Risk Association of Liver Cancer and Hepatitis B with Tree Ensemble and Lifestyle Features
Abstract
1. Introduction
2. Material and Methods
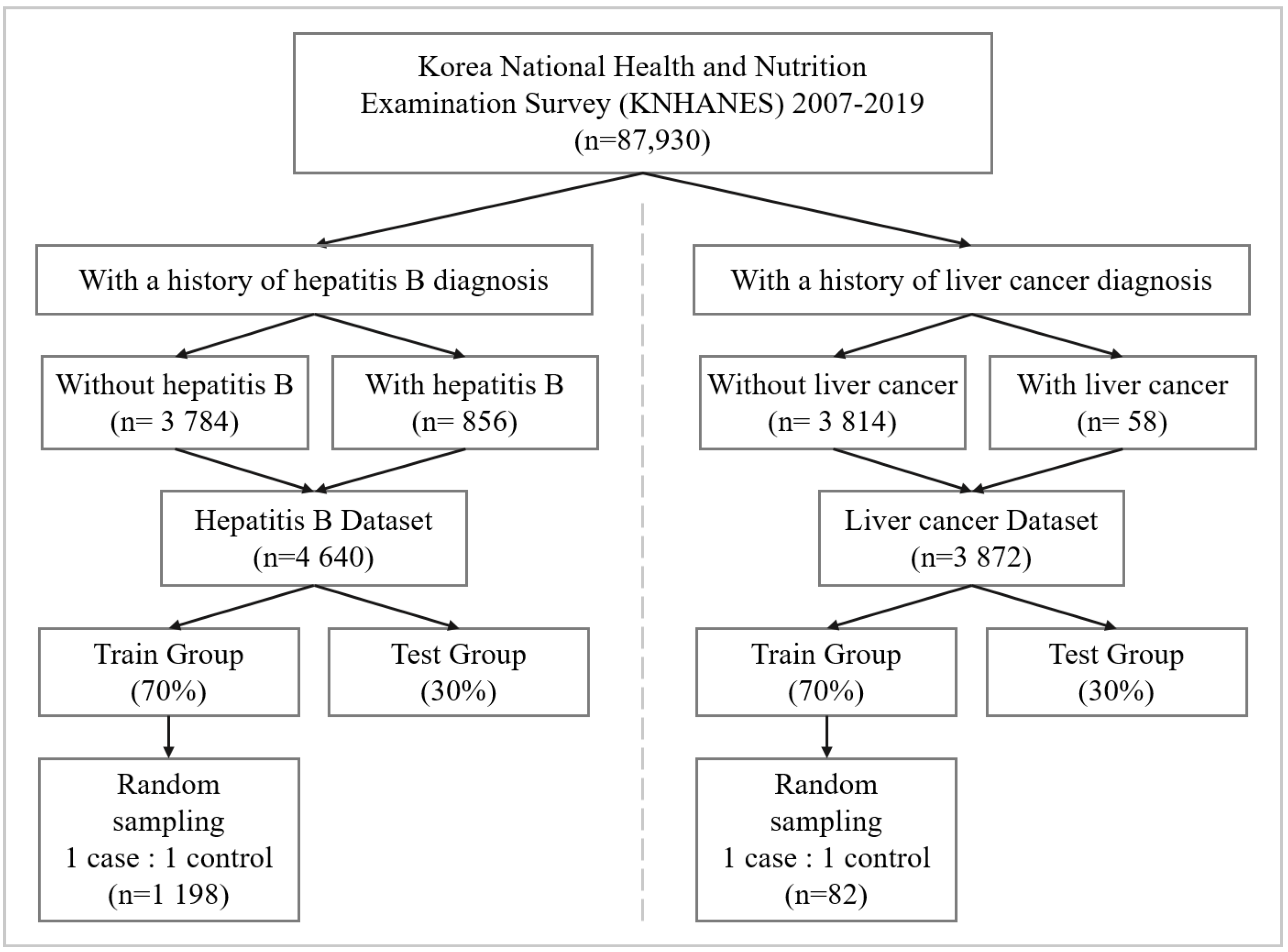
2.1. Data Source
2.2. Data Preprocessing
2.3. Risk Association Model
2.4. Performance Evaluation and Model Interpretation
3. Case Study Results
3.1. Results of Imputations
3.2. Performance Evaluation
3.3. Sensitivity Analysis
3.4. Ablation Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.S.; Irfan, M.; Chowdhury, A. Prediction of liver disease using classification algorithms. In Proceedings of the 2018 4th International Conference on Computing Communication and Automation (ICCCA), Greater Noida, India, 14–15 December 2018; pp. 1–3. [Google Scholar]
- Cornelius, C.E. Liver function. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1980; pp. 201–257. [Google Scholar]
- Schiff, L.; Schiff, E.R. Diseases of the Liver; Lippincott: Philadelphia, PA, USA, 1993; Volume 2. [Google Scholar]
- Parker, G.A.; Picut, C.A. Liver immunobiology. Toxicol. Pathol. 2005, 33, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Park, J.W. Epidemiology of liver cancer in South Korea. Clin. Mol. Hepatol. 2018, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Gwack, J.; Kang, S.; Koo, B.; Jung, S.J.; Dhamala, P.; Ko, K.P.; Lim, Y.K.; Yoo, K.Y. Viral hepatitis and liver cancer in Korea: An epidemiological perspective. Asian Pac. J. Cancer Prev. 2013, 14, 6227–6231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiollais, P.; Pourcel, C.; Dejean, A. The hepatitis B virus. Nature 1985, 317, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.J. Epidemiology and prevention of hepatitis B. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 2003; Volume 23, pp. 39–46. [Google Scholar]
- Venkatesh, R.; Balasubramanian, C.; Kaliappan, M. Development of Big Data Predictive Analytics Model for Disease Prediction using Machine learning Technique. J. Med. Syst. 2019, 43, 272. [Google Scholar] [CrossRef] [PubMed]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Dahiwade, D.; Patle, G.; Meshram, E. Designing disease prediction model using machine learning approach. In Proceedings of the 2019 3rd International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 27–29 March 2019; pp. 1211–1215. [Google Scholar]
- Shukla, S.; Gupta, D.L.; Prasad, B.R. Comparative study of recent trends on cancer disease prediction using data mining techniques. Int. J. Database Theory Appl. 2016, 9, 107–118. [Google Scholar] [CrossRef]
