Streptococcus sobrinus as a Predominant Oral Bacteria Related to the Occurrence of Dental Caries in Polish Children at 12 Years Old
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
- A decayed tooth (DT) defines a tooth in which a cavity could clearly be seen or a lesion could be felt with an explorer in a pit or fissure or on a smooth surface. It also includes temporary fillings in the teeth.
- Missing tooth (MT) defines a tooth that has been lost or extracted for dental caries, not at replacement age. Third molars were excluded.
- Filled tooth (FT) defines a tooth with one or more permanent restorations and no cavity anywhere on the tooth.
2.2. Sample Collection
2.3. Genomic DNA Isolation: Positive Control Microorganisms
2.4. Real-Time PCR Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Featherstone, J. Dental caries: A dynamic disease process. Aust. Dent. J. 2008, 53, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health Indicators for 2000. Dental Caries at 12 Years; 850503, 0373V/000A; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Robert, S.H.; Amid, I.; Nigel, P.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.; Kim, N.; Kim, J.-R.; Yoo, D.; Oh, D.-H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Yang, F.; Zeng, X.; Ning, K.; Liu, K.-L.; Lo, C.-C.; Wang, W.; Chen, J.; Wang, D.; Huang, R.; Chang, X.; et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012, 6, 1–10. [Google Scholar] [CrossRef]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- WHO Oral Health Surveys, 5th ed.; WHO: Geneva, Switzerland, 2013.
- World Health Organisation. Oral Health Surveys. Basic Methods, 3rd ed.; WHO: Geneva, Switzerland, 1987. [Google Scholar]
