Physiological Responses of a Diazotrophic Cyanobacterium to Acidification of Paddy Floodwater: N2 Fixation, Photosynthesis, and Oxidative–Antioxidative Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterial Strain
2.2. Simulation of Paddy Floodwater
2.3. Experimental Design
2.4. Determination of Chlorophyll Fluorescence Transients (CFT)
2.5. Determination of Respiration and Photosynthetic Oxygen Evolution
2.6. Dinitrogen–Fixation Rate Determination
2.7. Intracellular pH Determination
2.8. Extraction of Enzymatic and Non-Enzymatic Antioxidants
2.9. Activities of Enzymatic Antioxidant
2.10. Determination of Non-Enzymatic Antioxidants
2.11. Determinations of ROS and Thiobarbituric Acid Reactive Substances (TBARS)
2.12. Statistic Analyses
3. Results
3.1. Respiration Rate and Oxygen Evolution Rate
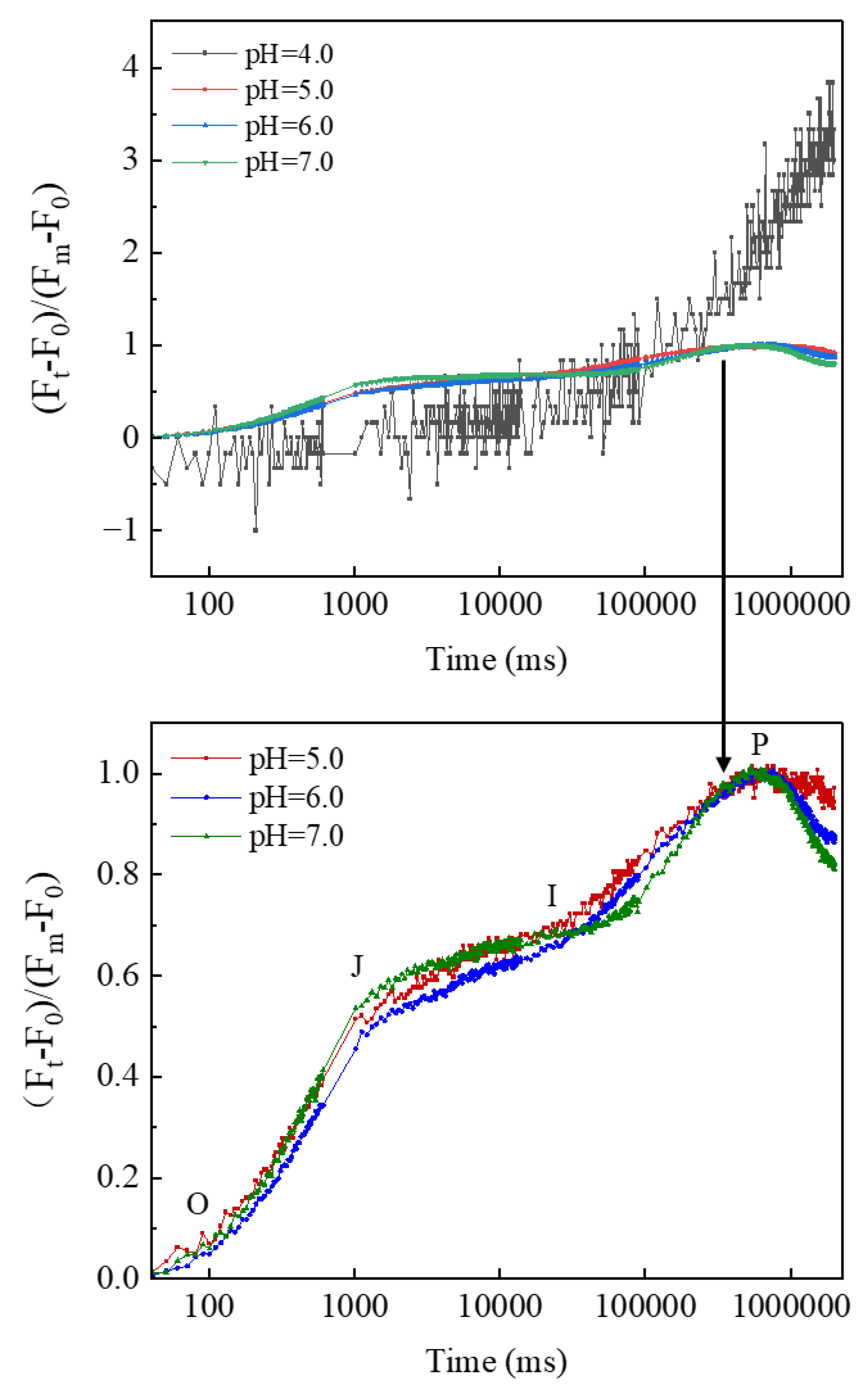
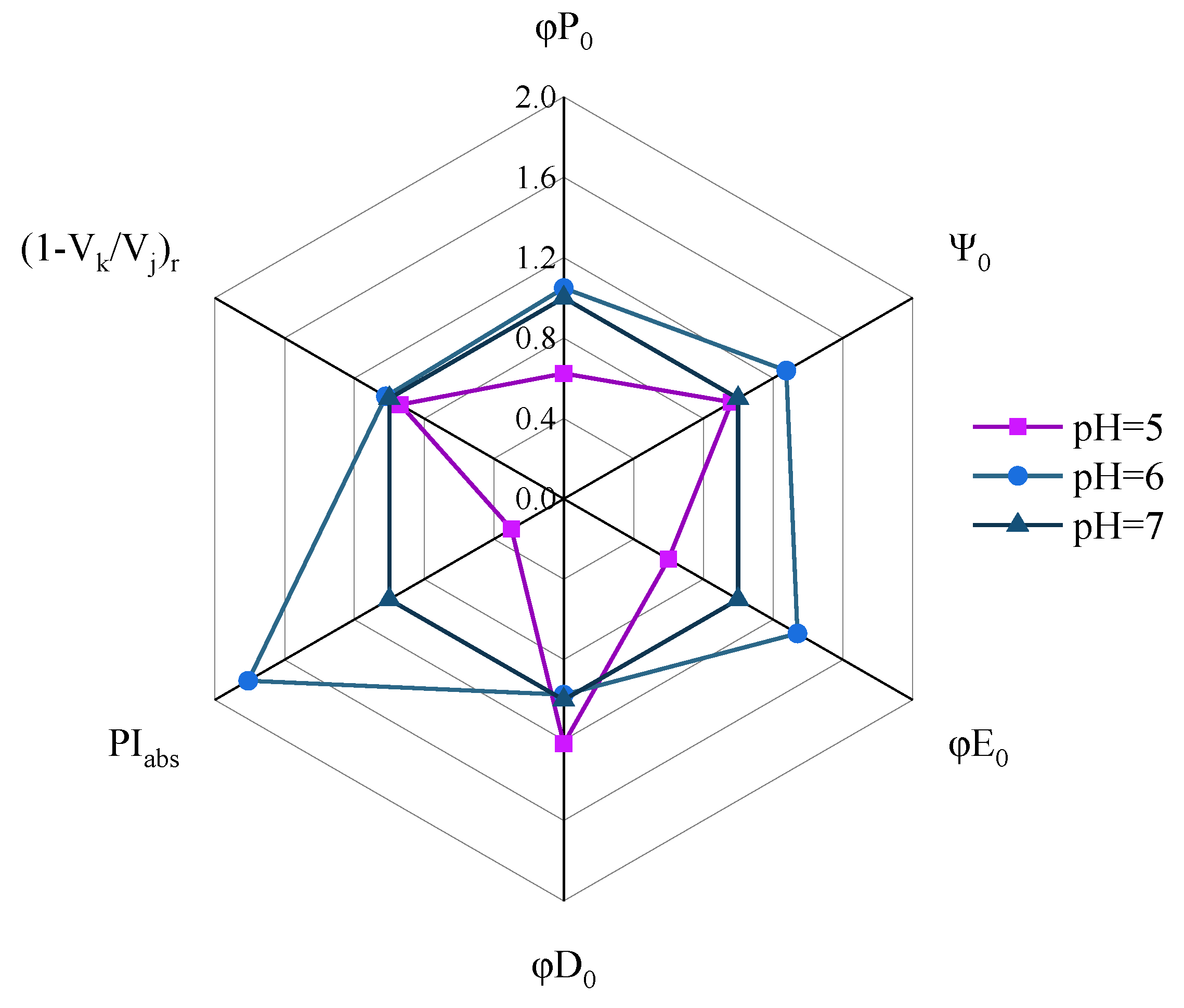
3.2. Characteristics of CFT
3.3. Intracellular pH and N2-Fixation Rate
3.4. Activities of Antioxidative Enzymes
3.5. Contents of Non-Enzymatic Antioxidants
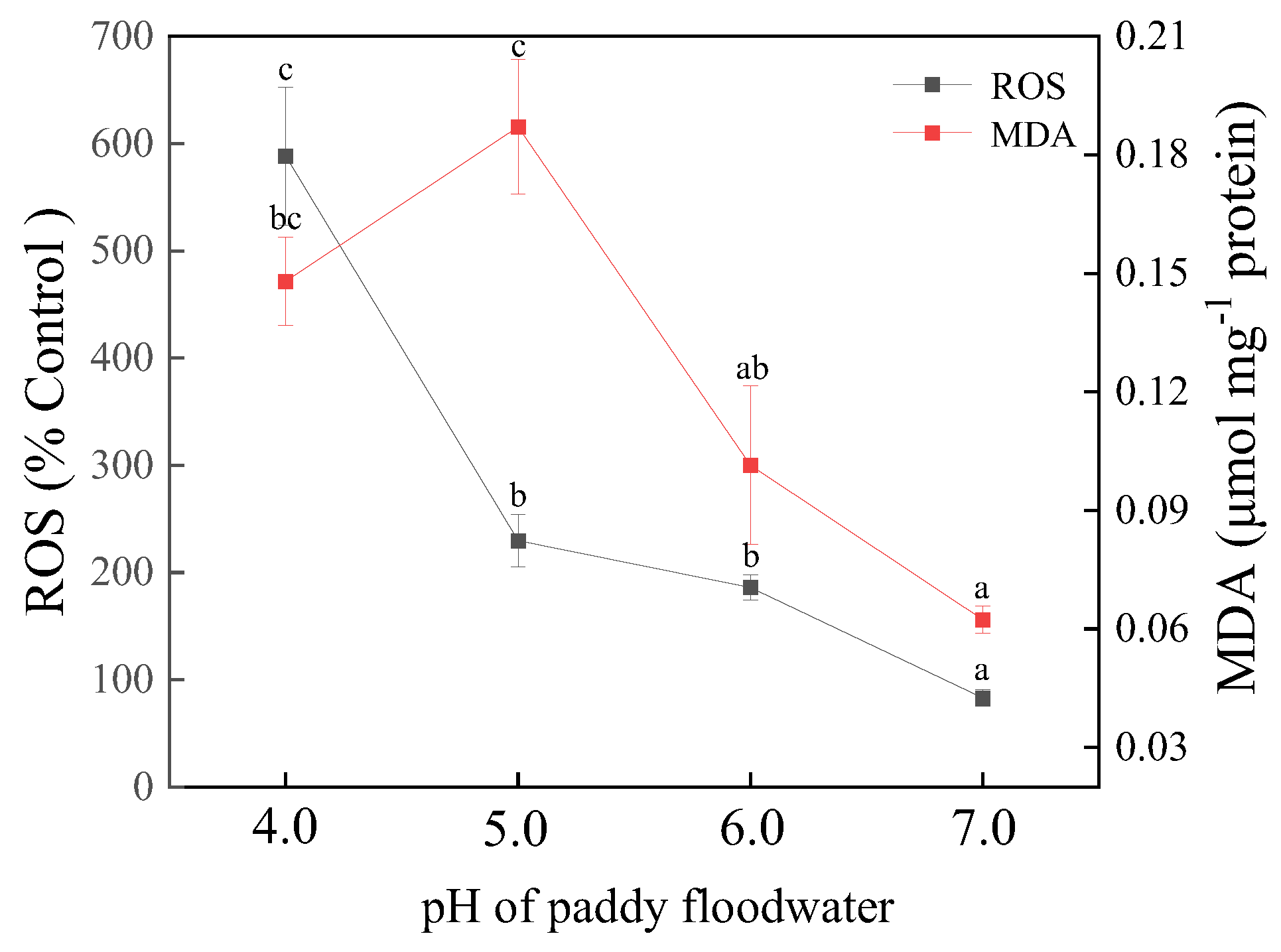
3.6. Contents of ROS and MDA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, T.; Lupwayi, N.; Marc, S.A.; Siddique, K.H.M.; Bainard, L.D. Anthropogenic drivers of soil microbial communities and impacts on soil biological functions in agroecosystems. Glob. Ecol. Conserv. 2021, 27, e01521. [Google Scholar] [CrossRef]
- Shi, R.Y.; Ni, N.; Nkoh, J.N.; Dong, Y.; Zhao, W.R.; Pan, X.Y.; Li, J.Y.; Xu, R.K.; Qian, W. Biochar retards Al toxicity to maize (Zea mays L.) during soil acidification: The effects and mechanisms. Sci. Total Environ. 2020, 719, 137448. [Google Scholar] [CrossRef] [PubMed]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Oanh, D.T.; Thuy, D.T.; Lien, N.T.T.; Dang, T.M.A.; Le, T.P.Q. Isolation and screening producing growth regulator cyanobacteria strains. Vietnam J. Biotechnol. 2020, 18, 571–579. [Google Scholar] [CrossRef]
