Home-Based Exercise to Improve Motor Functions, Cognitive Functions, and Quality of Life in People with Huntington’s Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Search and Study Selection
2.2. Data Extraction
2.3. Risk of Bias and Quality Assessment
2.4. Data Synthesis and Meta-Analysis
3. Results
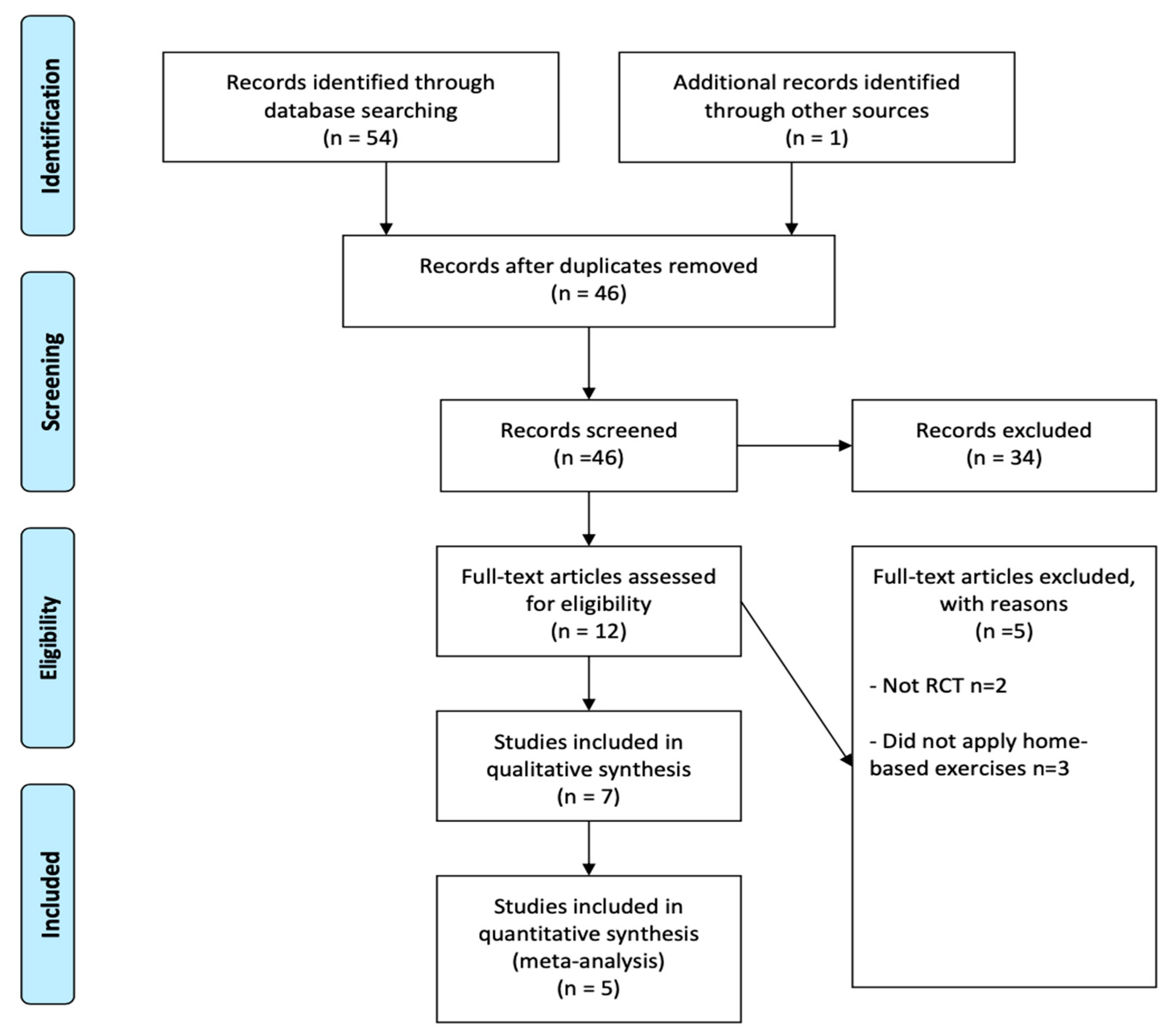
3.1. Study Selection
3.2. Study and Participant Characteristics
3.3. Quality and Risk of Bias
3.4. Intervention
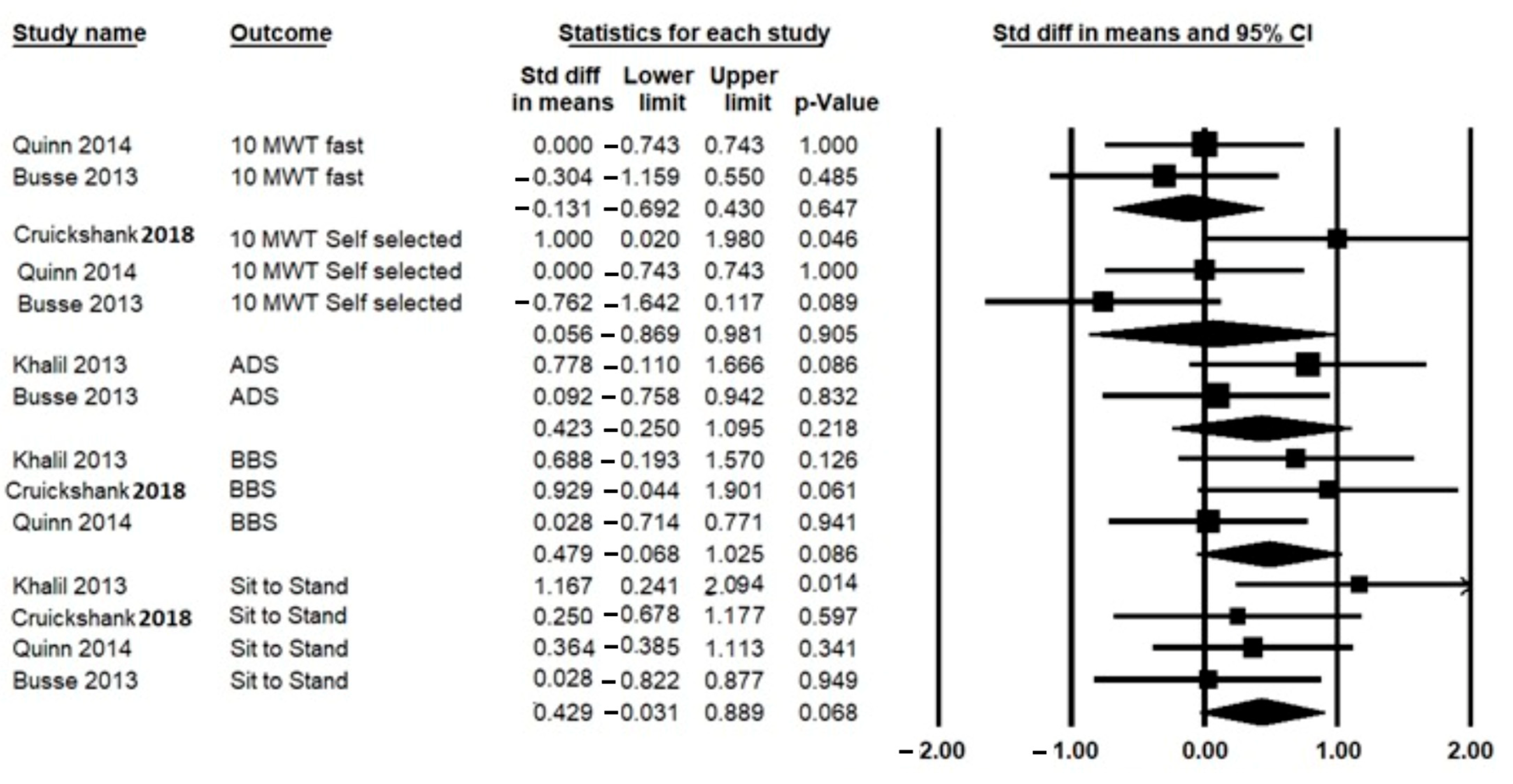
3.5. Effect of HBE on Primary Outcomes (Motor Function Ability)
3.6. Effect of HBE on Secondary Outcomes
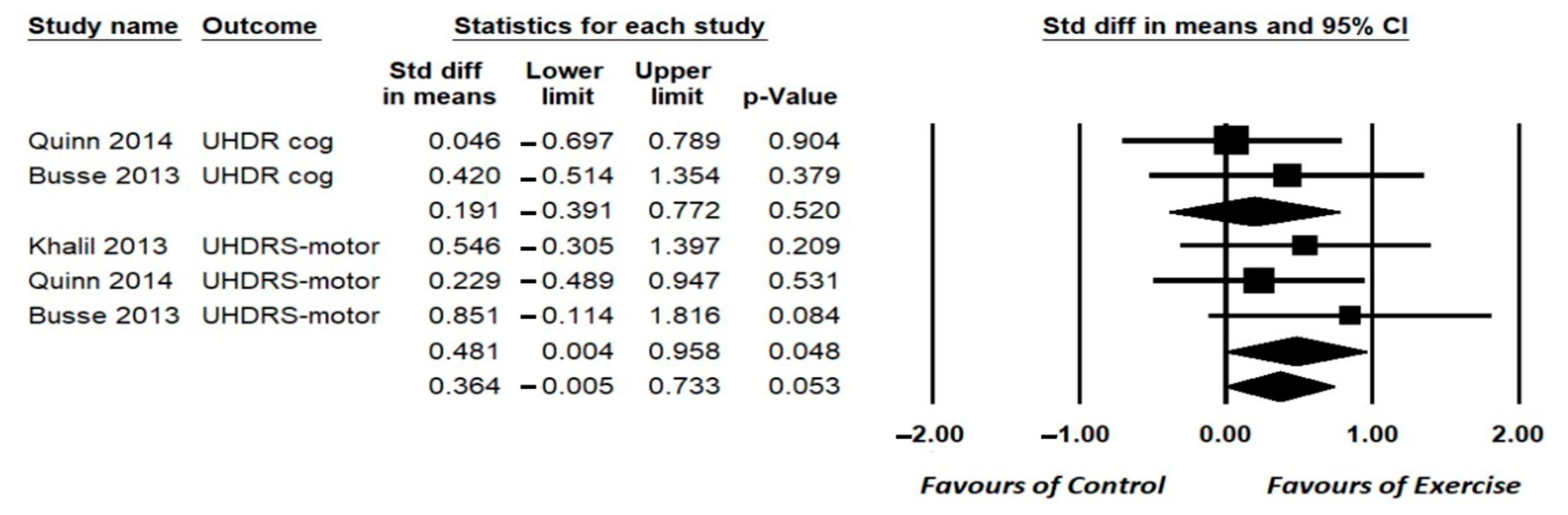
3.6.1. Cognition
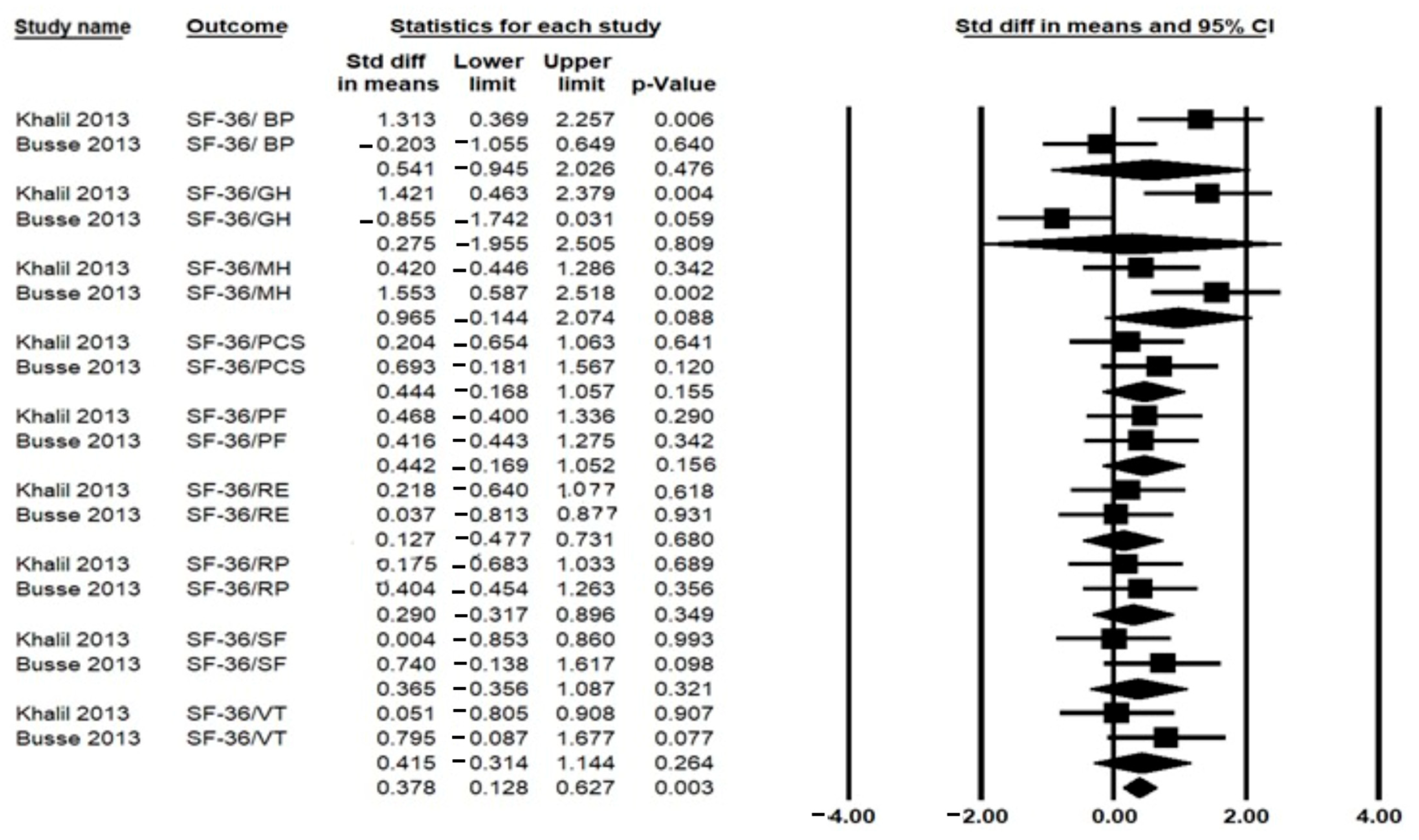
3.6.2. Health-Related Quality of Life
3.7. Adherence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nopoulos, P.C. Huntington Disease: A Single-Gene Degenerative Disorder of the Striatum. Dialogues Clin. Neurosci. 2022, 18, 91–98. [Google Scholar] [CrossRef]
- Varda, E.; Demetriou, C.A.; Heraclides, A.; Christou, Y.P.; Zamba-Papanicolaou, E. Quality of Life of Cypriot Patients Suffering with Huntington’s Disease. PLoS Curr. 2016, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Kegelmeyer, D.; Kloos, A.; Rao, A.K.; Busse, M.; Fritz, N.E. Clinical Recommendations to Guide Physical Therapy Practice for Huntington Disease. Neurology 2020, 94, 217–228. [Google Scholar] [CrossRef]
- Wyant, K.J.; Ridder, A.J.; Dayalu, P. Huntington’s Disease—Update on Treatments. Curr. Neurol. Neurosci. Rep. 2017, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Nance, M.A. Genetics of Huntington Disease. Handb. Clin. Neurol. 2017, 144, 3–14. [Google Scholar] [PubMed]