- Choi, E.-J.; Lee, S.-H.; Kim, Y.-J. Quantitative real-time polymerase chain reaction for Streptococcus mutans and Streptococcus sobrinus in dental plaque samples and its association with early childhood caries. Int. J. Paediatr. Dent. 2009, 19, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Haarman, M.; Knol, J. Quantitative Real-Time PCR Analysis of Fecal Lactobacillus Species in Infants Receiving a Prebiotic Infant Formula. Appl. Environ. Microbiol. 2006, 72, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, T.; Zhu, P.; Sun, Z.; Li, S.; Li, F.; Zhang, Y.; Tan, K.; Lu, J.; Yuan, R.; et al. Quantitative Analysis of Salivary Oral Bacteria Associated with Severe Early Childhood Caries and Construction of Caries Assessment Model. Sci. Rep. 2020, 10, 6365. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, E.; Kang, J.; Kim, H.-J.; Lee, J.-Y.; Choi, J.; Joo, J.-Y. Real-time PCR quantification of 9 periodontal pathogens in saliva samples from periodontally healthy Korean young adults. J. Periodontal Implant Sci. 2018, 48, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-N.; Lim, Y.K.; Kook, J.-K. Development of quantitative real-time PCR primers for detecting 42 oral bacterial species. Arch. Microbiol. 2013, 195, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, F.; Wang, R. Dental caries experience and related risk indicators of 12-year-old students in Jilin, China. Medicine 2020, 99, e20988. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yan, X.; Song, Y.; Ma, S.; Ma, J.; Zhu, G. Trends of dental caries in permanent teeth among 12-year-old Chinese children: Evidence from five consecutive national surveys between 1995 and 2014. BMC Oral Heal. 2021, 21, 467. [Google Scholar] [CrossRef]
