Toxicity Assessment of Curculigo orchioides Leaf Extract Using Drosophila melanogaster: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection of Plant Material and Extraction
2.3. Characterization of A.L. Extract
2.4. Fly Strain and Rearing
2.5. Exposure of A.L. Extract to Drosophila
2.6. Fly Developmental Assay and Phenotypic Analysis
2.7. Reproductive Performance
2.8. Climbing Assay
2.9. Survival Assay
2.10. RNA Isolation and Gene Expression Analysis
2.11. Quantitative Estimation of ROS
2.12. Immunostaining
2.13. Statistical Analysis
3. Results and Discussion
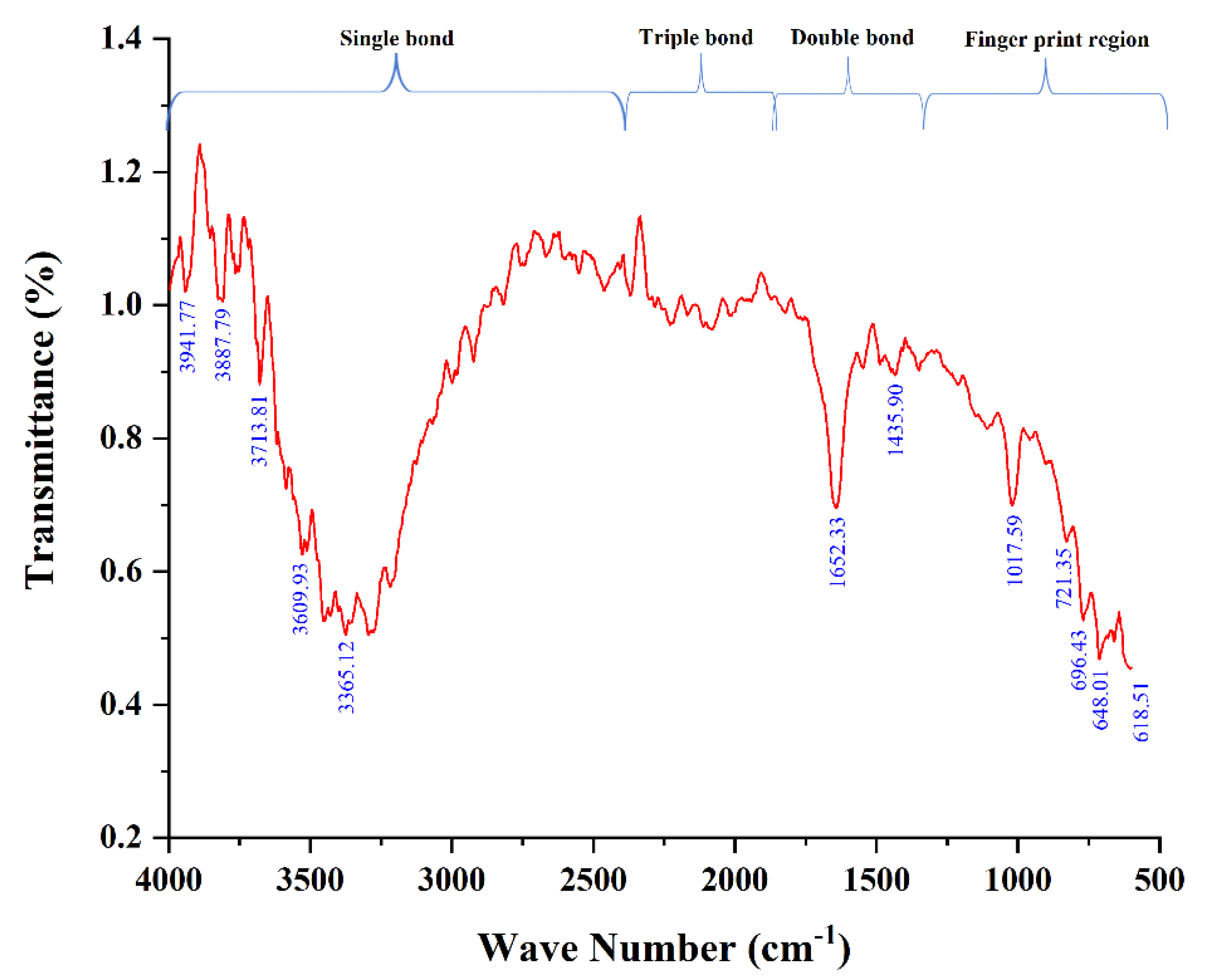
3.1. Characterization of A.L. Extract Using FTIR
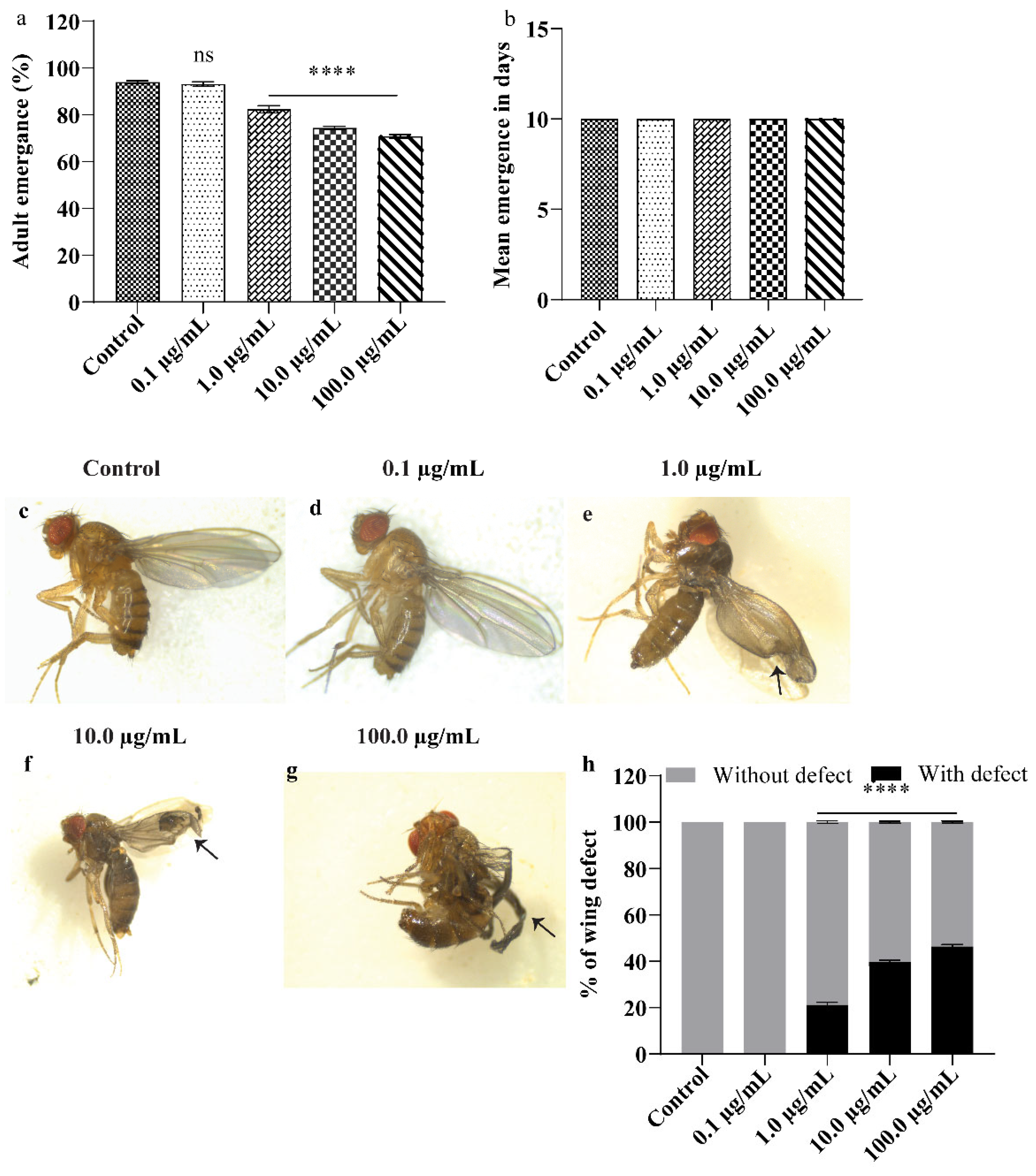
3.2. A.L. Extract Supplementation Causes Developmental Toxicity and Wing Deformity in Drosophila
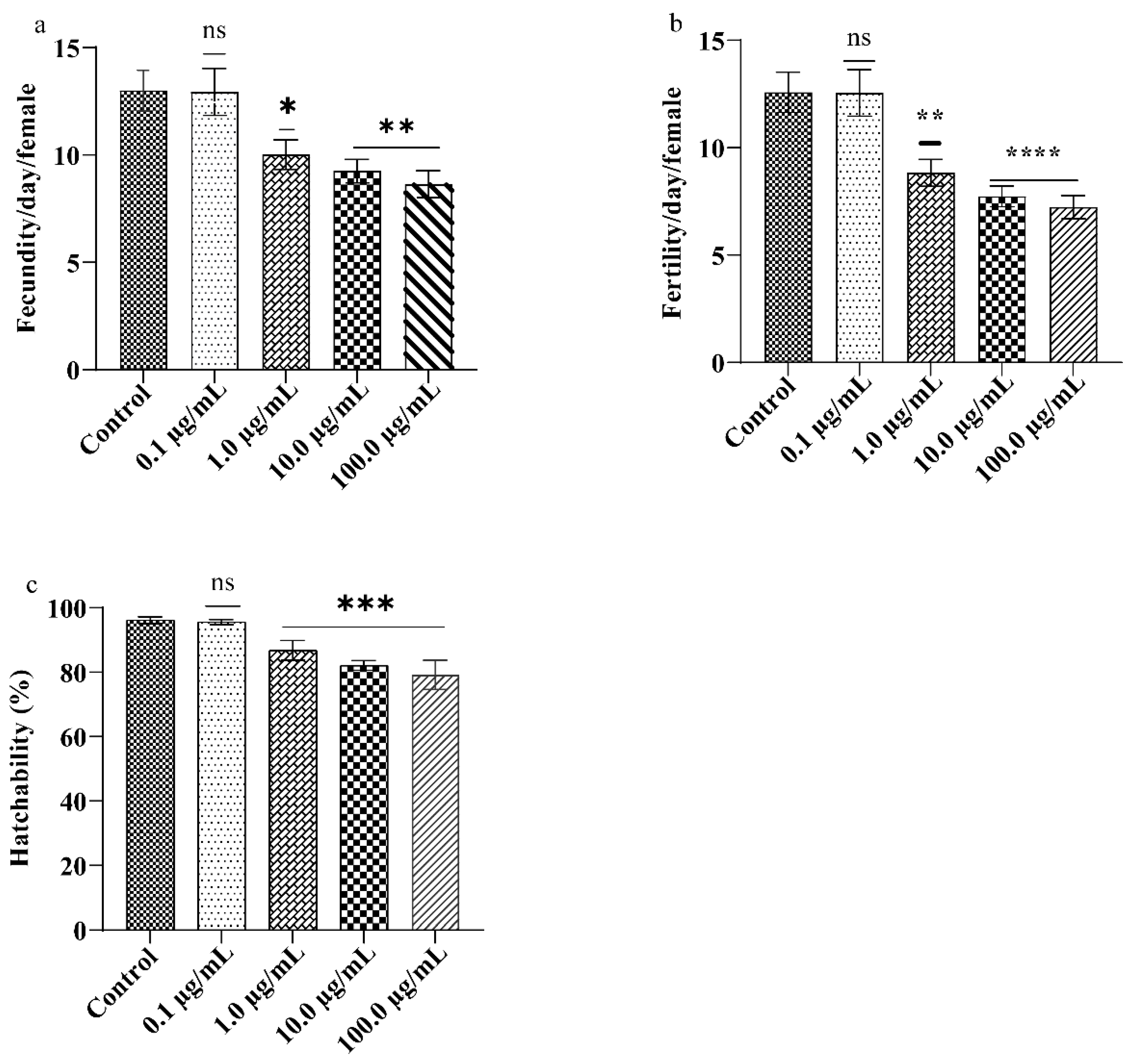
3.3. A.L. Extract Moderates the Drosophila Reproductive Performance