- Quinn, L.; Busse, M. Development of Physiotherapy Guidance and Treatment-Based Classifications for People with Huntington’s Disease. Neurodegener. Dis. Manag. 2012, 2, 11–19. [Google Scholar] [CrossRef]
- Fritz, N.; Rao, A.K.; Kegelmeyer, D.; Kloos, A.; Busse, M.; Hartel, L.; Carrier, J.; Quinn, L. Physical Therapy and Exercise Interventions in Huntington’s Disease: A Mixed Methods Systematic Review. J. Huntingtons. Dis. 2017, 6, 217–236. [Google Scholar] [CrossRef]
- Mueller, S.M.; Petersen, J.A.; Jung, H.H. Exercise in Huntington’s Disease: Current State and Clinical Significance. Tremor Other Hyperkinetic Mov. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Khalil, H.; Quinn, L.; van Deursen, R.; Dawes, H.; Playle, R.; Rosser, A.; Busse, M. What Effect Does a Structured Home-Based Exercise Programme Have on People with Huntington’s Disease? A Randomized, Controlled Pilot Study. Clin. Rehabil. 2013, 27, 646–658. [Google Scholar] [CrossRef]
- Busse, M.; Quinn, L.; Debono, K.; Jones, K.; Collett, J.; Playle, R.; Kelly, M.; Simpson, S.; Backx, K.; Wasley, D.; et al. A Randomized Feasibility Study of a 12-Week Community-Based Exercise Program for People with Huntington’s Disease. J. Neurol. Phys. Ther. 2013, 37, 149–158. [Google Scholar] [CrossRef]
- Kamieniarz, A.; Milert, A.; Grzybowska-Ganszczyk, D.; Opara, J.; Juras, G. Tai Chi and Qi Gong Therapies as a Complementary Treatment in Parkinson’s Disease—A Systematic Review. Complement. Ther. Med. 2021, 56, 102589. [Google Scholar] [CrossRef] [PubMed]