- Wu, D.; Cao, J.; Li, W.; Wang, X. Development of a Prediction Classifier for the Early Diagnosis of Liver Cancer. 2018. Available online: https://easychair.org/publications/preprint/sDf7 (accessed on 9 October 2018).
- Chen, K.H.; Wang, H.W.; Liu, C.M. Applying Artificial Intelligence to Survival Prediction of Hepatocellular Carcinoma Patients. In Proceedings of the 2020 4th International Conference on Deep Learning Technologies (ICDLT), Beijing, China, 10–12 July 2020; pp. 135–139. [Google Scholar]
- Ward, A.; Sarraju, A.; Chung, S.; Li, J.; Harrington, R.; Heidenreich, P.; Palaniappan, L.; Scheinker, D.; Rodriguez, F. Machine learning and atherosclerotic cardiovascular disease risk prediction in a multi-ethnic population. NPJ Digit. Med. 2020, 3, 125. [Google Scholar] [CrossRef]
- Perveen, S.; Shahbaz, M.; Keshavjee, K.; Guergachi, A. A systematic machine learning based approach for the diagnosis of non-alcoholic fatty liver disease risk and progression. Sci. Rep. 2018, 8, 2112. [Google Scholar] [CrossRef]
- Dimopoulos, A.C.; Nikolaidou, M.; Caballero, F.F.; Engchuan, W.; Sanchez-Niubo, A.; Arndt, H.; Ayuso-Mateos, J.L.; Haro, J.M.; Chatterji, S.; Georgousopoulou, E.N.; et al. Machine learning methodologies versus cardiovascular risk scores, in predicting disease risk. BMC Med. Res. Methodol. 2018, 18, 179. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, G.; Singh, R. Stark assessment of lifestyle based human disorders using data mining based learning techniques. IRBM 2017, 38, 305–324. [Google Scholar] [CrossRef]
- Ajana, S.; Cougnard-Grégoire, A.; Colijn, J.M.; Merle, B.M.; Verzijden, T.; de Jong, P.T.; Hofman, A.; Vingerling, J.R.; Hejblum, B.P.; Korobelnik, J.F.; et al. Predicting progression to advanced age-related macular degeneration from clinical, genetic, and lifestyle factors using machine learning. Ophthalmology 2021, 128, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Sollano, J.D. Factors influencing liver disease progression in chronic hepatitis B. Liver Int. 2006, 26, 23–29. [Google Scholar] [CrossRef]
- Chen, C.J.; Liang, K.Y.; Chang, A.S.; Chang, Y.C.; Lu, S.N.; Liaw, Y.F.; Chang, W.Y.; Sheen, M.C.; Lin, T.M. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology 1991, 13, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. The Seventh Korea National Health and Nutrition Examination Survey (KNHANES V-3); Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2018.