- Rodakowska, E.; Wilczyńska-Borawska, M.; Bagińska, J.; Stokowska, E. Epidemiological analysis of dental caries in 12-year-old children residing in urban and rural settings in the Podlaskie region of north-eastern Poland. Ann. Agric. Environ. Med. 2013, 20, 325–328. [Google Scholar]
- Broniarek-Machnik, M.; Colonna-Walewska, M.; Płuciennik-Stronias, M.; Bołtacz-Rzepkowska, E. Evaluation of dental status and effectiveness of treatment among children and adolescents in the reference groups aged 6, 12, and 18 years from Skierniewice and its region in 2017–2020. Prz. Epidemiol. 2021, 75, 119–127. [Google Scholar]
- Olczak-Kowalczyk, D.; Turska, A.; Gozdowski, D.; Kaczmarek, U. Dental caries level and sugar consumption in 12-year-old children from Poland. Adv. Clin. Exp. Med. 2016, 25, 545–550. [Google Scholar] [CrossRef]
- Onov, M.P.; Beltcheva, A.B. Caries Prevalence in 12-year-old Children from Plovdiv—A Multifactorial Regression Analysis. Folia Medica 2020, 62, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-Operation and Development. Health at the Glance 2007: OECD Indicatiors; OECD Publishing: Paris, France, 2007; pp. 19–44. [Google Scholar]
- Rybarczyk-Townsend, E.; Hilt, A.; Szczepańska, J. Dentition status in 12-year-old children in Łódzkie province in 2014. Przeg Epidemiol. 2016, 70, 129–132. [Google Scholar]
- Davenport, E.S. Caries in the preschool child: Aetiology. J. Dent. 1990, 18, 300–303. [Google Scholar] [CrossRef]
- Richards, V.P.; Alvarez, A.J.; Luce, A.R.; Bedenbaugh, M.; Mitchell, M.L.; Burne, R.A.; Nascimento, M.M. Microbiomes of Site-Specific Dental Plaques from Children with Different Caries Status. Infect. Immun. 2017, 85, e00106-17. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.C.R.; Kressirer, C.A.; Faller, L.L. Understanding Caries From the Oral Microbiome Perspective. J. Calif. Dent. Assoc. 2016, 44, 437–446. [Google Scholar]