- Alwardat, M.; Etoom, M.; Al Dajah, S.; Schirinzi, T.; Di Lazzaro, G.; Salimei, P.S.; Mercuri, N.B.; Pisani, A. Effectiveness of Robot-Assisted Gait Training on Motor Impairments in People with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Int. J. Rehabil. Res. 2018, 41, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hadoush, H.; Alawneh, A.; Kassab, M.; Al-Wardat, M.; Al-Jarrah, M. Effectiveness of Non-Pharmacological Rehabilitation Interventions in Pain Management in Patients with Multiple Sclerosis: Systematic Review and Meta-Analysis. NeuroRehabilitation 2022, 50, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Alwardat, M.; Schirinzi, T.; Di Lazzaro, G.; Sancesario, G.M.; Franco, D.; Imbriani, P.; Sinibaldi Salimei, P.; Bernardini, S.; Mercuri, N.B.; Pisani, A. Association between Physical Activity and Dementia’s Risk Factors in Patients with Parkinson’s Disease. J. Neural Transm. 2019, 126, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dolbow, J.D.; Ly, H.; Elwert, N.; Gassler, J. Effects of Exercise Environment and Protocol Intensity on the Efficacy of Rehabilitation Care for Patients with Huntington’s Disease: A Comprehensive Review. Int. J. Exerc. Sci. 2019, 12, 456–470. [Google Scholar]
- Busse, M.; Quinn, L.; Drew, C.; Kelson, M.; Trubey, R.; McEwan, K.; Jones, C.; Townson, J.; Dawes, H.; Tudor-Edwards, R.; et al. Physical Activity Self-Management and Coaching Compared to Social Interaction in Huntington Disease: Results from the Engage-Hd Randomized, Controlled Pilot Feasibility Trial. Phys. Ther. 2017, 97, 625–639. [Google Scholar] [CrossRef]
- Reyes, A.; Cruickshank, T.; Nosaka, K.; Ziman, M. Respiratory Muscle Training on Pulmonary and Swallowing Function in Patients with Huntington’s Disease: A Pilot Randomised Controlled Trial. Clin. Rehabil. 2015, 29, 961–973. [Google Scholar] [CrossRef]
