Impact of Vitiligo on Life Quality of Patients: Assessment of Currently Available Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Instruments for Outcome Measurements
2.3. Quality of Life
2.4. Disease Severity and Location of Involvement
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Quality of Life
3.3. Willingness to Pay
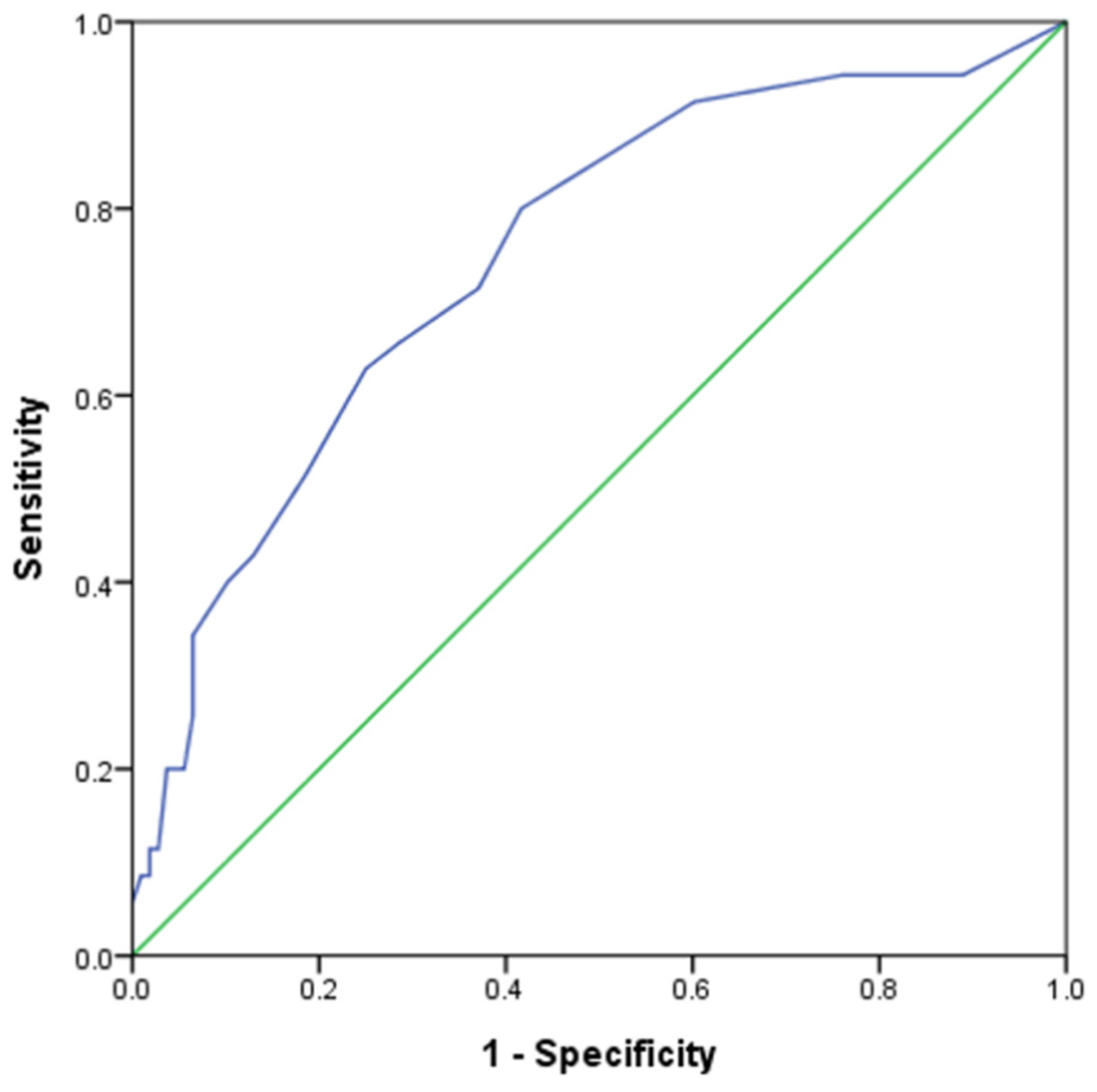
3.4. Deriving New Cutoff Value for DLQI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, A.; Pawaskar, M.; Taylor, S.L.; Balkrishnan, R.; Feldman, S.R. Prevalence of pigmentary disorders and their impact on quality of life: A prospective cohort study. J. Cosmet. Dermatol. 2008, 7, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, K.; Harris, J.E. Vitiligo. In Fitzpatrick’s Dermatology, 9th ed.; Kang, S., Amagai, M., Bruckner, A.L., Enk, A.H., Margolis, D.J., McMichael, A.J., Orringer, J.S., Eds.; McGraw Hill: New York, NY, USA, 2019; Volume 1, pp. 1330–1350. [Google Scholar]
- Bhandarkar, S.S.; Kundu, R.V. Quality-of-life issues in vitiligo. Dermatol. Clin. 2012, 30, 255. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Ahmed, S.; Nasreen, S. Frequency and pattern of psychiatric disorders in patients with vitiligo. J. Ayub. Med. Coll. Abbottabad. 2007, 19, 19–21. [Google Scholar] [PubMed]
- Chan, M.F.; Chua, T.L.; Goh, B.K.; Aw, C.W.; Thng, T.G.; Lee, S.M. Investigating factors associated with depression of vitiligo patients in Singapore. J. Clin. Nurs. 2012, 21, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Hongbo, Y.; Thomas, C.L.; Harrison, M.A.; Salek, M.S.; Finlay, A.Y. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J. Invest. Dermatol. 2005, 125, 659–664. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, P.V.; Tomas-Aragones, L.; Manolache, L.; Pustisek, N.; Salavastru, C.M.; Marron, S.E.; Bewley, A.; Svensson, A.; Poot, F.; Suru, A.; et al. Quality of life measurement in vitiligo. Position statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes with external experts. J. Eur. Acad. Dermatol. Venereol. 2022. ahead of print. [Google Scholar] [CrossRef]
- Ko, W.C.; Tsai, T.F.; Tang, C.H. Health state utility, willingness to pay, and quality of life among Taiwanese patients with psoriasis. Dermatol. Sinica. 2016, 34, 185–191. [Google Scholar] [CrossRef]
- Lundberg, L.; Johannesson, M.; Silverdahl, M.; Hermansson, C.; Lindberg, M. Quality of life, health-state utilities and willingness to pay in patients with psoriasis and atopic eczema. Br. J. Dermatol. 1999, 141, 1067–1075. [Google Scholar] [CrossRef]
- Seidler, A.M.; Bayoumi, A.M.; Goldstein, M.K.; Cruz, P.D., Jr.; Chen, S.C. Willingness to pay in dermatology: Assessment of the burden of skin diseases. J. Invest. Dermatol. 2012, 132, 1785–1790. [Google Scholar] [CrossRef]