- Zhang, J.L.; Song, X.N.; Wei, H.; Zhou, W.C.; Peng, C.R.; Li, D.H. Effect of substituting nitrogen fertilizer with nitrogen-fixing cyanobacteria on yield in a double-rice cropping system in southern China. J. Appl. Phycol. 2021, 33, 2221–2232. [Google Scholar] [CrossRef]
- Song, X.N.; Zhang, J.L.; Peng, C.R.; Li, D.H. Replacing nitrogen fertilizer with nitrogen-fixing cyanobacteria reduced nitrogen leaching in red soil paddy fields. Agr. Ecosyst. Environ. 2021, 312, 107320. [Google Scholar] [CrossRef]
- Hu, T.; Chen, A.W.; Jiang, Y.X.; Sun, C.M.; Luo, S.; Shao, J.H. Application of a newly recorded diazotrophic cyanobacterium in acidified and Cd contaminated paddy soil: Promotes rice yield and decreases Cd accumulation. Sci. Total Environ. 2022, 814, 152630. [Google Scholar] [CrossRef]
- Iida, Y.; Takatani, Y.; Kojima, A.; Ishii, M. Global trends of ocean CO2 sink and ocean acidification: An observation-based reconstruction of surface ocean inorganic carbon variables. J. Oceanogr. 2020, 77, 323–358. [Google Scholar] [CrossRef]
- Hong, H.; Shen, R.; Zhang, F.; Wen, Z.; Chang, S.; Lin, W.; Kranz, S.A.; Luo, Y.W.; Morel, F.M.M. The complex effects of ocean acidification on the prominent N2-fixing cyanobacterium Trichodesmium. Science 2017, 356, 527–531. [Google Scholar] [CrossRef]
- Kolzau, S.; Dolman, A.M.; Voss, M.; Wiedner, C. The response of nitrogen fixing cyanobacteria to a reduction in nitrogen loading. Int. Rev. Hydrobiol. 2018, 103, 5–14. [Google Scholar] [CrossRef]
- Zavřel, T.; Schoffman, H.; Lukeš, M.; Fedorko, J.; Keren, N.; Červený, J. Monitoring fitness and productivity in cyanobacteria batch cultures. Algal Res. 2021, 56, 102328. [Google Scholar] [CrossRef]
- Lin, Y.Q.; Chen, A.W.; Wu, G.Y.; Peng, L.; Xu, Z.C.; Shao, J.H. Growth, microcystins synthesis, and cell viability of Microcystis aeruginosa FACHB905 to dissolved organic matter originated from cattle manure. Int. Biodeter. Biodegr. 2017, 118, 126–133. [Google Scholar] [CrossRef]