- Quinn, L.; Debono, K.; Dawes, H.; Rosser, A.E.; Nemeth, A.H.; Rickards, H.; Tabrizi, S.J.; Quarrell, O.; Trender-Gerhard, I.; Kelson, M.J.; et al. Task-Specific Training in Huntington Disease: A Randomized Controlled Feasibility Trial. Phys. Ther. 2014, 94, 1555–1568. [Google Scholar] [CrossRef]
- Quinn, L.; Hamana, K.; Kelson, M.; Dawes, H.; Collett, J.; Townson, J.; Roos, R.; van der Plas, A.A.; Reilmann, R.; Frich, J.C.; et al. A Randomized, Controlled Trial of a Multi-Modal Exercise Intervention in Huntington’s Disease. Park. Relat. Disord. 2016, 31, 46–52. [Google Scholar] [CrossRef]
- Cruickshank, T.M.; Reyes, A.P.; Penailillo, L.E.; Pulverenti, T.; Bartlett, D.M.; Zaenker, P.; Blazevich, A.J.; Newton, R.U.; Thompson, J.A.; Lo, J.; et al. Effects of Multidisciplinary Therapy on Physical Function in Huntington’s Disease. Acta Neurol. Scand. 2018, 138, 500–507. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Abreu, D.; Damásio, J.; Goncalves, N.; Correia-Guedes, L.; Coelho, M.; Ferreira, J.J. Survival, Mortality, Causes and Places of Death in a European Huntington’s Disease Prospective Cohort. Mov. Disord. Clin. Pract. 2017, 4, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Di Lazzaro, G.; Salimei, C.; Cerroni, R.; Liguori, C.; Scalise, S.; Alwardat, M.; Mercuri, N.B.; Pierantozzi, M.; Stefani, A.; et al. Physical Activity Changes and Correlate Effects in Patients with Parkinson’s Disease during COVID-19 Lockdown. Mov. Disord. Clin. Pract. 2020, 7, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Ghahfarrokhi, M.M.; Banitalebi, E.; Negaresh, R.; Motl, R.W. Home-Based Exercise Training in Multiple Sclerosis: A Systematic Review with Implications for Future Research. Mult. Scler. Relat. Disord. 2021, 55, 103177. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.; Higgins, J.R.H. Comprehensive Meta-Analysis, Version 2; Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Oxford, UK, 2019. [Google Scholar]
