Distribution of Trace Metals in Ice and Water of Liaodong Bay, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Analytical Method
3. Results
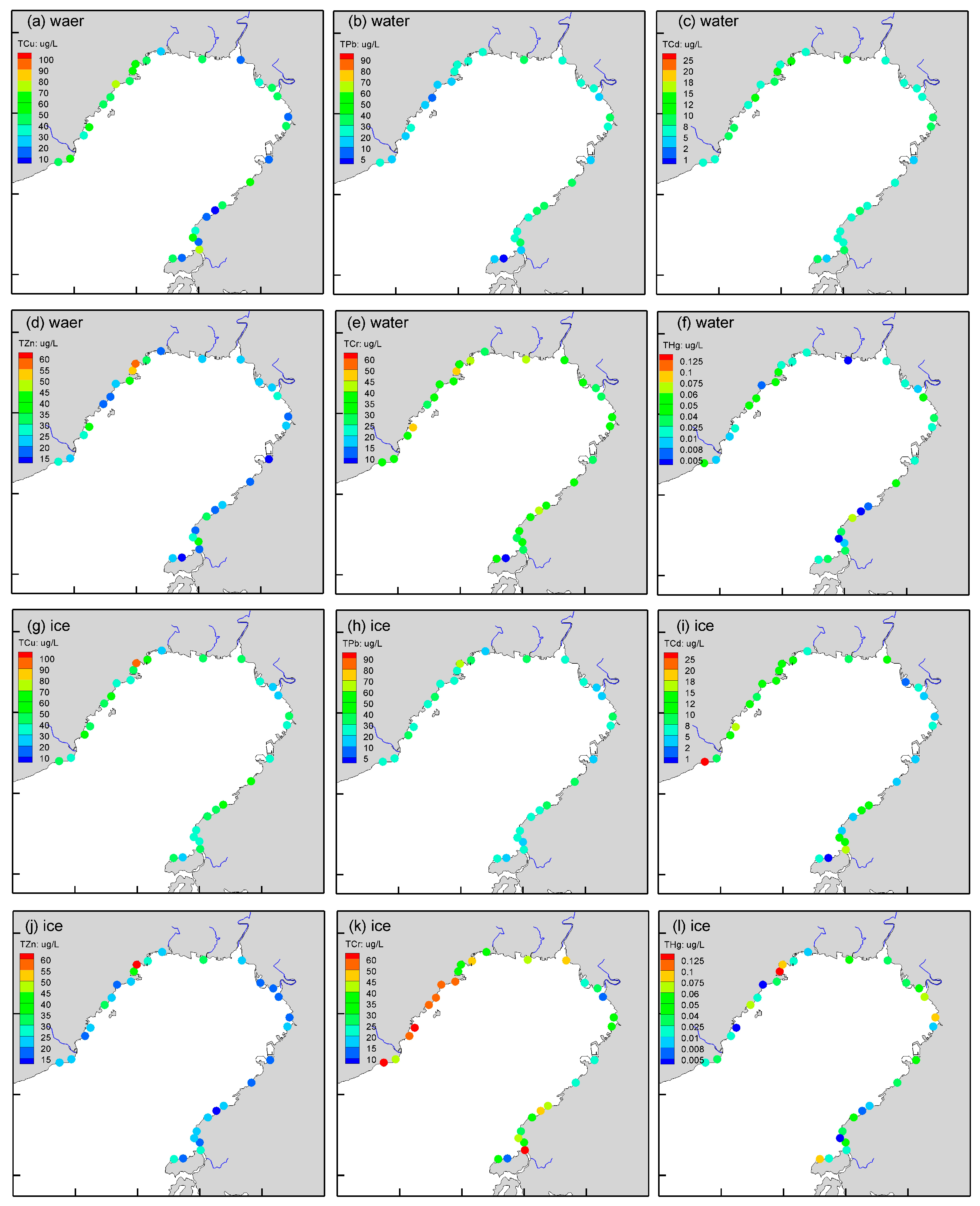
3.1. Spatial Distribution of Trace Metals
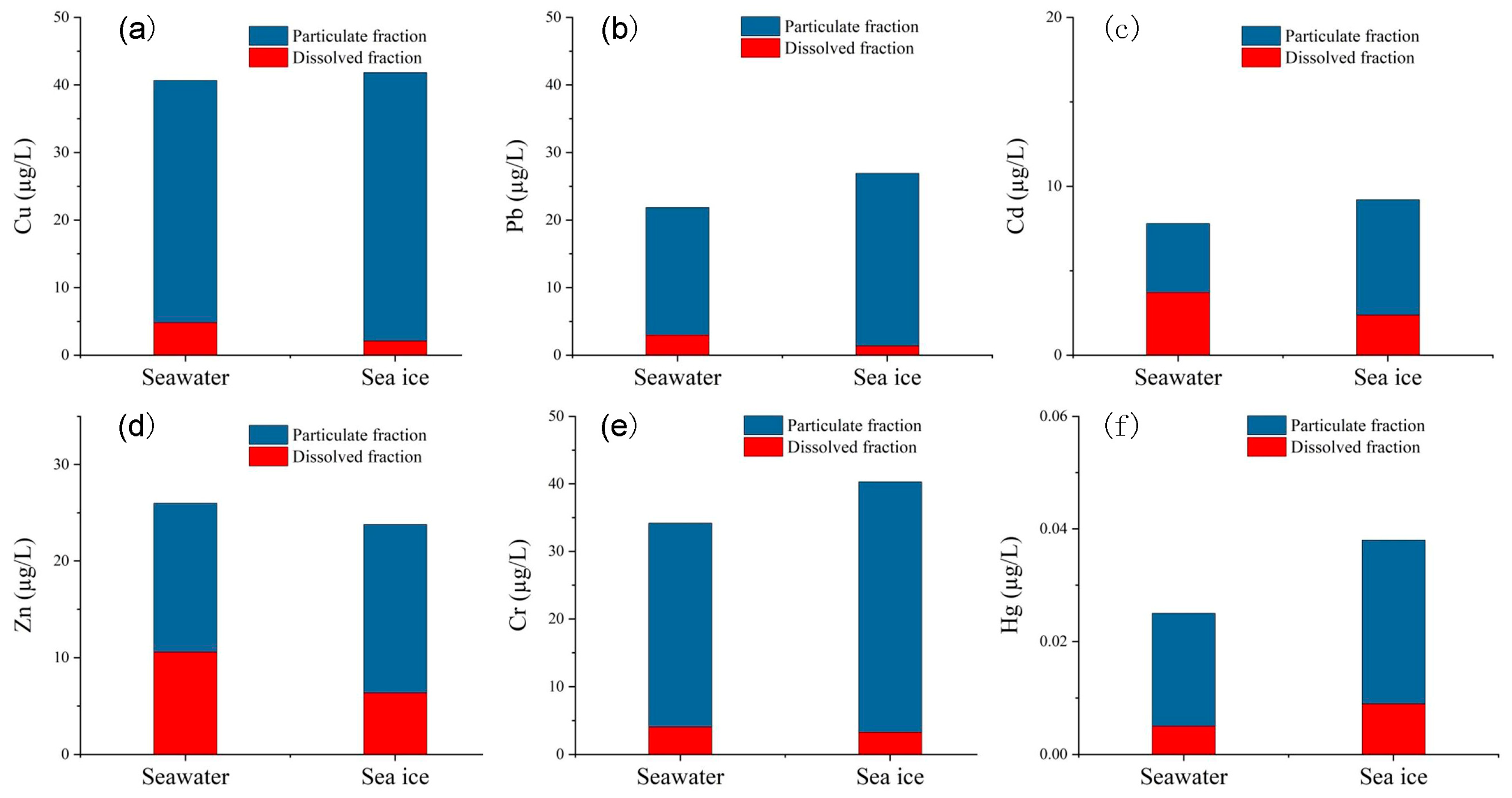
3.2. Partitioning of Trace Metals in Coastal Water and Sea Ice
4. Discussion
4.1. Factors Affecting Trace Metal Distribution
4.2. Trace-Metal Partitioning, Kd
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leppäranta, M. The Drift of Sea Ice; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Bilge, T.A.; Fournier, N.; Mignac, D.; Hume-Wright, L.; Bertino, L.; Williams, T.; Tietsche, S. An Evaluation of the Performance of Sea Ice Thickness Forecasts to Support Arctic Marine Transport. J. Mar. Sci. Eng. 2022, 10, 265. [Google Scholar] [CrossRef]
- Squire, V.A.; Kovalev, P.D.; Kovalev, D.P. Resonance and interactions of infragravity waves with sea ice. Cold Reg. Sci. Technol. 2021, 182, 103217. [Google Scholar] [CrossRef]
- Pfirman, S.L.; Eicken, H.; Bauch, D.; Weeks, W.F. The potential transport of pollutants by Arctic sea ice. Sci. Total Environ. 1995, 159, 129–146. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; González-Ortegón, E.; Duarte, C.M. Trace metal partitioning in the top meter of the ocean. Sci. Total Environ. 2019, 652, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Sedwick, P.N.; DiTullio, G.R. Regulation of algal blooms in Antarctic shelf waters by the release of iron from melting sea ice. Geophys. Res. Lett. 1997, 24, 2515–2518. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Duarte, C.M.; Alonso, J.C.; Lacorte, S.; Tauler, R.; Galbán-Malagón, C. Impacts of metals and nutrients released from melting multiyear Arctic sea ice. J. Geophys. Res. Oceans 2010, 115. [Google Scholar] [CrossRef]
- Edwards, R.; Sedwick, P. Iron in East Antarctic snow: Implications for atmospheric iron deposition and algal production in Antarctic waters. Geophys. Res. Lett. 2001, 28, 3907–3910. [Google Scholar] [CrossRef]
- Kanna, N.; Toyota, T.; Nishioka, J. Iron and macro-nutrient concentrations in sea ice and their impact on the nutritional status of surface waters in the southern Okhotsk Sea. Prog. Oceanogr. 2014, 126, 44–57. [Google Scholar] [CrossRef]
- Evans, L.K.; Nishioka, J. Accumulation processes of trace metals into Arctic sea ice: Distribution of Fe, Mn and Cd associated with ice structure. Mar. Chem. 2019, 209, 36–47. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Abelmoschi, M.L.; Rivaro, P.; Magi, E.; Frache, R. Temporal distribution of trace metals in Antarctic coastal waters. Mar. Chem. 2001, 76, 189–209. [Google Scholar] [CrossRef]
- Janssens, J.; Meiners, K.M.; Townsend, A.T.; Lannuzel, D. Organic matter controls of iron incorporation in growing sea ice. Front. Earth Sci. 2018, 6, 22. [Google Scholar] [CrossRef]