- Schmitt, F.J.; Renger, G.; Friedrich, T.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Los, D.A.; Kuznestov, V.V.; Allakhverdiev, S.I. Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef]
- Brutemark, A.; Engström-Öst, J.; Vehmaa, A.; Gorokhova, E. Growth, toxicity and oxidative stress of a cultured cyanobacterium (Dolichospermum sp.) under different CO2/pH and temperature conditions. Phycol. Res. 2015, 63, 56–63. [Google Scholar] [CrossRef]
- Rippka, R.; Desrulles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignment, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1977, 79, 544–552. [Google Scholar] [CrossRef]
- Beauchamp, C.; Frodovich, I. Superoxide dismutase: Improved assays and an assays applicable acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavengedfg by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Chen, J.X.; Wang, X.F. Guide in Plant Physiological Experiments; South China University of Technology Press: Guangzhou, China, 2006; pp. 76–77. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.Y.; Li, F.M. Physiological and biochemical effects of allelochemical ethyl 2-methyl acetoacetate (EMA) on cyanobacterium Microcystis aeruginosa. Ecotox. Environ. Safe. 2008, 71, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Del, R.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Bartling, J.; Kotzerke, A.; Mai, M.; Esperschütz, J.; Buegger, F.; Schloter, M.; Wilke, B.M. Microbial community structure and function during abnormal curve development of substrate-induced respiration measurements. Chemosphere 2009, 77, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.H.; Yu, G.L.; Wang, Z.J.; Wu, Z.X.; Peng, X.; Li, R.H. Towards clarification of the inhibitory mechanism of wheat bran leachate on Microcystis aeruginosa NIES-843 (cyanobacteria): Physiological responses. Ecotoxicology 2010, 19, 1634–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, J.H.; Wu, X.Q.; Li, R.H. Physiological Responses of Microcystis aeruginosa PCC7806 to nonanoic acid Stress. Environ. Toxicol. 