- Thompson, J.A.; Cruickshank, T.M.; Penailillo, L.E.; Lee, J.W.; Newton, R.U.; Barker, R.A.; Ziman, M.R. The Effects of Multidisciplinary Rehabilitation in Patients with Early-to-Middle-Stage Huntington’s Disease: A Pilot Study. Eur. J. Neurol. 2013, 20, 1325–1329. [Google Scholar] [CrossRef]
- Kloos, A.D.; Fritz, N.E.; Kostyk, S.K.; Young, G.S.; Kegelmeyer, D.A. Video Game Play (Dance Dance Revolution) as a Potential Exercise Therapy in Huntington’s Disease: A Controlled Clinical Trial. Clin. Rehabil. 2013, 27, 972–982. [Google Scholar] [CrossRef]
- Mestre, T.A.; Forjaz, M.J.; Mahlknecht, P.; Cardoso, F.; Ferreira, J.J.; Reilmann, R.; Sampaio, C.; Goetz, C.G.; Cubo, E.; Martinez-Martin, P.; et al. Rating Scales for Motor Symptoms and Signs in Huntington’s Disease: Critique and Recommendations. Mov. Disord. Clin. Pract. 2018, 5, 111. [Google Scholar] [CrossRef]
- Zinzi, P.; Salmaso, D.; de Grandis, R.; Graziani, G.; Maceroni, S.; Bentivoglio, A.; Zappata, P.; Frontali, M.; Jacopini, G. Effects of an Intensive Rehabilitation Programme on Patients with Huntington’s Disease: A Pilot Study. Clin. Rehabil. 2007, 21, 603–613. [Google Scholar] [CrossRef]
- Piira, A.; van Walsem, M.R.; Mikalsen, G.; Nilsen, K.H.; Knutsen, S.; Frich, J.C. Effects of a One Year Intensive Multidisciplinary Rehabilitation Program for Patients with Huntington’s Disease: A Prospective Intervention Study. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Playle, R.; Dimitropoulou, P.; Kelson, M.; Quinn, L.; Busse, M. Exercise Interventions in Huntington’s Disease: An Individual Patient Data Meta-Analysis. Mov. Disord. Clin. Pract. 2019, 6, 567–575. [Google Scholar] [CrossRef]