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Waljee, A.K.; Mukherjee, A.; Singal, A.G.; Zhang, Y.; Warren, J.; Balis, U.; Marrero, J.; Zhu, J.; Higgins, P.D. Comparison of imputation methods for missing laboratory data in medicine. BMJ Open 2013, 3, e002847. [Google Scholar] [CrossRef]
- Batista, G.E.; Monard, M.C. An analysis of four missing data treatment methods for supervised learning. Appl. Artif. Intell. 2003, 17, 519–533. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, J.; Zhang, Q.; Wei, X. Risk prediction of type II diabetes based on random forest model. In Proceedings of the 2017 Third International Conference on Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), Chennai, India, 27–28 February 2017; pp. 382–386. [Google Scholar]
- Li, R.; Shen, S.; Zhang, X.; Li, R.; Wang, S.; Zhou, B.; Wang, Z. Cardiovascular disease risk prediction based on random forest. In Proceedings of the The International Conference on Healthcare Science and Engineering, Guilin, China, 10–12 September 2018; pp. 31–43. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011, 48, 277–287. [Google Scholar] [CrossRef]
- Menard, S. Applied Logistic Regression Analysis; SAGE: Thousand Oaks, CA, USA, 2002; Volume 106. [Google Scholar]
- Safavian, S.R.; Landgrebe, D. A survey of decision tree classifier methodology. IEEE Trans. Syst. Man Cybern. 1991, 21, 660–674. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; McClelland, J.L.; PDP Research Group. Parallel Distributed Processing; IEEE: New York, NY, USA, 1988; Volume 1. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.Y. Lightgbm: A highly efficient gradient boosting decision tree. In Advances in Neural Information Processing Systems 30 (NIPS 2017); Curran Associates: Montreal, QC, Canada, 2017. [Google Scholar]
- Suthaharan, S. Support vector machine. In Machine Learning Models and Algorithms for Big Data Classification; Springer: Berlin/Heidelberg, Germany, 2016; pp. 207–235. [Google Scholar]
- Yala, A.; Lehman, C.; Schuster, T.; Portnoi, T.; Barzilay, R. A deep learning mammography-based model for improved breast cancer risk prediction. Radiology 2019, 292, 60–66. [Google Scholar] [CrossRef]
- Dite, G.S.; MacInnis, R.J.; Bickerstaffe, A.; Dowty, J.G.; Allman, R.; Apicella, C.; Milne, R.L.; Tsimiklis, H.; Phillips, K.A.; Giles, G.G.; et al. Breast cancer risk prediction using clinical models and 77 independent risk-associated SNPs for women aged under 50 years: Australian Breast Cancer Family Registry. Cancer Epidemiol. Prev. Biomarkers 2016, 25, 359–365. [Google Scholar] [CrossRef]
- Sipeky, C.; Talala, K.M.; Tammela, T.L.; Taari, K.; Auvinen, A.; Schleutker, J. Prostate cancer risk prediction using a polygenic risk score. Sci. Rep. 2020, 10, 17075. [Google Scholar] [CrossRef]
- Weng, S.F.; Reps, J.; Kai, J.; Garibaldi, J.M.; Qureshi, N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS ONE 2017, 12, e0174944. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, Q.; Gao, F.; Mao, D.; Li, J.; Gong, Z.; Luo, X.; Chen, G.; Li, Y.; Yang, Z.; et al. Artificial neural network-based models used for predicting 28-and 90-day mortality of patients with hepatitis B-associated acute-on-chronic liver failure. BMC Gastroenterol. 2020, 20, 75. [Google Scholar] [CrossRef]