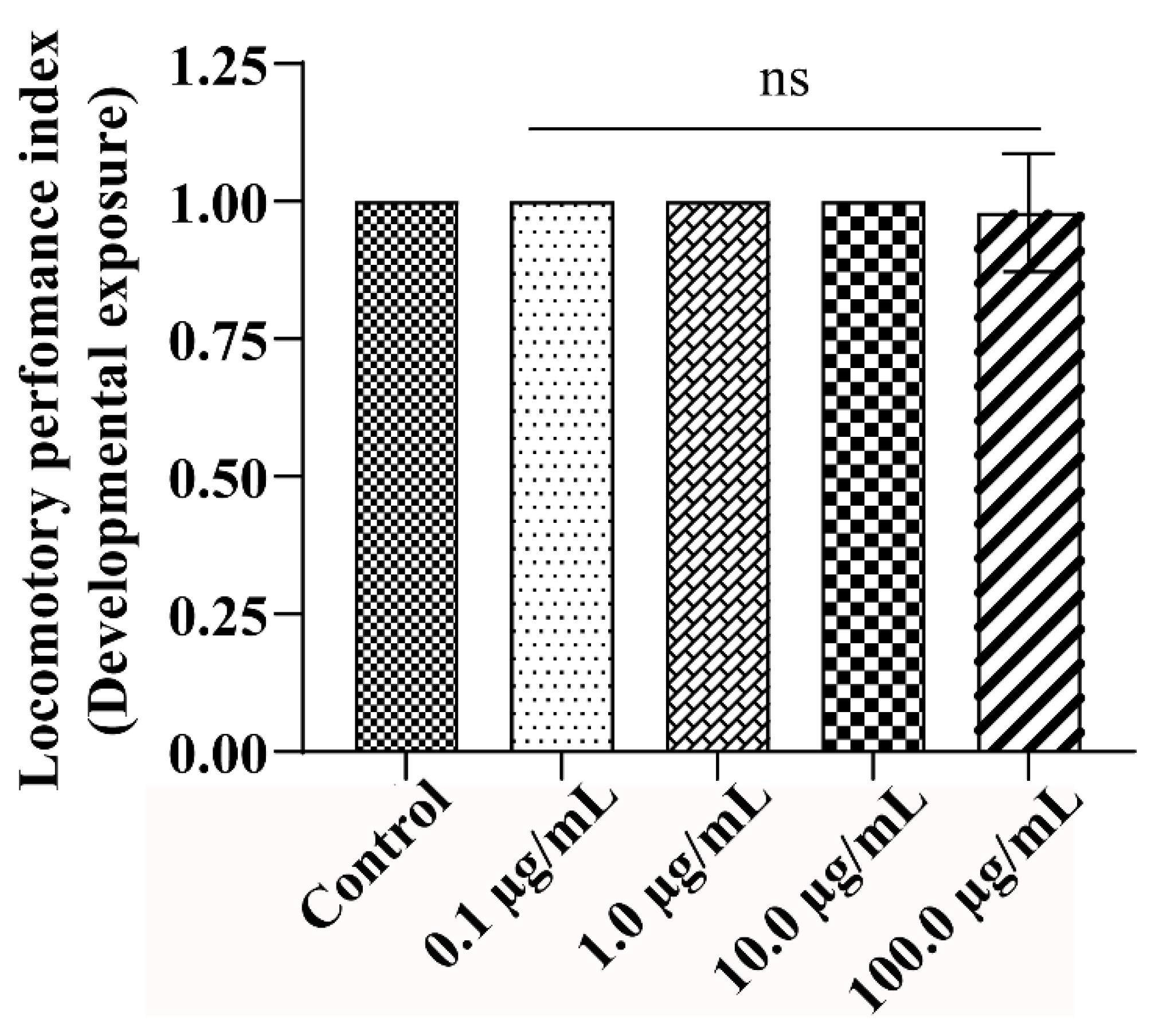
3.4. A.L. Extract Supplementation Does Not Affect the Locomotory Behaviour of the Exposed Organism
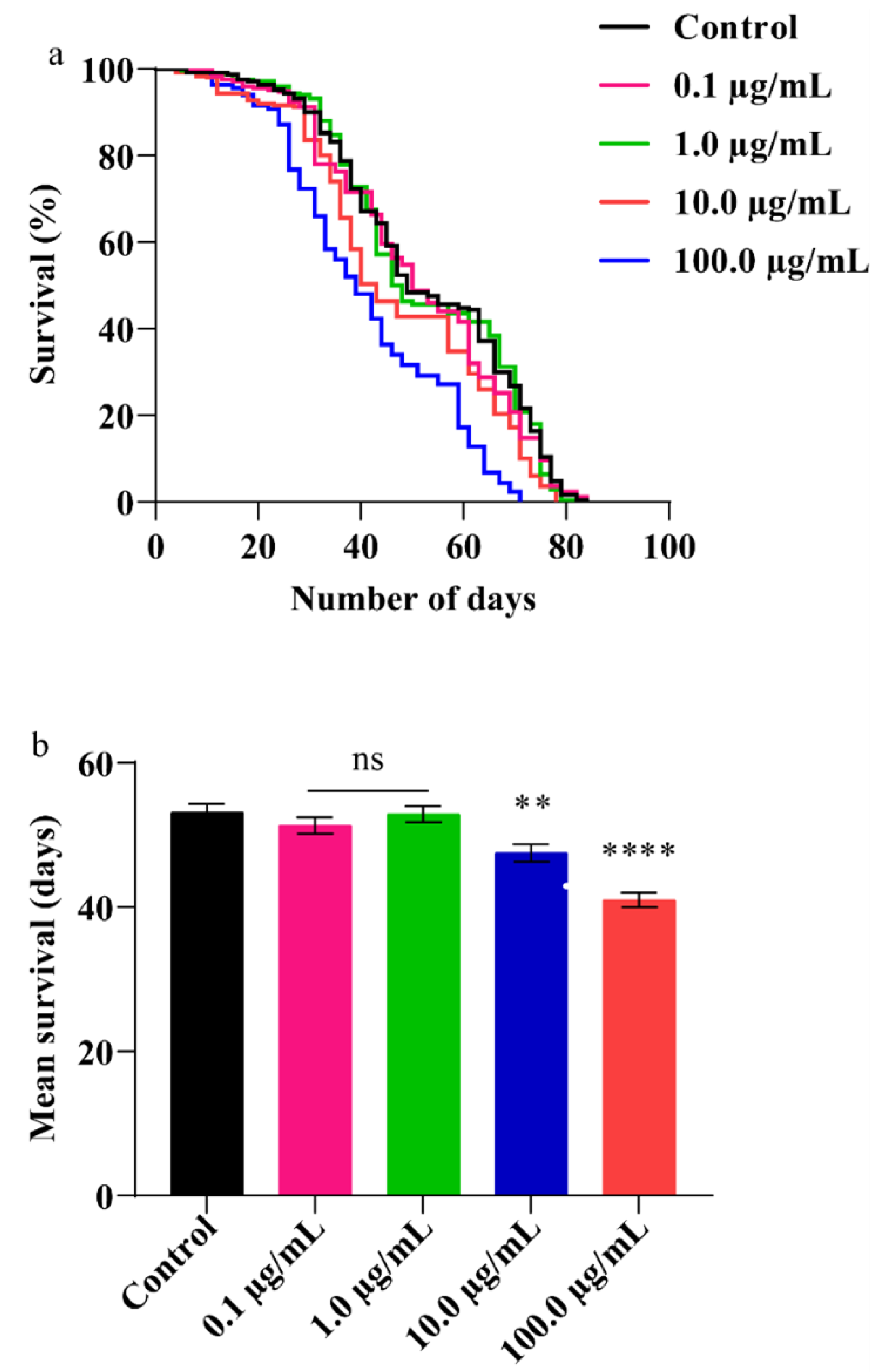
3.5. A.L. Extract Decreases Lifespan in Flies
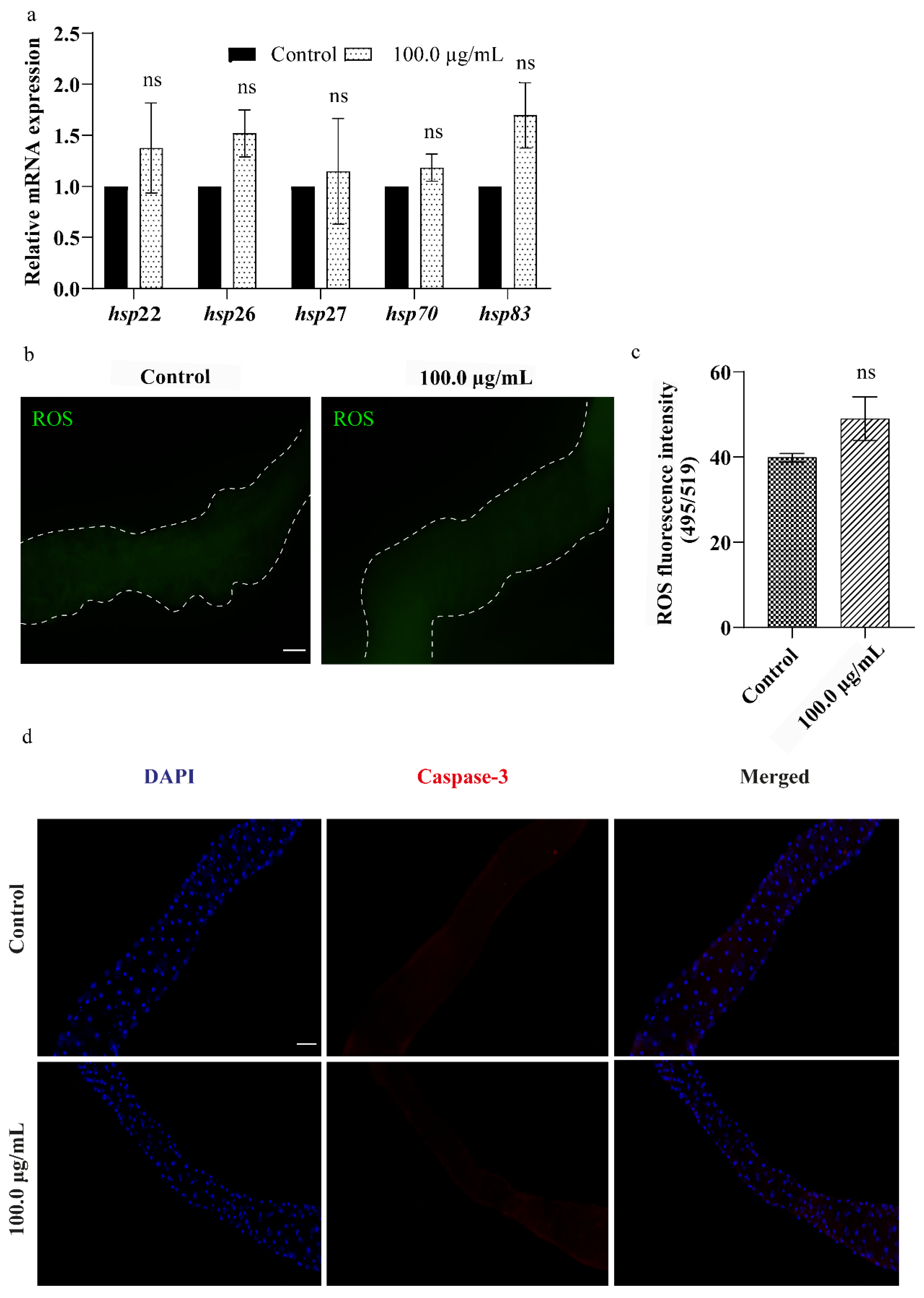
3.6. A.L. Extract Does Not Elevate Cellular Stress in Drosophila Larval Gut
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.S.B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use among Adults: United States, 2017–2018; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. [CrossRef]
- Zahn, R.; Perry, N.; Perry, E.; Mukaetova-Ladinska, E.B. Use of herbal medicines: Pilot survey of UK users’ views. Complement. Med. 2019, 44, 83–90. [Google Scholar] [CrossRef]
- Cecilia, N.C.; Al Washali, A.Y.; Albishty, A.A.A.M.M.; Suriani, I.; Rosliza, A.M. The use of herbal medicine in Arab countries: A Review. Int. J. Public Health Clin. Sci. 2017, 4, 1–4. [Google Scholar]
- Insights, F.B. Herbal Medicine Market Size, Share & COVID-19 Impact Analysis, by Application (Pharmaceutical & Nutraceutical, Food & Beverages, and Personal Care & Beauty Products), Form (Powder, Liquid & Gel, and Tablets & Capsules) and Regional Forecast, 2021–2028. 2022. Available online: https://www.fortunebusinessinsights.com/herbal-medicine-market-106320 (accessed on 10 October 2022).