- Iwano, Y.; Sugano, N.; Matsumoto, K.; Nishihara, R.; Iizuka, T.; Yoshinuma, N.; Ito, K. Salivary microbial levels in relation to periodontal status and caries development. J. Periodontal Res. 2010, 45, 165–169. [Google Scholar] [CrossRef]
- Sioson, P.B.; Furgang, D.; Steinberg, L.M.; Fine, D.H. Proximal Caries in Juvenile Periodontitis Patients. J. Periodontol. 2000, 71, 710–716. [Google Scholar] [CrossRef]
- Kleinberg, I. A Mixed-bacteria Ecological Approach to Understanding the Role of the Oral Bacteria in Dental Caries Causation: An Alternative to Streptococcus mutans and the Specific-plaque Hypothesis. Crit. Rev. Oral Biol. Med. 2002, 13, 108–125. [Google Scholar] [CrossRef]
- van Ruyven, F.O.; Lingström, P.; van Houte, J.; Kent, R. Relationship among mutans streptococci, “low-ph” bacteria, and lodophilic polysaccharide-producing bacteria in dental plaque and early enamel caries in humans. J. Dent. Res. 2000, 79, 778–784. [Google Scholar] [CrossRef]
- Keijser, B.J.F.; Zaura, E.; Huse, S.M.; Van Der Vossen, J.M.B.M.; Schuren, F.H.J.; Montijn, R.C.; ten Cate, J.M.; Crielaard, W. Pyrosequencing analysis of the Oral Microflora of healthy adults. J. Dent. Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef]
- De Soet, J.J.; Van Loveren, C.; Lammens, A.J.; Pavičić, M.J.A.M.P.; Homburg CH, E.; Ten Cate, J.M.; De Graaff, J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Soda, Y.; Hayashi, F.; Doi, T.; Suzuki, J.; Miura, K.; Kozai, K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J. Med. Microbiol. 