- Adler, E.D.; Voors, A.A.; Klein, L.; Macheret, F.; Braun, O.O.; Urey, M.A.; Zhu, W.; Sama, I.; Tadel, M.; Campagnari, C.; et al. Improving risk prediction in heart failure using machine learning. Eur. J. Heart Fail. 2020, 22, 139–147. [Google Scholar] [CrossRef]
- Saltelli, A. Sensitivity analysis for importance assessment. Risk Anal. 2002, 22, 579–590. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems 30 (NIPS 2017); Curran Associates: Montreal, QC, Canada, 2017. [Google Scholar]
- Litwak, S.A.; Pang, L.; Galic, S.; Igoillo-Esteve, M.; Stanley, W.J.; Turatsinze, J.V.; Loh, K.; Thomas, H.E.; Sharma, A.; Trepo, E.; et al. JNK activation of BIM promotes hepatic oxidative stress, steatosis, and insulin resistance in obesity. Diabetes 2017, 66, 2973–2986. [Google Scholar] [CrossRef]
- Choi, J.; Han, S.; Kim, N.; Lim, Y.S. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology 2017, 66, 1454–1463. [Google Scholar] [CrossRef]
- Åberg, F.; Helenius-Hietala, J.; Puukka, P.; Jula, A. Binge drinking and the risk of liver events: A population-based cohort study. Liver Int. 2017, 37, 1373–1381. [Google Scholar] [CrossRef]
- Karpagavalli, S.; Jamuna, K.; Vijaya, M. Machine learning approach for preoperative anaesthetic risk prediction. Int. J. Recent Trends Eng. 2009, 1, 19. [Google Scholar]
- Aleksandrova, K.; Reichmann, R.; Kaaks, R.; Jenab, M.; Bueno-de Mesquita, H.B.; Dahm, C.C.; Eriksen, A.K.; Tjønneland, A.; Artaud, F.; Boutron-Ruault, M.C.; et al. Development and validation of a lifestyle-based model for colorectal cancer risk prediction: The LiFeCRC score. BMC Med. 2021, 19, 1. [Google Scholar] [CrossRef]
- Song, Y.J. The South Korean health care system. Jpn. Med. Assoc. J. 2009, 52, 206–209. [Google Scholar]
- Walker, T.Y.; Elam-Evans, L.D.; Yankey, D.; Markowitz, L.E.; Williams, C.L.; Fredua, B.; Singleton, J.A.; Stokley, S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2018. Morb. Mortal. Wkly. Rep. 2019, 68, 718. [Google Scholar] [CrossRef]
- Sinn, D.H.; Cho, E.J.; Kim, J.H.; Kim, D.Y.; Kim, Y.J.; Choi, M.S. Current status and strategies for viral hepatitis control in Korea. Clin. Mol. Hepatol. 2017, 23, 189. [Google Scholar] [CrossRef]
- Wilkins, T.; Zimmerman, D.; Schade, R.R. Hepatitis B: Diagnosis and treatment. Am. Fam. Physician 2010, 81, 965–972. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: Berlin/Heidelberg, Germany, 2013; Volume 112. [Google Scholar]
- Kabiraj, S.; Raihan, M.; Alvi, N.; Afrin, M.; Akter, L.; Sohagi, S.A.; Podder, E. Breast cancer risk prediction using XGBoost and random forest algorithm. In Proceedings of the 2020 11th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kharagpur, India, 1–3 July 2020; pp. 1–4. [Google Scholar]