- Directorate-General of Budget, Accounting and Statistics, Executive Yuan. Employees’ Earnings Survey, 2017 (AA220031) [data file]. Available from Survey Research Data Archive, Academia Sinica. 2018. Available online: https://srda.sinica.edu.tw/datasearch_detail.php?id=2858 (accessed on 10 November 2022).
- Beikert, F.C.; Langenbruch, A.K.; Radtke, M.A.; Kornek, T.; Purwins, S.; Augustin, M. Willingness to pay and quality of life in patients with atopic dermatitis. Arch. Dermatol. Res. 2014, 306, 279–286. [Google Scholar] [CrossRef]
- Beikert, F.C.; Langenbruch, A.K.; Radtke, M.A.; Augustin, M. Willingness to pay and quality of life in patients with rosacea. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 734–738. [Google Scholar] [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef]
- Taft, C.; Karlsson, J.; Sullivan, M. Do SF-36 summary component scores accurately summarize subscale scores? Qual. Life Res. 2001, 10, 395–404. [Google Scholar] [CrossRef]
- Ware, J.E.; Snow, K.K.; Kosinski, M.; Gandek, B. SF-36 Health Survey Manual and Interpretation Guide; New England Medical Center, The Health Institute: Boston, MA, USA, 1993. [Google Scholar]
- Van Geel, N.; Lommerts, J.; Bekkenk, M.; Wolkerstorfer, A.; Prinsen, C.A.; Eleftheriadou, V.; Taïeb, A.; Picardo, M.; Ezzedine, K.; Speeckaert, R. Development and Validation of the Vitiligo Extent Score (VES): An International Collaborative Initiative. J. Invest. Dermatol. 2016, 136, 978–984. [Google Scholar] [CrossRef]
- Chin, Y.-T.; Lin, W.-T.; Wu, P.-W.; Tsai, S.; Lee, C.-Y.; Seal, D.; Chen, T.; Huang, H.-L.; Lee, C.-H. Characteristic-Grouped Adiposity Indicators for Identifying Metabolic Syndrome in Adolescents: Develop and Valid Risk Screening Tools Using Dual Population. Nutrients 2020, 12, 3165. [Google Scholar] [CrossRef] [PubMed]
- Lilly, E.; Lu, P.D.; Borovicka, J.H.; Victorson, D.; Kwasny, M.; West, D.; Kundu, R.V. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J. Am. Acad. Dermatol. 2013, 69, e11–e18. [Google Scholar] [CrossRef]
- Gupta, V.; Sreenivas, V.; Mehta, M.; Khaitan, B.K.; Ramam, M. Measurement properties of the Vitiligo Impact Scale-22 (VIS-22), a vitiligo-specific quality-of-life instrument. Br. J. Dermatol. 2014, 171, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Salzes, C.; Abadie, S.; Seneschal, J.; Whitton, M.; Meurant, J.-M.; Jouary, T.; Ballanger, F.; Boralevi, F.; Taieb, A.; Taieb, C.; et al. The Vitiligo Impact Patient Scale (VIPs): Development and Validation of a Vitiligo Burden Assessment Tool. J. Invest. Dermatol. 2016, 136, 52–58. [Google Scholar] [CrossRef]