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharm. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Grujicic, D.; Markovic, A.; Vukajlovic, J.T.; Stankovic, M.; Jakovljevic, M.R.; Ciric, A.; Djordjevic, K.; Planojevic, N.; Milutinovic, M.; Milosevic-Djordjevic, O. Genotoxic and cytotoxic properties of two medical plants (Teucrium arduini L. and Teucrium flavum L.) in relation to their polyphenolic contents. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2020, 852, 503168. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Alqahtani, A.S.; Almarfadi, O.M.; Ullah, R.; Nasr, F.A.; Noman, O.M.; Siddiqui, N.A.; Shahat, A.A.; Ahamad, S.R. The Reproductive Toxicity Associated with Dodonaea viscosa, a Folk Medicinal Plant in Saudi Arabia. Evid. Based Complement. Altern. Med. 2021, 2021, 6689110. [Google Scholar] [CrossRef]
- Teshome, D.; Tiruneh, C.; Berhanu, L.; Berihun, G.; Belete, Z.W. Developmental Toxicity of Ethanolic Extracts of Leaves of Achyranthes aspera, Amaranthaceae in Rat Embryos and Fetuses. J. Exp. Pharm. 2021, 13, 555–563. [Google Scholar] [CrossRef]
- Junior, F.E.; Macedo, G.E.; Zemolin, A.P.; Silva, G.F.; Cruz, L.C.; Boligon, A.A.; de Menezes, I.R.; Franco, J.L.; Posser, T. Oxidant effects and toxicity of Croton campestris in Drosophila melanogaster. Pharm. Biol. 2016, 54, 3068–3077. [Google Scholar] [CrossRef]
- Mohd-Fuat, A.R.; Kofi, E.A.; Allan, G.G. Mutagenic and cytotoxic properties of three herbal plants from Southeast Asia. Trop. Biomed. 2007, 24, 49–59. [Google Scholar]
- Chaturvedi, P.; Briganza, V. Enhanced Synthesis of Curculigoside by Stress and Amino Acids in Static Culture of Curculigo orchioides Gaertn (Kali Musli). Pharmacogn. Res. 2016, 8, 193–198. [Google Scholar] [CrossRef]
- Wala, B.B.; Jasrai, Y.T. Micropropagation of an endangered medicinal plant: Curculigo orchioides Gaertn. Plant Tissue Cult. 2003, 13, 13–19. [Google Scholar]
- Nie, Y.; Dong, X.; He, Y.; Yuan, T.; Han, T.; Rahman, K.; Qin, L.; Zhang, Q. Medicinal plants of genus Curculigo: Traditional uses and a phytochemical and ethnopharmacological review. J. Ethnopharmacol. 2013, 147, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Li, N. Phytochemistry and Pharmacological Activity of Plants of Genus Curculigo: An Updated Review Since 2013. Molecules 2021, 26, 3396. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yu, W.; Liu, H.B.; Zhang, N.; Li, X.B.; Zhao, M.G.; Liu, S.B. Neuroprotective effects of curculigoside against NMDA-induced neuronal excitoxicity in vitro. Food Chem. Toxicol. 2012, 50, 4010–4015. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, J.; Ni, Y.; Yin, W.; Hou, Q.; Zhang, Y.; Yan, S.; Quan, R. Curculigoside Protects against Titanium Particle-Induced Osteolysis through the Enhancement of Osteoblast Differentiation and Reduction of Osteoclast Formation. J. Immunol. Res. 2021, 2021, 5707242. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, A.K.; Panda, S.K.; Meher, A.; Padhan, A.R. Studies on the anti-inflammatory properties of Curculigo orchioides gaertn. Root Tubers. Int. J. Pharm. Sci. Res. 2010, 1, 139–143. [Google Scholar]
- Madhavan, V.; Joshi, R.; Murali, A.; Yoganarasimhan, S.N. Antidiabetic Activity of Curculigo orchioides. Root Tuber. Pharm. Biol. 2008, 45, 18–21. [Google Scholar] [CrossRef]
- Chauhan, N.S.; Rao, C.V.; Dixit, V.K. Effect of Curculigo orchioides rhizomes on sexual behaviour of male rats. Fitoterapia 2007, 78, 530–534. [Google Scholar] [CrossRef]
- Kushalan, S.; Yathisha, U.G.; Khyahrii, S.A.; Hegde, S. Phytochemical and anti-oxidant evaluation of in vitro and in vivo propagated plants of Curculigo orchioides. In Vitro Cell. Dev. Biol.-Plant 2022, 58, 382–391. [Google Scholar] [CrossRef]
- Nagesh, K.S.; Shanthamma, C. Antibacterial activity of Curculigo orchioides rhizome extract on pathogenic bacteria. Afr. J. Microbiol. Res. 2009, 3, 5–9. [Google Scholar] [CrossRef]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Festing, M.F.W.; Baumans, V.; Combes, R.D.; Haider, M.; Hendriksen, C.F.M.; Howard, B.R.; Lovell, D.P.; Moore, G.J.; Overend, P.; Wilson, M.S. Reducing the Use of Laboratory Animals in Biomedical Research: Problems and Possible Solutions:The Report and Recommendations of ECVAM Workshop 29. Altern. Lab. Anim. 1998, 26, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Aloysius, K.S.; Sharanya, K.; Kini, S.; Milan, G.R.; Hegde, S. Phytochemical analysis of Curculigo orchioides and its cytotoxic effect on lung adenocarcinoma cancer cell line (NCI-H522). Med. Plants-Int. J. Phytomed. Relat. Ind. 2020, 12, 400–404. [Google Scholar] [CrossRef]