2009, 24, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Chen, A.W.; Li, J.L.; Liu, D.M.; Shao, J.H. Control of Microcystis (Cyanobacteria) using the fruit of Macleaya cordata: From laboratory experiment to in situ field test. Phycologia 2017, 56, 382–389. [Google Scholar] [CrossRef]
- Shao, J.H.; He, Y.X.; Li, F.; Zhang, H.L.; Chen, A.W.; Luo, S.; Gu, J.D. Growth inhibition and possible mechanism of oleamide against the toxin-producing cyanobacterium Microcystis aeruginosa NIES-843. Ecotoxicology 2016, 25, 225–233. [Google Scholar] [CrossRef]
- Christen, D.; Schönmann, S.; Jermini, M.; Strasser, R.J.; Defago, G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, D.; Xu, X.T.; Gao, X.X.; Sun, X.; Xu, N.J. Physiological responses of a green algae (Ulva prolifera) exposed to simulated acid rain and decreased salinity. Photosynthetica 2017, 55, 623–629. [Google Scholar] [CrossRef]
- Debnath, B.; Hussain, M.; Irshad, M.; Mitra, S.; Li, M.; Liu, S.; Qiu, D. Exogenous melatonin mitigates acid rain stress to tomato plants through modulation of leaf ultrastructure, photosynthesis and antioxidant potential. Molecules 2018, 23, 388. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 2, a000315. [Google Scholar] [CrossRef] [PubMed]
- Cardona, T.; Battchikova, N.; Zhang, P.; Stensjö, K.; Aro, E.-M.; Lindblad, P.; Magnuson, A. Electron transfer protein complexes in the thylakoid membranes of heterocysts from the cyanobacterium Nostoc punctiforme. Biochim. Biophys. Acta 2009, 1787, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Kulasooriya, S.A.; Magana-Arachchi, D.N. Nitrogen fixing cyanobacteria: Their diversity, ecology and utilization with special reference to rice cultivation. J. Natl. Sci. Found Sri. 2016, 44, 111–128. [Google Scholar] [CrossRef]