- Hashem, E.M.; Aboel-fotouh, M.R. A Random Forest-Genetic algorithm integration approach for Hepatocellular Carcinoma Early prediction. Ann. Rom. Soc. Cell Biol. 2021, 25, 13500–13512. [Google Scholar]


| Type | Feature |
|---|---|
| Continuous | Age |
| Height | |
| Weight | |
| BMI | |
| Drinking age | |
| Average weekday sleep time per day | |
| Average weekend sleep time per day | |
| Total monthly household income | |
| Average weekly working hours | |
| Categorical | National health insurance type |
| Private health insurance status | |
| Hepatitis B | |
| Sex |
| Model | Parameter | Liver Cancer | Hepatitis B |
|---|---|---|---|
| Random forest | N estimators | 300 | 100 |
| Max depth | 30 | 10 | |
| Criterion | entropy | entropy | |
| Min samples split | 5 | 10 | |
| Min sample leaf | 1 | 1 | |
| Bootstrap | True | True | |
| Warm strat | True | False | |
| Max features | auto | auto |
| Model | Parameter | Liver Cancer | Hepatitis B |
|---|---|---|---|
| Decision tree | Max depth | 10 | 10 |
| Criterion | entropy | entropy | |
| Min samples split | 2 | 5 | |
| Min sample leaf | 1 | 1 | |
| Logistic regression | Class weight | none | balanced |
| Max iter | 50 | 50 | |
| ANN | Slover | sgd | adam |
| Activation | tanh | tanh | |
| Alpha | 0.0001 | 0001 | |
| Learning rate | constant | constant | |
| Hidden layer size | (100, 50) | (50, 25) | |
| XGboost | Eta | 0.1 | 0.3 |
| Min child weight | 0 | 5 | |
| Max depth | 6 | 6 | |
| Gamma | 0 | 5 | |
| Colsample bytree | 0.5 | 0.6 | |
| N estimators | 100 | 100 | |
| LightGBM | Num leaves | 31 | 5 |
| Max depth | −1 | −1 | |
| N estimator | 1 | 100 | |
| Learning rate | 0.05 | 0.1 | |
| Min data in leaf | 10 | 20 | |
| Num iteration | 100 | 100 | |
| Colsample bytree | 0.6 | 1.0 | |
| Boosting | dart | gbdt | |
| SVM | Kernel | sigmoid | rbf |
| Degree | 1 | 1 | |
| Gamma | auto | scale |
| Risk Association Model for Liver Cancer | |||
|---|---|---|---|
| Simple Imputation | K-NN Imputation | Mice Imputation | |
| Random forest | 0.8317 (0.0345) | 0.8062 (0.0501) | 0.8248 (0.0276) |
| Decision tree | 0.7671 (0.0531) | 0.7546 (0.0569) | 0.7710 (0.0543) |
| Logistic regression | 0.8211 (0.0447) | 0.8186 (0.0469) | 0.8199 (0.0539) |
| ANN | 0.8129 (0.0431) | 0.8066 (0.0463) | 0.8129 (0.0393) |
| XGboost | 0.8153 (0.0433) | 0.7788 (0.0587) | 0.8154 (0.0411) |
| lightGBM | 0.7992 (0.0464) | 0.7636 (0.0419) | 0.7925 (0.0447) |
| SVM | 0.8098 (0.0555) | 0.8025 (0.0581) | 0.8055 (0.0470) |
| Risk Association Model for Hepatitis B | |||
|---|---|---|---|
| Simple Imputation | K-NN Imputation | Mice Imputation | |
| Random forest | 0.8567 (0.0124) | 0.7050 (0.0147) | 0.8632 (0.0113) |
| Decision tree | 0.7818 (0.0132) | 0.6295 (0.0178) | 0.7947 (0.0157) |
| Logistic regression | 0.6384 (0.0144) | 0.6313 (0.0147) | 0.6338 (0.0137) |
| ANN | 0.8015 (0.0121) | 0.6548 (0.0120) | 0.7990 (0.0150) |
| XGboost | 0.8469 (0.0108) | 0.7505 (0.0119) | 0.8611 (0.0094) |
| lightGBM | 0.8534 (0.0115) | 0.7520 (0.0126) | 0.8636 (0.0098) |
| SVM | 0.7401 (0.0149) | 0.6560 (0.0167) | 0.7235 (0.0127) |