- Chen, D.; Tuan, H.; Zhou, E.Y.; Liu, D.; Zhao, Y. Quality of life of adult vitiligo patients using camouflage: A survey in a Chinese vitiligo community. PLoS ONE 2019, 14, e0210581. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, M.; Yamamoto, Y.; Kato, M.; Miyachi, Y. Camouflage for patients with vitiligo vulgaris improved their quality of life. J. Cosmet. Dermatol. 2010, 9, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Wang, K.H.; Zhang, Z.P. Health-related quality of life and marital quality of vitiligo patients in China. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ongenae, K.; Van Geel, N.; De Schepper, S.; Naeyaert, J.M. Effect of vitiligo on self-reported health-related quality of life. Br. J. Dermatol. 2005, 152, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.T.; Oh, E.H.; Kim, J.E.; Ko, J.Y.; Ro, Y.S. Comparative study of quality of life between psoriasis, vitiligo and autoimmune bullous disease. Hong Kong J. Dermatol. Venereol. 2017, 25, 57–64. [Google Scholar]
- Lewis, V.; Finlay, A.Y. 10 years experience of the Dermatology Life Quality Index (DLQI). J. Investig. Dermatol. Symp. Proc. 2004, 9, 169–180. [Google Scholar] [CrossRef]
- Tseng, H.M.; Lu, J.F.R.; Tsai, Y.J. Assessment of health-related quality of life in Taiwan (II): Norming and validation of SF-36 Taiwan version. Taiwan J. Public Health 2003, 22, 512–518. [Google Scholar] [CrossRef]
- Kostopoulou, P.; Jouary, T.; Quintard, B.; Ezzedine, K.; Marques, S.; Boutchnei, S.; Taïeb, A. Objective vs. subjective factors in the psychological impact of vitiligo: The experience from a French referral centre. Br. J. Dermatol. 2009, 161, 128–133. [Google Scholar] [CrossRef]
- Salzer, B.A.; Schallreuter, K.U. Investigation of the personality structure in patients with vitiligo and a possible association with impaired catecholamine metabolism. Dermatology 1995, 190, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hamidizadeh, N.; Ranjbar, S.; Ghanizadeh, A.; Parvizi, M.M.; Jafari, P.; Handjani, F. Evaluating prevalence of depression, anxiety and hopelessness in patients with Vitiligo on an Iranian population. Health Qual. Life Outcomes 2020, 18, 20. [Google Scholar] [CrossRef]
- Lai, Y.C.; Yew, Y.W.; Kennedy, C.; Schwartz, R.A. Vitiligo and depression: A systematic review and meta-analysis of observational studies. Br. J. Dermatol. 2017, 177, 708–718. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, J.E.; Lee, R.W.; Ju, H.J.; Han, J.H.; Lee, J.H.; Woo, Y.R.; Lee, J.H.; Bang, C.H.; Park, C.J.; et al. Beyond quality of life: A call for patients’ own willingness to pay in chronic skin disease to assess psychosocial burden-A multicenter, cross-sectional, prospective survey. J. Am. Acad. Dermatol. 2021, 85, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) |
|---|---|
| Sex (female), n = 143 | 86 (60.1) |
| Age, mean (SD), n = 143 | 44.9 (14.8) |
| Severity, n = 143 | |
| VES, median (IQR) Mean (SD) | 0.56 (0.24–1.35) 1.26 (2.15) |
| Location, n = 143 | |
| Exposed | 123 (86) |
| Non-exposed | 20 (14) |
| Disease duration, n = 143 | |
| <6 months | 18 (12.6) |
| 6 months–1 year | 15 (10.5) |
| 1 year–3 years | 33 (23.1) |
| 3 years–5 years | 15 (10.5) |