- Grotti, M.; Soggia, F.; Ardini, F.; Magi, E. Major and trace element partitioning between dissolved and particulate phases in Antarctic surface snow. J. Environ. Monit. 2011, 13, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, C.; Liu, J.; Shi, X.; Zhao, S.; Wu, Y.; Tian, W. First-Principles study on the migration of heavy metal ions in ice-water medium from Ulansuhai lake. Water 2018, 10, 1149. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Zhao, W.; Liu, T.; Liu, Y. The migration law of iron during the process of water icing. Water 2020, 12, 441. [Google Scholar] [CrossRef]
- Bianchi, T.S. Biogeochemistry of Estuaries; Oxford University Press on Demand: Oxford, UK, 2007. [Google Scholar]
- Zhang, A.; Wang, L.; Zhao, S.; Yang, X.; Zhao, Q.; Zhang, X.; Yuan, X. Heavy metals in seawater and sediments from the northern Liaodong Bay of China: Levels, distribution and potential risks. Reg. Stud. Mar. Sci. 2017, 11, 32–42. [Google Scholar] [CrossRef]
- Liu, L.; Wang, L.; Yang, Z.; Hu, Y.; Ma, M. Spatial and temporal variations of heavy metals in marine sediments from Liaodong Bay, Bohai Sea in China. Mar. Pollut. Bull. 2017, 124, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Jiao, D.; Wang, J.; Lei, K.; Lin, C. Trophic transfer of toxic elements in the estuarine invertebrate and fish food web of Daliao River, Liaodong Bay, China. Mar. Pollut. Bull. 2017, 113, 258–265. [Google Scholar] [CrossRef]
- GB17378.3; The Speciation for the Marine Monitoring-Part 3: Sample Collection, Storage and Transportation. Standardization Administration of P.R.C.: Beijing, China, 2007.
- GB17378.4; The Speciation for the Marine Monitoring-Part 4: Seawater Analysis. Standardization Administration of P.R.C.: Beijing, China, 2007.