2005, 54, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, S.; Balasouli, C.; Tsuzukibashi, O.; Argyropoulou, A.; Menexes, G.; Kotsanos, N.; Kalfas, S. Streptococcus mutans, Streptococcus sobrinus and Candida albicans in oral samples from caries-free and caries-active children. Eur. Arch. Paediatr. Dent. 2016, 17, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kawamura, M.; Oda, Y.; Yasuda, R.; Kojima, T.; Kurihara, H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int. J. Paediatr. Dent. 2012, 22, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Rodis, O.M.; Matsumura, S.; Kariya, N.; Okazaki, Y.; Ogata, S.; Reißmann, D.R. Culture-based PCR analysis of plaque samples of Japanese school children to assess the presence of six common cariogenic bacteria and its association with caries risk. Mol. Cell. Probes 2009, 23, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. Caries Ecology Revisited: Microbial Dynamics and the Caries Process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Rupf, S.; Merte, K.; Eschrich, K.; Kneist, S. Streptococcus sobrinus in children and its influence on caries activity. Eur. Arch. Paediatr. Dent. 2006, 1, 17–22. [Google Scholar] [CrossRef]
- Hughes, C.V.; Dahlan, M.; Papadopolou, E.; Loo, C.Y.; Pradhan, N.S.; Lu, S.C.; Mathney, J.M.J.; Bravoco, A.; Kent, R.L.; Tanner, A.C.R. Aciduric microbiota and mutans streptococci in severe and recurrent severe early childhood caries. Int. J. Clin. Pediatr. Dent. 2012, 34, e16–e23. [Google Scholar]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef]
- Kneist, S.; Nietzsche, S.; Küpper, H.; Raser, G.; Willershausen, B.; Callaway, A. Penetration of Streptococcus sobrinus and Streptococcus sanguinis into dental enamel. Anaerobe 2015, 35, 54–59. [Google Scholar] [CrossRef]