| Liver Cancer Risk Association | Hepatitis B Risk Association | |||
|---|---|---|---|---|
| AUC | Accuracy | AUC | Accuracy | |
| Random forest | 0.8367 (0.0357) | 0.8190 (0.0384) | 0.8803 (0.0030) | 0.9245 (0.0060) |
| Decision tree | 0.7921 (0.0568) | 0.7807 (0.0481) | 0.8224 (0.0206) | 0.8423 (0.0305) |
| Logistic regression | 0.8211 (0.0447) | 0.8302 (0.0289) | 0.6341 (0.0136) | 0.6541 (0.0159) |
| ANN | 0.8206 (0.0526) | 0.8312 (0.0311) | 0.8306 (0.0142) | 0.8466 (0.0111) |
| XGboost | 0.8260 (0.0392) | 0.8075 (0.0409) | 0.8778 (0.0103) | 0.9309 (0.0057) |
| LightGBM | 0.8234 (0.0474) | 0.8118 (0.0485) | 0.8764 (0.0117) | 0.9299 (0.0068) |
| SVM | 0.8130 (0.0507) | 0.8408 (0.0351) | 0.7235 (0.0127) | 0.6919 (0.0137) |
| p-Value | ||
|---|---|---|
| Liver Cancer Risk Association | Hepatitis B Risk Association | |
| Decision tree | 0.0000 | 0.0000 |
| Logistic regression | 0.0210 | 0.0000 |
| ANN | 0.0336 | 0.0000 |
| XGboost | 0.0074 | 0.0000 |
| LightGBM | 0.0408 | 0.0022 |
| SVM | 0.0042 | 0.0000 |
| Permutated Feature | Mean of AUC |
|---|---|
| - | 0.8803 (0.0030) |
| Age | 0.8750 (0.0069) |
| Sex | 0.8799 (0.0050) |
| Height | 0.8795 (0.0051) |
| Weight | 0.8803 (0.0051) |
| BMI | 0.8788 (0.0062) |
| Total monthly household income | 0.8773 (0.0070) |
| Average weekly working hours | 0.8664 (0.0060) |
| Average weekday sleep time per day | 0.8516 (0.0090) |
| Average weekend sleep time per day | 0.8492 (0.0087) |
| Drinking age | 0.8710 (0.0154) |
| Private health insurance status | 0.8767 (0.0064) |
| National health insurance type | 0.8790 (0.0054) |
| Feature | Mean of AUC |
|---|---|
| - | 0.8367 (0.0357) |
| Age | 0.8126 (0.0483) |
| Sex | 0.8216 (0.0368) |
| Height | 0.8327 (0.0376) |
| Weight | 0.8255 (0.0429) |
| BMI | 0.8253 (0.0424) |
| Total monthly household income | 0.8229 (0.0444) |
| Average weekly working hours | 0.8312 (0.0352) |
| Average weekday sleep time per day | 0.8288 (0.0429) |
| Average weekend sleep time per day | 0.8301 (0.0428) |
| Drinking age | 0.8307 (0.0378) |
| Private health insurance status | 0.8327 (0.0374) |
| Hepatitis B | 0.7935 (0.0379) |
| National health insurance type | 0.8344 (0.0374) |
| Feature | SHAP Value |
|---|---|
| Age | 0.0476 |
| Sex | 0.0093 |
| Height | 0.0089 |
| Weight | 0.0122 |
| BMI | 0.0116 |
| Total monthly household income | 0.0482 |
| Average weekly working hours | 0.0349 |
| Average weekday sleep time per day | 0.1240 |
| Average weekend sleep time per day | 0.1175 |
| Drinking age | 0.0190 |
| Private health insurance status | 0.0290 |
| National health insurance type | 0.0019 |
| Feature | SHAP Value |
|---|---|
| Age | 0.0940 |
| Sex | 0.0090 |
| Height | 0.0159 |
| Weight | 0.0219 |
| BMI | 0.0147 |
| Total monthly household income | 0.0421 |
| Average weekly working hours | 0.0499 |
| Average weekday sleep time per day | 0.0327 |
| Average weekend sleep time per day | 0.0748 |
| Drinking age | 0.0200 |
| Private health insurance status | 0.0229 |
| Hepatitis B | 0.0776 |
| National health insurance type | 0.0021 |
| Sensitivity Analysis | SHAP |