- Thiel, T.; Hartnett, T.; Pakrasi, H.B. Examination of photosystem II in heterocysts of the cyanobacterium Nostoc sp. ATCC 29150. In Current Research in Photosynthesis; Baltscheffsky, M., Ed.; Springer: Dordrecht, The Netherlands, 1990; pp. 291–294. [Google Scholar]
- ShylajaNaciyar, M.; Karthick, L.; Prakasam, P.A.; Deviram, G.; Uma, L.; Prabaharan, D.; Saha, S.K. Diversity of glutathione S-transferases (GSTs) in cyanobacteria with reference to their structures, substrate recognition and catalytic functions. Microorganisms 2020, 8, 712. [Google Scholar] [CrossRef]
- Kammerscheit, X.; Chauvat, F.; Cassier-Chauvat, C. First in vivo evidence that glutathione-S-transferase operates in photo-oxidative stress in cyanobacteria. Front. Microbiol. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Pandey, T.; Singh, S.K.; Chhetri, G.; Tripathi, T.; Singh, A.K. Characterization of a highly pH stable chi-class glutathione S-transferase from Synechocystis PCC 6803. PLoS ONE 2017, 10, e0126811. [Google Scholar] [CrossRef]
- Hamed, S.M.; Hassan, S.H.; Selim, S.; Kumar, A.; Khalaf, S.M.H.; Wadaan, M.A.M.; Hozzein, W.N.; Abdelgawad, H. Physiological and biochemical responses to aluminum-induced oxidative stress in two cyanobacterial species. Environ. Pollut. 2019, 251, 961–969. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.Y.; Xie, X.; Li, F.M. Responses of enzymatic antioxidants and non-enzymatic antioxidants in the cyanobacterium Microcystis aeruginosa to the allelochemical ethyl 2-methyl acetoacetate (EMA) isolated from reed (Phragmites communis). J. Plant Physiol. 2008, 165, 1264–1273. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, S.M. Regulation of insecticide toxicity by kinetin in two paddy field cyanobacteria: Physiological and biochemical assessment. Environ. Pollut. 2020, 259, 113806. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, A.; Peng, L.; Luo, S.; Zeng, Q.; Shao, J. Physiological characteristics and toxin production of Microcystis aeruginosa (Cyanobacterium) in response to DOM in anaerobic digestion effluent. Sci. Total Environ. 2019, 685, 902–910. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Häder, D.P. Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem. Photobiol. 2002, 1, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. The rise and fall of photosynthesis: Hormetic dose response in plants. J. For. Res. 2021, 32, 889–898. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Xiao, P.; Li, J.; He, Y.; Shao, J. Physiological Responses of a Diazotrophic Cyanobacterium to Acidification of Paddy Floodwater: N2 Fixation, Photosynthesis, and Oxidative–Antioxidative Characteristics. Int. J. Environ. Res. Public Health 2022, 19, 15070. https://doi.org/10.3390/ijerph192215070
Yan Q, Xiao P, Li J, He Y, Shao J. Physiological Responses of a Diazotrophic Cyanobacterium to Acidification of Paddy Floodwater: N2 Fixation, Photosynthesis, and Oxidative–Antioxidative Characteristics. International Journal of Environmental Research and Public Health. 2022; 19(22):15070. https://doi.org/10.3390/ijerph192215070
Chicago/Turabian StyleYan, Qiong, Peng Xiao, Jun Li, Yaxian He, and Jihai Shao. 2022. "Physiological Responses of a Diazotrophic Cyanobacterium to Acidification of Paddy Floodwater: N2 Fixation, Photosynthesis, and Oxidative–Antioxidative Characteristics" International Journal of Environmental Research and Public Health 19, no. 22: 15070. https://doi.org/10.3390/ijerph192215070
APA StyleYan, Q., Xiao, P., Li, J., He, Y., & Shao, J. (2022). Physiological Responses of a Diazotrophic Cyanobacterium to Acidification of Paddy Floodwater: N2 Fixation, Photosynthesis, and Oxidative–Antioxidative Characteristics. International Journal of Environmental Research and Public Health, 19(22), 15070. https://doi.org/10.3390/ijerph192215070