- Qudeimat, M.A.; Alyahya, A.; Karched, M.; Behbehani, J.; Salako, N.O. Dental plaque microbiota profiles of children with caries-free and caries-active dentition. J. Dent. 2021, 104, 103539. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Paster, B.J.; Fiehn, N.E.; Bardow, A.; Holmstrup, P. Salivary bacterial fingerprints of established oral disease revealed by the human oral microbe identification using next generation sequencing (homings) technique. J. Oral. Microbiol. 2016, 14, 30170. [Google Scholar] [CrossRef] [PubMed]
- Mitrakul, K.; Vongsavan, K.; Suratanachaikul, P. Prevalence of Streptococcus mutans and Lactobacillus fermentum and their association with caries and dietary habits in preschool Thai children. Eur. Arch. Paediatr. Dent. 2013, 14, 83–87. [Google Scholar] [CrossRef]
- ElSalhy, M.; Söderling, E.; Honkala, E.; Fontana, M.; Flannagan, S.; Kokaras, A.; Paster, B.J.; Varghese, A.; Honkala, S. Salivary microbiota and caries occurrence in Mutans Streptococci-positive school children. Eur. J. Paediatr. Dent. 2016, 17, 188–192. [Google Scholar] [PubMed]
- Gross, E.L.; Leys, E.J.; Gasparovich, S.R.; Firestone, N.D.; Schwartzbaum, J.A.; Janies, D.A.; Asnani, K.; Griffen, A.L. Bacterial 16S sequence analysis of severe caries in young permanent teeth. J. Clin. Microbiol. 2010, 48, 4121–4128. [Google Scholar] [CrossRef]
- Ling, Z.; Kong, J.; Jia, P.; Wei, C.; Wang, Y.; Pan, Z.; Huang, W.; Li, L.; Chen, H.; Xiang, C. Analysis of Oral Microbiota in Children with Dental Caries by PCR-DGGE and Barcoded Pyrosequencing. Microb. Ecol. 2010, 60, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, L.; Yan, S.; Chen, X.; Que, G. The impact of caries status on supragingival plaque and salivary microbiome in children with mixed dentition: A cross-sectional survey. BMC Oral Heal. 2021, 21, 319. [Google Scholar] [CrossRef]
- Lee, E.; Park, S.; Um, S.; Kim, S.; Lee, J.; Jang, J.; Jeong, H.-O.; Shin, J.; Kang, J.; Lee, S.; et al. Microbiome of Saliva and Plaque in Children According to Age and Dental Caries Experience. Diagnostics 2021, 11, 1324. [Google Scholar] [CrossRef]