| >5 years | 62 (43.4) |
| Concomitant diseases, n = 143 | |
| None | 117 (81.8) |
| Malignancy | 2 (1.4) |
| Thyroid disorders | 7 (4.9) |
| Diabetes mellitus | 2 (1.4) |
| Hypertension | 6 (4.2) |
| Sjogren’s syndrome | 1 (0.7) |
| Coronary artery disease | 3 (2.1) |
| * Other diseases | 8 (5.6) |
| Monthly income (NTD), n = 81 | |
| <26,000 | 23 (28.4) |
| 26,000–39,000 | 16 (19.8) |
| 39,000–50,000 | 22 (27.2) |
| 50,000–100,000 | 14 (17.3) |
| >100,000 | 6 (7.4) |
| Mean (SD) | Median (IQR) | |
|---|---|---|
| DLQI, n = 143 | 5.32 (4.67) | 4 (2–8) |
| SF-36, n = 143 | ||
| Physical functioning (PF) | 93.6 (10.18) | 100 (90–100) |
| Role-physical (RP) | 91.61 (22.58) | 100 (100–100) |
| Bodily pain (BP) | 88.86 (16.38) | 100 (82–100) |
| General health (GH) | 61.97 (18.61) | 62 (48.5–72) |
| Vitality (VT) | 62.21 (16.21) | 60 (50–70) |
| Social function (SF) | 84.79 (15.78) | 87.5 (75–100) |
| Role-emotional (RE) | 84.62 (30.58) | 100 (100–100) |
| Mental health (MH) | 64.62 (16.30) | 64 (56–76) |
| n (%) | ||
| Willingness to pay, n = 81 | ||
| <10% | 29 (35.8) | |
| 10–19% | 32 (39.5) | |
| 20–29% | 8 (9.9) | |
| 30–39% | 3 (3.7) | |
| 40% | 9 (11.1) | |
| DLQI | PF | RP | BP | GH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | Rho | p-Value | |
| DLQI | - | - | −0.079 | 0.351 | −0.173 | 0.039 | −0.134 | 0.112 | −0.280 | 0.001 |
| Severity | 0.25 | 0.003 | −0.016 | 0.854 | −0.105 | 0.217 | −0.094 | 0.267 | 0.091 | 0.282 |
| VT | SF | RE | MH | WTP | ||||||
| Rho | p-value | Rho | p-value | Rho | p-value | Rho | p-value | Rho | p-value | |
| DLQI | −0.331 | <0.001 | −0.284 | 0.001 | −0.289 | <0.001 | −0.466 | <0.001 | - | - |
| Severity | 0.010 | 0.903 | −0.232 | 0.006 | −0.048 | 0.569 | −0.133 | 0.115 | 0.055 | 0.623 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.-T.; Lee, C.-H.; Lan, C.-C.E. Impact of Vitiligo on Life Quality of Patients: Assessment of Currently Available Tools. Int. J. Environ. Res. Public Health 2022, 19, 14943. https://doi.org/10.3390/ijerph192214943
Yang T-T, Lee C-H, Lan C-CE. Impact of Vitiligo on Life Quality of Patients: Assessment of Currently Available Tools. International Journal of Environmental Research and Public Health. 2022; 19(22):14943. https://doi.org/10.3390/ijerph192214943
Chicago/Turabian StyleYang, Ting-Ting, Chien-Hung Lee, and Cheng-Che E. Lan. 2022. "Impact of Vitiligo on Life Quality of Patients: Assessment of Currently Available Tools" International Journal of Environmental Research and Public Health 19, no. 22: 14943. https://doi.org/10.3390/ijerph192214943
APA StyleYang, T.-T., Lee, C.-H., & Lan, C.-C. E. (2022). Impact of Vitiligo on Life Quality of Patients: Assessment of Currently Available Tools. International Journal of Environmental Research and Public Health, 19(22), 14943. https://doi.org/10.3390/ijerph192214943