- Khan, M.F.; Abutaha, N.; Nasr, F.A.; Alqahtani, A.S.; Noman, O.M.; Wadaan, M.A.M. Bitter gourd (Momordica charantia) possess developmental toxicity as revealed by screening the seeds and fruit extracts in zebrafish embryos. BMC Complement. Altern. Med. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mishra, M.; Shukla, A.K.; Kumar, R.; Abdin, M.Z.; Chowdhuri, D.K. Organochlorine pesticide, endosulfan induced cellular and organismal response in Drosophila melanogaster. J. Hazard. Mater. 2012, 221–222, 275–287. [Google Scholar] [CrossRef]
- Misra, S.; Singh, A.; CH, R.; Sharma, V.; Reddy Mudiam, M.K.; Ram, K.R. Identification of Drosophila-based endpoints for the assessment and understanding of xenobiotic-mediated male reproductive adversities. Toxicol. Sci. 2014, 141, 278–291. [Google Scholar] [CrossRef]
- Paithankar, J.G.; Kushalan, S.; Nijil, S.; Hegde, S.; Kini, S.; Sharma, A. Systematic toxicity assessment of CdTe quantum dots in Drosophila melanogaster. Chemosphere 2022, 295, 133836. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Dwivedi, S.; Raihan, F.; Yathisha, U.G.; Raghu, S.V.; Mamatha, B.S.; Sharma, A. Hsp70 overexpression in Drosophila hemocytes attenuates benzene-induced immune and developmental toxicity via regulating ROS/JNK signaling pathway. Environ. Toxicol. 2022, 37, 1723–1739. [Google Scholar] [CrossRef]
- Dwivedi, S.; D’Souza, L.C.; Shetty, N.G.; Raghu, S.V.; Sharma, A. Hsp27, a potential EcR target, protects nonylphenol-induced cellular and organismal toxicity in Drosophila melanogaster. Environ. Pollut. 2022, 293, 118484. [Google Scholar] [CrossRef]
- Mabasa, X.E.; Mathomu, L.M.; Madala, N.E.; Musie, E.M.; Sigidi, M.T.; Patra, J.K. Molecular Spectroscopic (FTIR and UV-Vis) and Hyphenated Chromatographic (UHPLC-qTOF-MS) Analysis and In Vitro Bioactivities of the Momordica balsamina Leaf Extract. Biochem. Res. Int. 2021, 2021, 2854217. [Google Scholar] [CrossRef]
- Umar, A.H.; Ratnadewi, D.; Rafi, M.; Sulistyaningsih, Y.C. Untargeted Metabolomics Analysis Using FTIR and UHPLC-Q-Orbitrap HRMS of Two Curculigo Species and Evaluation of their Antioxidant and alpha-Glucosidase Inhibitory Activities. Metabolites 2021, 11, 42. [Google Scholar] [CrossRef]
- Green, B.T.; Lee, S.T.; Welch, K.D.; Panter, K.E. Plant alkaloids that cause developmental defects through the disruption of cholinergic neurotransmission. Birth Defects Res. C Embryo Today 2013, 99, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. Plant Toxins 2015, 2, 1–15. [Google Scholar]
- Kumar, A.; Dave, M.; Pant, D.C.; Laxkar, R.; Tiwari, A.K. Vinca rosea leaf extract supplementation leads to developmental delay and several phenotypic anomalies in Drosophila melanogaster. Toxicol. Environ. Chem. 2013, 95, 635–645. [Google Scholar] [CrossRef]
- Gutierrez-Pajares, J.L.; Zuniga, L.; Pino, J. Ruta graveolens aqueous extract retards mouse preimplantation embryo development. Reprod. Toxicol. 2003, 17, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Abusufyan Shaikh, K.K.; Menna Ibrahim, M.K. Teratogenic effects of aqueous extract of Ficus glomerata leaf during embryonic development in zebrafish (Danio rerio). J. Appl. Pharm. Sci. 2019, 9, 107–111. [Google Scholar] [CrossRef]
- Pb, B.; Rani, S.; Kim, Y.O.; Ahmed Al-Ghamdi, A.; Elshikh, M.S.; Al-Dosary, M.A.; Hatamleh, A.A.; Arokiyaraj, S.; Kim, H.J. Prophylactic efficacy of Boerhavia diffusa L. aqueous extract in toluene induced reproductive and developmental toxicity in Drosophila melanogaster. J. Infect. Public Health 2020, 13, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hieu, L.T.; Son, L.L.; Nguyet, N.T.; Nhung, N.M.; Vu, H.X.A.; Man, N.Q.; Trang, L.T.; Minh, T.T.; Thi, T.T.V. In vitro antioxidant activity and Content of compounds from Curculigo orchioides rhizome. Hue Univ. J. Sci. Nat. Sci. 2020, 129, 71–77. [Google Scholar] [CrossRef]
- Pandit, P.; Singh, A.; Bafna, A.R.; Kadam, P.V.; Patil, M.J. Evaluation of Antiasthmatic Activity of Curculigo orchioides Gaertn. Rhizomes. Indian J. Pharm. Sci. 2008, 70, 440–444. [Google Scholar] [CrossRef]
- Yakubu, M.T. Effect of a 60-day oral gavage of a crude alkaloid extract from Chromolaena odorata leaves on hormonal and spermatogenic indices of male rats. J. Androl. 2012, 33, 1199–1207. [Google Scholar] [CrossRef]
- Shu, Y.; Cao, M.; Yin, Z.Q.; Li, P.; Li, T.Q.; Long, X.F.; Zhu, L.F.; Jia, R.Y.; Dai, S.J.; Zhao, J. The reproductive toxicity of saponins isolated from Cortex Albiziae in female mice. Chin. J. Nat. Med. 2015, 13, 119–126. [Google Scholar] [CrossRef]