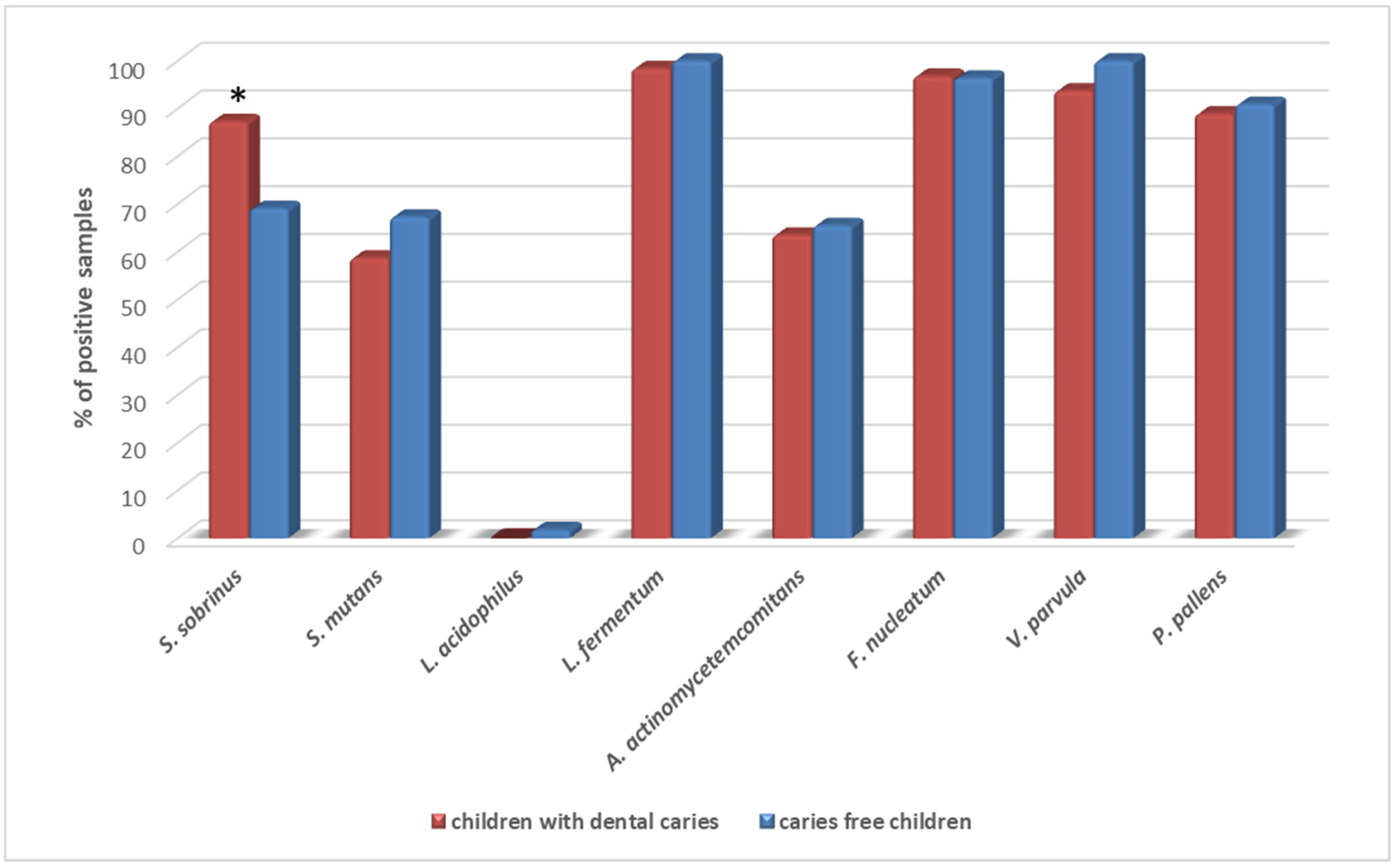
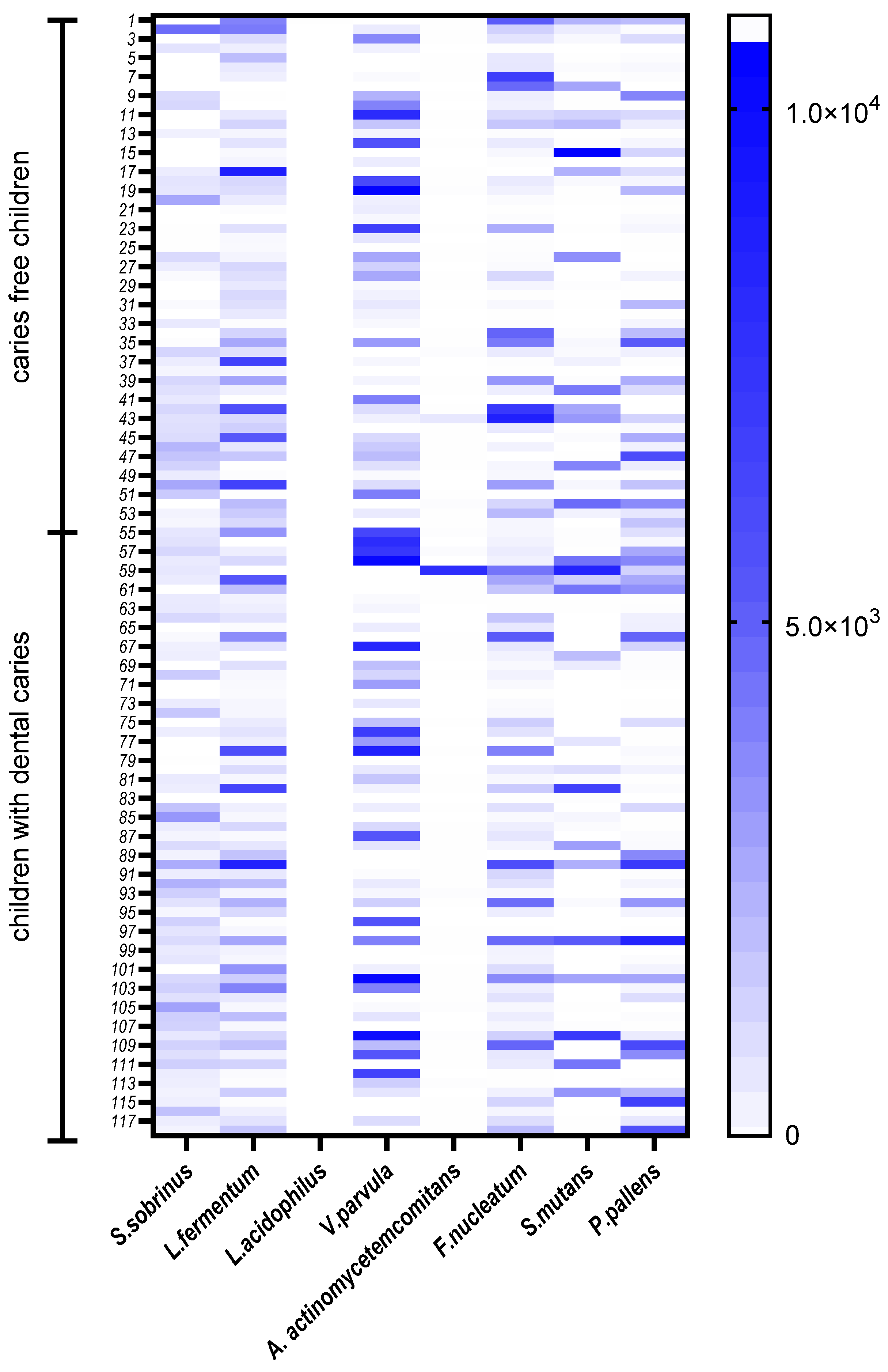
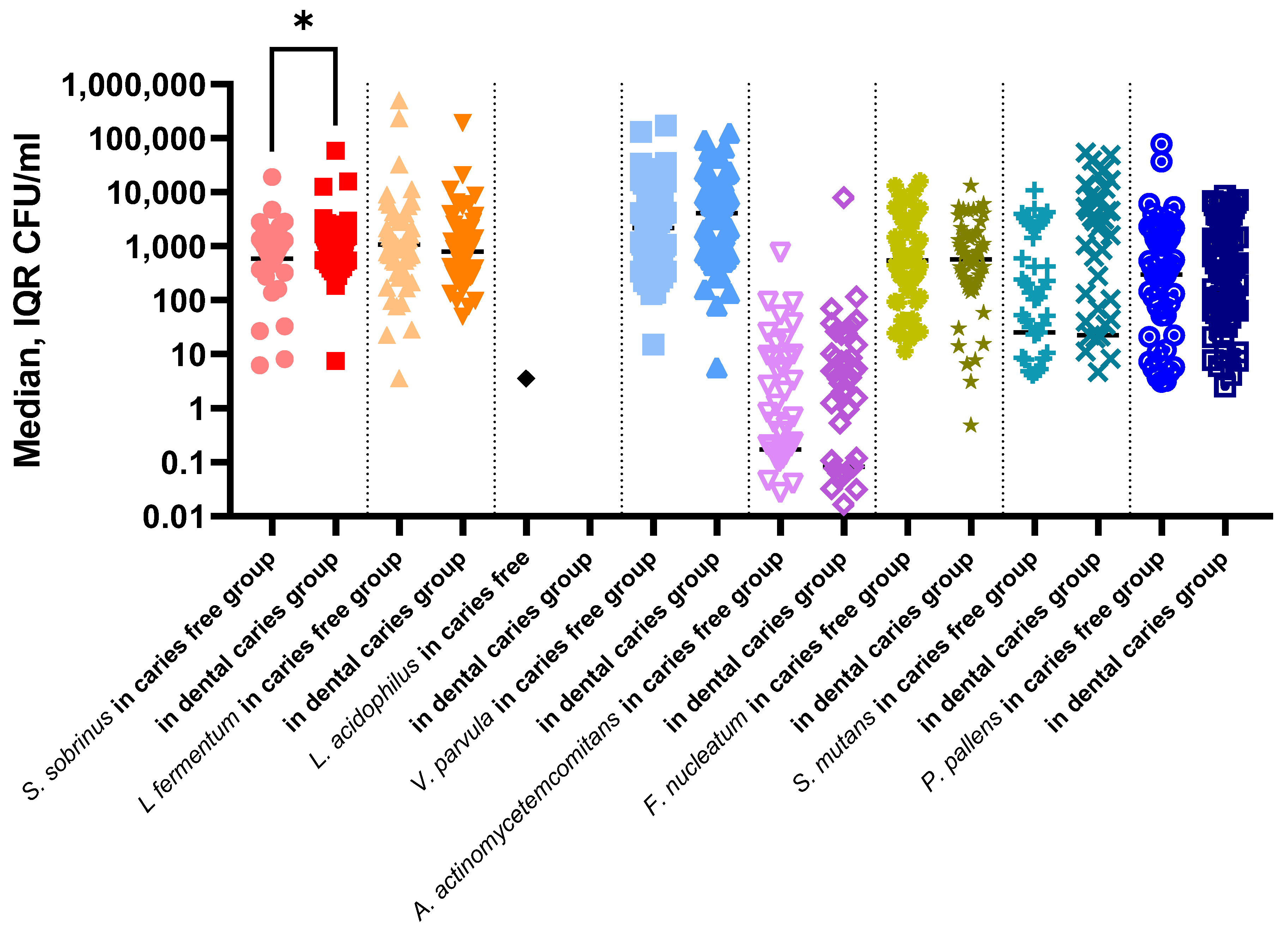
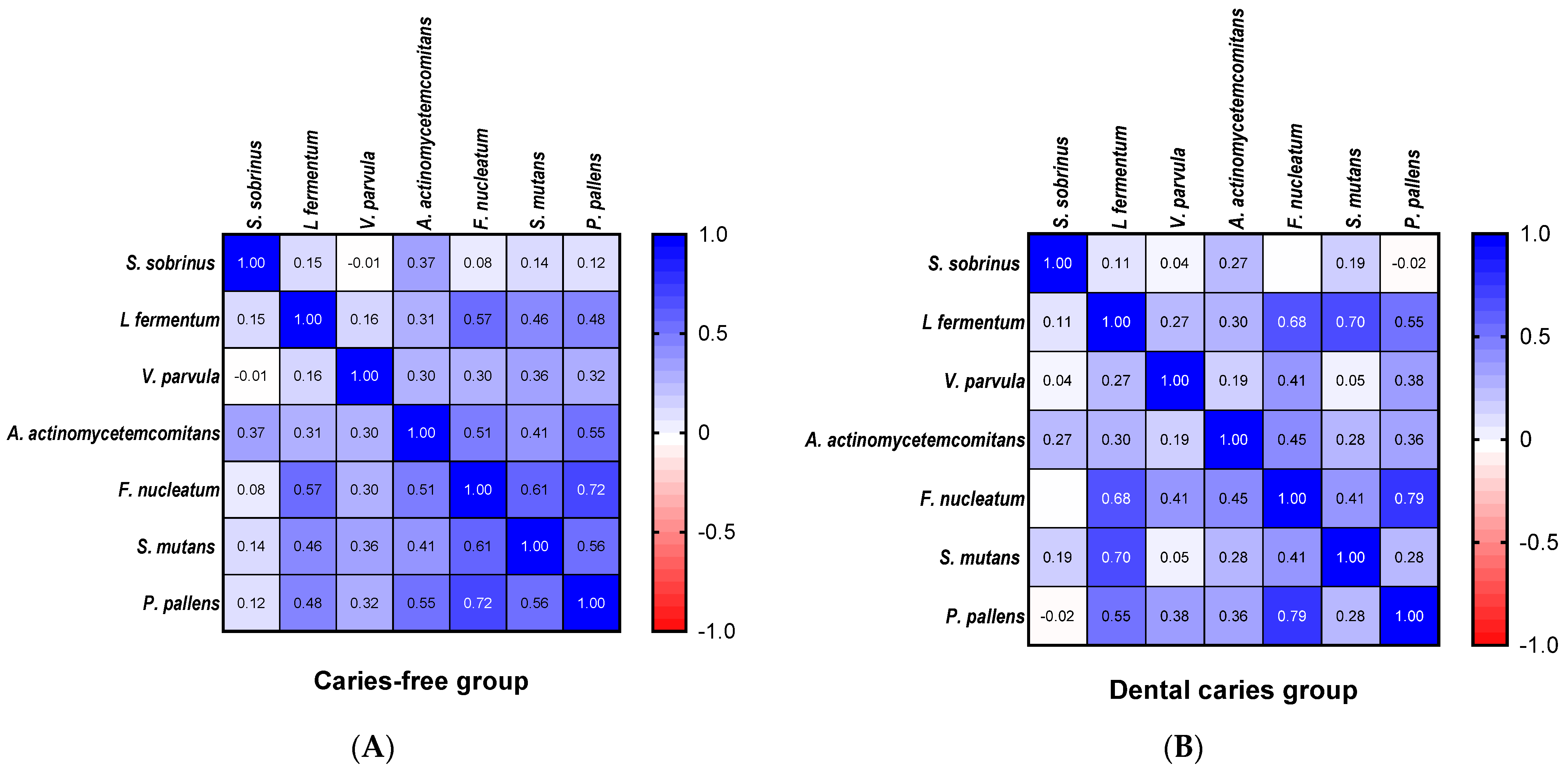
| Variables | Total (n = 118) | Boys (n = 61) | Girls (n = 57) | p Value 1 |
|---|---|---|---|---|
| DT | 1.47 ± 1.9 | 1.21 ± 1.7 | 1.74 ± 2.07 | 0.24 |
| MT | 0 | 0 | 0 | - |
| FT | 0.12 ± 0.47 | 0.18 ± 0.59 | 0.05 ± 0.29 | 0.17 |
| DMFT | 1.58 ± 1.98 | 1.39 ± 1.86 | 1.79 ± 2.07 | 0.36 |
| Bacterial Species | Total (n = 118) | Girls (n = 57) | Boys (n = 61) | p Value |
|---|---|---|---|---|
| Median (IQR) | ||||
| S. sobrinus | 661.85 (32.85–1272.0) | 937.6 (553.1–1482.0) | 539.8 (0.0–918.2) | 0.0039 * |
| L. fermentum | 934.0 (316.0–2103.0) | 738.2 (270.3–2816.0) | 986.2 (393.3–1867.0) | 0.88 |
| V. parvula | 2823.0 (615.6–12080.0) | 2174.0 (488.1–12080.0) | 4054.0 (730.7–10970.0) | 0.35 |
| A. actinomycetemcomitans | 0.13 (0.0–4.93) | 0.54 (0.0–8.11) | 0.077 (0.0–2.67) | 0.17 |
| F. nucleatum | 569.6 (164.4–1561.0) | 537.0 (144.5–1496.0) | 637.0 (200.6–1693.0) | 0.58 |
| S. mutans | 24.25 (0.0–2197.0) | 43.6 (0.0–3121.0) | 19.9 (0.0–632.9) | 0.22 |
| P. pallens | 281.2 (21.21–1448.0) | 144.8 (7.8–1167.0) | 401.9 (64.6–2156.0) | 0.13 |
| DMFT Index in Correlation to: | Spearman R | t (N − 2) | p-Value |
|---|---|---|---|
| S. sobrinus | 0.26 | 2.91 | 0.0043 |
| L. fermentum | −0.044 | −0.48 | 0.63 |
| L. acidophilus | −0.093 | −1.01 | 0.32 |
| V. parvula | 0.093 | 0.98 | 0.33 |
| A. actinomycetemcomitans | 0.095 | 1.02 | 0.31 |
| F. nucleatum | 0.072 | 0.78 | 0.44 |
| S. mutans | 0.1 | 1.09 | 0.28 |