|---|---|
| Average weekend sleep time per day | Average weekday sleep time per day |
| Average weekday sleep time per day | Average weekend sleep time per day |
| Average weekly working hours | Total monthly household income |
| Drinking age | Age |
| Age | Average weekly working hours |
| Sensitivity Analysis | SHAP |
|---|---|
| Hepatitis B | Age |
| Age | Hepatitis B |
| Sex | Average weekend sleep time per day |
| Total monthly household income | Average weekly working hours |
| BMI | Total monthly household income |
| Liver Cancer | Non-Liver Cancer | |||
|---|---|---|---|---|
| Feature | Value | SHAP Value | Value | SHAP Value |
| Age | 72 | 0.1451 | 53 | −0.0269 |
| Sex | Man | 0.0087 | Man | 0.0094 |
| Height | 169.7 | 0.0180 | 168.3 | −0.0088 |
| Weight | 71 | 0.0193 | 70.4 | 0.0072 |
| BMI | 24.7 | 0.0042 | 24.9 | −0.0104 |
| Total monthly household income | 250 | 0.0172 | 390 | −0.0281 |
| Average weekly working hours | 21 | 0.0206 | 40 | 0.0286 |
| Average weekday sleep time per day | 420 | 0.0319 | 440 | 0.0134 |
| Average weekend sleep time per day | 480 | 0.0590 | 440 | −0.0268 |
| Drinking age | 26 | 0.0217 | 21 | 0.0153 |
| Private health insurance status | Subscriber | −0.0096 | Subscriber | −0.0326 |
| Hepatitis B | Yes | 0.1450 | No | −0.0639 |
| National health insurance type | Employee | −0.0015 | Employee | −0.0073 |
| Hepatitis B | Non-Hepatitis B | |||
|---|---|---|---|---|
| Feature | Value | SHAP Value | Value | SHAP Value |
| Age | 52 | 0.0295 | 57 | 0.0093 |
| Sex | Man | 0.0063 | Woman | −0.0121 |
| Height | 164.1 | 0.0063 | 155.9 | −0.0204 |
| Weight | 56.7 | 0.0138 | 58.6 | −0.0038 |
| BMI | 21.1 | 0.0036 | 24.1 | −0.0027 |
| Total monthly household income | 126.7 | 0.0846 | 166.7 | 0.0159 |
| Average weekly working hours | 20 | −0.0770 | 60 | −0.0315 |
| Average weekday sleep time per day | 426.5 | 0.1811 | 540 | −0.1255 |
| Average weekend sleep time per day | 460.7 | 0.2252 | 540 | −0.1111 |
| Drinking age | 16 | −0.0034 | 45 | 0.0186 |
| Private health insurance status | Subscriber | −0.0070 | Subscriber | −0.0246 |
| National health insurance type | Non-employee | 0.0011 | Non-employee | −0.0058 |
| Train Dataset Ratio | 10% | 20% | 30% | 40% | 50% | 60% | 70% (Default) |
|---|---|---|---|---|---|---|---|
| Random forest | 0.8288 (0.0025) | 0.8513 (0.0043) | 0.8614 (0.0023) | 0.8691 (0.0038) | 0.8732 (0.0044) | 0.8771 (0.0022) | 0.8803 (0.0030) |
| Decision tree | 0.7723 (0.0269) | 0.7854 (0.0241) | 0.8039 (0.0133) | 0.8066 (0.0130) | 0.8161 (0.0147) | 0.8172 (0.0135) | 0.8224 (0.0206) |
| Logistic regression | 0.6040 (0.0173) | 0.6223 (0.0101) | 0.6274 (0.0119) | 0.6309 (0.0086) | 0.6351 (0.0100) | 0.6343 (0.0121) | 0.6341 (0.0136) |
| ANN | 0.6633 (0.0273) | 0.7390 (0.0253) | 0.7808 (0.0161) | 0.8055 (0.0105) | 0.8148 (0.0084) | 0.8250 (0.0091) | 0.8306 (0.0143) |
| XGboost | 0.8157 (0.0108) | 0.8415 (0.0075) | 0.8515 (0.0066) | 0.8567 (0.0066) | 0.8610 (0.0074) | 0.8647 (0.0099) | 0.8778 (0.0103) |
