Prenatal Exposure to Phthalates and Features of the Metabolic Syndrome in Males from Childhood into Adulthood" International Journal of Environmental Research and Public Health 19, no. 22: 15244. https://doi.org/10.3390/ijerph192215244
APA StyleBerman, Y. E., Doherty, D. A., Mori, T. A., Beilin, L. J., Ayonrinde, O. T., Adams, L. A., Huang, R.-C., Olynyk, J. K., Keelan, J. A., Newnham, J. P., & Hart, R. J. (2022). Associations between Prenatal Exposure to Phthalates and Features of the Metabolic Syndrome in Males from Childhood into Adulthood. International Journal of Environmental Research and Public Health, 19(22), 15244. https://doi.org/10.3390/ijerph192215244