| P. pallens | 0.032 | 0.35 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korona-Glowniak, I.; Skawinska-Bednarczyk, A.; Wrobel, R.; Pietrak, J.; Tkacz-Ciebiera, I.; Maslanko-Switala, M.; Krawczyk, D.; Bakiera, A.; Borek, A.; Malm, A.; et al. Streptococcus sobrinus as a Predominant Oral Bacteria Related to the Occurrence of Dental Caries in Polish Children at 12 Years Old. Int. J. Environ. Res. Public Health 2022, 19, 15005. https://doi.org/10.3390/ijerph192215005
Korona-Glowniak I, Skawinska-Bednarczyk A, Wrobel R, Pietrak J, Tkacz-Ciebiera I, Maslanko-Switala M, Krawczyk D, Bakiera A, Borek A, Malm A, et al. Streptococcus sobrinus as a Predominant Oral Bacteria Related to the Occurrence of Dental Caries in Polish Children at 12 Years Old. International Journal of Environmental Research and Public Health. 2022; 19(22):15005. https://doi.org/10.3390/ijerph192215005
Chicago/Turabian StyleKorona-Glowniak, Izabela, Agnieszka Skawinska-Bednarczyk, Rafal Wrobel, Justyna Pietrak, Izabela Tkacz-Ciebiera, Monika Maslanko-Switala, Dorota Krawczyk, Adrian Bakiera, Anna Borek, Anna Malm, and et al. 2022. "Streptococcus sobrinus as a Predominant Oral Bacteria Related to the Occurrence of Dental Caries in Polish Children at 12 Years Old" International Journal of Environmental Research and Public Health 19, no. 22: 15005. https://doi.org/10.3390/ijerph192215005
APA StyleKorona-Glowniak, I., Skawinska-Bednarczyk, A., Wrobel, R., Pietrak, J., Tkacz-Ciebiera, I., Maslanko-Switala, M., Krawczyk, D., Bakiera, A., Borek, A., Malm, A., & Mielnik-Blaszczak, M. (2022). Streptococcus sobrinus as a Predominant Oral Bacteria Related to the Occurrence of Dental Caries in Polish Children at 12 Years Old. International Journal of Environmental Research and Public Health, 19(22), 15005. https://doi.org/10.3390/ijerph192215005