- Guo, W.; Zou, J.; Liu, S.; Chen, X.; Kong, X.; Zhang, H.; Xu, T. Seasonal and spatial variation in dissolved heavy metals in Liaodong Bay, China. Int. J. Environ. Res. Public Health 2022, 19, 608. [Google Scholar] [CrossRef]
- Zaborska, A.; Strzelewicz, A.; Rudnicka, P.; Moskalik, M. Processes driving heavy metal distribution in the seawater of an Arctic fjord (Hornsund, southern Spitsbergen). Mar. Pollut. Bull. 2020, 161, 111719. [Google Scholar] [CrossRef]
- Lannuzel, D.; Bowie, A.R.; van der Merwe, P.C.; Townsend, A.T.; Schoemann, V. Distribution of dissolved and particulate metals in Antarctic sea ice. Mar. Chem. 2011, 124, 134–146. [Google Scholar] [CrossRef]
- Evans, L.K.; Nishioka, J. Quantitative analysis of Fe, Mn and Cd from sea ice and seawater in the Chukchi Sea, Arctic Ocean. Polar Sci. 2018, 17, 50–58. [Google Scholar] [CrossRef]
- Tang, D.; Warnken, K.W.; Santschi, P.H. Distribution and partitioning of trace metals (Cd, Cu, Ni, Pb, Zn) in Galveston bay waters. Mar. Chem. 2002, 78, 29–45. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Zhang, Y.; Tian, J.; Song, L.; Han, J.; Zhang, Y. Spatial distribution and ecological risks of polycyclic aromatic hydrocarbons in sea ice and seawater from northern Liaodong Bay, China. Mar. Pollut. Bull. 2022, 174, 113319. [Google Scholar] [CrossRef] [PubMed]
- Schoellhamer, D.H.; Mumley, T.E.; Leatherbarrow, J.E. Suspended sediment and sediment-associated contaminants in San Francisco Bay. Environ. Res. 2007, 105, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Feng, C.; Wang, D.; Liu, Y.; Shen, Z. Salinity increases the mobility of Cd, Cu, Mn, and Pb in the sediments of Yangtze Estuary: Relative role of sediments’ properties and metal speciation. Chemosphere 2013, 91, 977–984. [Google Scholar] [CrossRef]
- Liang, X.; Tian, C.; Zong, Z.; Wang, X.; Jiang, W.; Chen, Y.; Zhang, G. Flux and source-sink relationship of heavy metals and arsenic in the Bohai Sea, China. Environ. Pollut. 2018, 242, 1353–1361. [Google Scholar] [CrossRef]
- Honeyman, B.D.; Santschi, P.H. A Brownian-pumping model for oceanic trace metal scavenging: Evidence from Th isotopes. J. Mar. Res. 1989, 47, 951–992. [Google Scholar] [CrossRef]
- Wen, L.S.; Santschi, P.; Gill, G.; Paternostro, C. Estuarine trace metal distributions in Galveston Bay: Importance of colloidal forms in the speciation of the dissolved phase. Mar. Chem. 1999, 63, 185–212. [Google Scholar] [CrossRef]


| Cu | Pb | Cd | Zn | Cr | Hg | |
|---|---|---|---|---|---|---|
| Cu | 1 | |||||
| Pb | −0.95 | 1 | ||||
| Cd | 0.48 ** | 0.22 | 1 | |||
| Zn | 0.25 | 0.22 | 0.44 * | 1 | ||
| Cr | 0.23 | 0.46 ** | 0.77 ** | 0.39 * | 1 | |
| Hg | −0.02 | −0.15 | −0.19 | 0.16 | −0.22 | 1 |
| COD | −0.04 | −0.24 | −0.26 | −0.27 | −0.35 | 0.15 |
| Sal. | 0.15 | 0.58 ** | 0.57 ** | 0.246 | 0.79 ** | −0.12 |
| SPM | −0.44 * | 0.082 | 0.045 | 0.12 | 0.077 | 0.004 |
| Cu | Pb | Cd | Zn | Cr | Hg | |
|---|---|---|---|---|---|---|
| Cu | 1 | |||||
| Pb | 0.99 ** | 1 | ||||
| Cd | 0.29 | 0.29 | 1 | |||
| Zn | 0.72 ** | 0.72 ** | 0.17 | 1 | ||
| Cr | 0.33 | 0.34 | 0.88 ** | 0.13 | 1 | |
| Hg | 0.29 | 0.28 | 0.12 | 0.59 ** | 0.10 | 1 |
| COD | 0.43 * | 0.44 * | −0.28 | 0.51 ** | −0.03 | 0.50 ** |
| Sal. | 0.28 | 0.28 | 0.92 ** | 0.07 | 0.91 ** | −0.05 |
| SPM | 0.23 | 0.24 | −0.12 | 0.38 * | −0.10 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Liu, S.; Kong, X.; Sun, L.; Zou, J. Distribution of Trace Metals in Ice and Water of Liaodong Bay, China. Int. J. Environ. Res. Public Health 2022, 19, 15241. https://doi.org/10.3390/ijerph192215241
Guo W, Liu S, Kong X, Sun L, Zou J. Distribution of Trace Metals in Ice and Water of Liaodong Bay, China. International Journal of Environmental Research and Public Health. 2022; 19(22):15241. https://doi.org/10.3390/ijerph192215241
Chicago/Turabian StyleGuo, Weijun, Sihong Liu, Xiangpeng Kong, Lixin Sun, and Jibing Zou. 2022. "Distribution of Trace Metals in Ice and Water of Liaodong Bay, China" International Journal of Environmental Research and Public Health 19, no. 22: 15241. https://doi.org/10.3390/ijerph192215241
APA StyleGuo, W., Liu, S., Kong, X., Sun, L., & Zou, J. (2022). Distribution of Trace Metals in Ice and Water of Liaodong Bay, China. International Journal of Environmental Research and Public Health, 19(22), 15241. https://doi.org/10.3390/ijerph192215241