- Arika, W.M.; Ogola, P.E.; Abdirahman, Y.A.; Mawia, A.M.; Wambua, F.K.; Nyamai, D.W.; Kiboi, N.G.; Wambani, J.R.; Njagi, S.M.; Rachuonyo, H.O.; et al. In Vivo Safety of Aqueous Leaf Extract of Lippia javanica in Mice Models. Biochem. Physiol. 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Vijayanarayana, K.; Rodrigues, R.S.; Chandrashekhar, K.S.; Subrahmanyam, E.V. Evaluation of estrogenic activity of alcoholic extract of rhizomes of Curculigo orchioides. J. Ethnopharmacol. 2007, 114, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Barik, B.K. Behavioral Teratogenesis in Drosophila melanogaster. Methods Mol. Biol. 2018, 1797, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.-L.; Gao, L. Anti-depressant-like effect of curculigoside isolated from Curculigo orchioides Gaertn root. Trop. J. Pharm. Res. 2016, 15, 2165–2172. [Google Scholar] [CrossRef][Green Version]
- Villeponteau, B.; Matsagas, K.; Nobles, A.C.; Rizza, C.; Horwitz, M.; Benford, G.; Mockett, R.J. Herbal supplement extends life span under some environmental conditions and boosts stress resistance. PLoS ONE 2015, 10, e0119068. [Google Scholar] [CrossRef]
- Li, H.; Liang, B.; Cao, Y.; Xu, Y.; Chen, J.; Yao, Y.; Shen, J.; Yao, D. Effects of Chinese herbal medicines on lifespan in Drosophila. Exp. Gerontol. 2021, 154, 111514. [Google Scholar] [CrossRef]
- Nenaah, G. Toxicity and growth inhibitory activities of methanol extract and the β-carboline alkaloids of Peganum harmala L. against two coleopteran stored-grain pests. J. Stored Prod. Res. 2011, 47, 255–261. [Google Scholar] [CrossRef]
- Dutta, N.; Garcia, G.; Higuchi-Sanabria, R. Hijacking Cellular Stress Responses to Promote Lifespan. Front. Aging 2022, 3, 20. [Google Scholar] [CrossRef]
- Dwivedi, S.; Kushalan, S.; Paithankar, J.G.; D’Souza, L.C.; Hegde, S.; Sharma, A. Environmental toxicants, oxidative stress and health adversities: Interventions of phytochemicals. J. Pharm. Pharm. 2022, 74, 516–536. [Google Scholar] [CrossRef]
- Subapriya, R.; Bhuvaneswari, V.; Nagini, S. Ethanolic neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Asian Pac. J. Cancer Prev. 2005, 6, 515–520. [Google Scholar]
- Campos, J.F.; Espindola, P.P.T.; Torquato, H.F.V.; Vital, W.D.; Justo, G.Z.; Silva, D.B.; Carollo, C.A.; de Picoli Souza, K.; Paredes-Gamero, E.J.; Dos Santos, E.L. Leaf and Root Extracts from Campomanesia adamantium (Myrtaceae) Promote Apoptotic Death of Leukemic Cells via Activation of Intracellular Calcium and Caspase-3. Front Pharm. 2017, 8, 466. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (5′-3′) |
|---|---|
| hsp22 | Forward primer: TGGCTATAGCTCCAGGCACT Reverse primer: GCTTTGTCATTTGGCTCCTC |
| hsp26 | Forward primer: GAGCGCATCATTCAAATTCA Reverse primer: TCCACACCAGGTGAACAAAA |
| hsp27 | Forward primer: GACTGGGTCGTCGTCGTTAT Reverse primer: TTGAACTGCGACACATCCAT |
| hsp70 | Forward primer: CATTCCGTGCAAGCAGACTA Reverse primer: GCTGACGTTCAGGATTCCAT |
| hsp83 | Forward primer: CAGCTGGTCTCTGTCACCAA Reverse primer: TGGACTTCATCAGCTTGCAC |
| β-Actin | Forward primer: GTGCCCATCTACGAGGGTTA Reverse primer: AGGGCAACATAGCAGCTT |
| Frequency Range (cm−1) | Frequency Peak (cm−1) | Bond | Functional Group |
|---|---|---|---|
| 600–650 | 618.51 | C-Br stretch | Alkyl halides |
| 648.01 | |||
| 680–720 | 696.43 | C-H “oop” | Aromatics |
| 721.35 | C-H rock | Alkanes | |
| 1000–1500 | 1017.59 | C-O stretch | Alcohols, carboxylic acids |
| 1453.90 | C-C stretch | Aromatics | |
| 1600–1700 | 1652.33 | C=C | Alkenes |
| 3300–3610 | 3365.12 | N-H stretch | 1° Amines |
| 3609.93 | |||
| 3700–4000 | 3713.81 | O-H stretch, free hydroxyl | Alcohols, Phenols |
| 3887.79 | |||
| 3941.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushalan, S.; D’Souza, L.C.; Aloysius, K.; Sharma, A.; Hegde, S. Toxicity Assessment of Curculigo orchioides Leaf Extract Using Drosophila melanogaster: A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 15218. https://doi.org/10.3390/ijerph192215218
Kushalan S, D’Souza LC, Aloysius K, Sharma A, Hegde S. Toxicity Assessment of Curculigo orchioides Leaf Extract Using Drosophila melanogaster: A Preliminary Study. International Journal of Environmental Research and Public Health. 2022; 19(22):15218. https://doi.org/10.3390/ijerph192215218
Chicago/Turabian StyleKushalan, Sharanya, Leonard Clinton D’Souza, Khyahrii Aloysius, Anurag Sharma, and Smitha Hegde. 2022. "Toxicity Assessment of Curculigo orchioides Leaf Extract Using Drosophila melanogaster: A Preliminary Study" International Journal of Environmental Research and Public Health 19, no. 22: 15218. https://doi.org/10.3390/ijerph192215218
APA StyleKushalan, S., D’Souza, L. C., Aloysius, K., Sharma, A., & Hegde, S. (2022). Toxicity Assessment of Curculigo orchioides Leaf Extract Using Drosophila melanogaster: A Preliminary Study. International Journal of Environmental Research and Public Health, 19(22), 15218. https://doi.org/10.3390/ijerph192215218