| Light GBM | 0.8253 (0.0167) | 0.8497 (0.0098) | 0.8597 (0.0086) | 0.8659 (0.0079) | 0.8678 (0.0071) | 0.8712 (0.0107) | 0.8764 (0.0117) |
| SVM | 0.6576 (0.0165) | 0.6855 (0.0119) | 0.7001 (0.0092) | 0.7083 (0.0106) | 0.7156 (0.0129) | 0.7202 (0.0119) | 0.7235 (0.0127) |
| Train Dataset Ratio | 10% | 20% | 30% | 40% | 50% | 60% | 70% (Default) |
|---|---|---|---|---|---|---|---|
| Random forest | 0.7963 (0.0102) | 0.8011 (0.0371) | 0.8151 (0.0276) | 0.8191 (0.0321) | 0.8199 (0.0326) | 0.8296 (0.0418) | 0.8367 (0.0357) |
| Decision tree | 0.6855 (0.0941) | 0.7345 (0.0628) | 0.7523 (0.0531) | 0.7541 (0.0536) | 0.7557 (0.0493) | 0.7574 (0.0582) | 0.7921 (0.0568) |
| Logistic regression | 0.7343 (0.0575) | 0.7847 (0.0312) | 0.7981 (0.0356) | 0.7984 (0.0371) | 0.7951 (0.0529) | 0.7986 (0.0440) | 0.8211 (0.0447) |
| ANN | 0.7362 (0.0625) | 0.7770 (0.0423) | 0.7970 (0.0326) | 0.8023 (0.0308) | 0.8136 (0.0435) | 0.8141 (0.0436) | 0.8206 (0.0526) |
| XGboost | 0.7428 (0.0545) | 0.7884 (0.0312) | 0.8045 (0.0301) | 0.8083 (0.0304) | 0.8074 (0.0375) | 0.8207 (0.0283) | 0.8026 (0.0392) |
| Light GBM | 0.5000 (0.0000) | 0.7194 (0.0683) | 0.7564 (0.0410) | 0.7923 (0.0396) | 0.8104 (0.0410) | 0.8267 (0.0338) | 0.8234 (0.0474) |
| SVM | 0.7319 (0.0482) | 0.7745 (0.0490) | 0.7891 (0.0304) | 0.7943 (0.0408) | 0.7965 (0.0452) | 0.8005 (0.0569) | 0.8130 (0.0507) |
| p-Value | ||
|---|---|---|
| Liver Cancer Risk Association | Hepatitis B Risk Association | |
| Decision tree | 0.0000 | 0.0000 |
| Logistic regression | 0.0000 | 0.0000 |
| ANN | 0.0000 | 0.0000 |
| XGboost | 0.0000 | 0.0000 |
| LightGBM | 0.0000 | 0.0000 |
| SVM | 0.0000 | 0.0000 |
| Liver Cancer | Hepatitis B | |
|---|---|---|
| Random forest | 0.7680 (0.0535) | 0.5350 (0.0579) |
| Decision tree | 0.7430 (0.0577) | 0.5437 (0.0669) |
| Logistic regression | 0.7518 (0.0580) | 0.6019 (0.0632) |
| ANN | 0.7106 (0.0625) | 0.5480 (0.0591) |
| XGboost | 0.7944 (0.0531) | 0.5472 (0.0444) |
| LightGBM | 0.8037 (0.0482) | 0.5564 (0.0450) |
| SVM | 0.6949 (0.0696) | 0.5183 (0.0344) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, E.; Kim, Y. Risk Association of Liver Cancer and Hepatitis B with Tree Ensemble and Lifestyle Features. Int. J. Environ. Res. Public Health 2022, 19, 15171. https://doi.org/10.3390/ijerph192215171
Koh E, Kim Y. Risk Association of Liver Cancer and Hepatitis B with Tree Ensemble and Lifestyle Features. International Journal of Environmental Research and Public Health. 2022; 19(22):15171. https://doi.org/10.3390/ijerph192215171
Chicago/Turabian StyleKoh, Eunji, and Younghoon Kim. 2022. "Risk Association of Liver Cancer and Hepatitis B with Tree Ensemble and Lifestyle Features" International Journal of Environmental Research and Public Health 19, no. 22: 15171. https://doi.org/10.3390/ijerph192215171
APA StyleKoh, E., & Kim, Y. (2022). Risk Association of Liver Cancer and Hepatitis B with Tree Ensemble and Lifestyle Features. International Journal of Environmental Research and Public Health, 19(22), 15171. https://doi.org/10.3390/ijerph192215171