- Kosinski, C.M.; Schlangen, C.; Gellerich, F.N.; Gizatullina, Z.; Deschauer, M.; Schiefer, J.; Young, A.B.; Landwehrmeyer, G.B.; Toyka, K.V.; Sellhaus, B.; et al. Myopathy as a First Symptom of Huntington’s Disease in a Marathon Runner. Mov. Disord. 2007, 22, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, D.; Piotrowska, I.; Marcinkowski, J.T.; Mielcarek, M. Skeletal Muscle Pathology in Huntington’s Disease. Front. Physiol. 2014, 5, 380. [Google Scholar] [CrossRef]
- Dawes, H.; Collett, J.; Debono, K.; Quinn, L.; Jones, K.; Kelson, M.J.; Simpson, S.A.; Playle, R.; Backx, K.; Wasley, D.; et al. Exercise Testing and Training in People with Huntington’s Disease. Clin. Rehabil. 2015, 29, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Steventon, J.J.; Collett, J.; Furby, H.; Hamana, K.; Foster, C.; O’Callaghan, P.; Dennis, A.; Armstrong, R.; Németh, A.H.; Rosser, A.E.; et al. Alterations in the Metabolic and Cardiorespiratory Response to Exercise in Huntington’s Disease. Park. Relat. Disord. 2018, 54, 56. [Google Scholar] [CrossRef] [PubMed]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical Exercise Improves Quality of Life, Depressive Symptoms, and Cognition across Chronic Brain Disorders: A Transdiagnostic Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Neurol. 2021, 268, 1222–1246. [Google Scholar] [CrossRef] [PubMed]
- Mestre, T.A.; Carlozzi, N.E.; Ho, A.K.; Burgunder, J.M.; Walker, F.; Davis, A.M.; Busse, M.; Quinn, L.; Rodrigues, F.B.; Sampaio, C.; et al. Quality of Life in Huntington’s Disease: Critique and Recommendations for Measures Assessing Patient Health-Related Quality of Life and Caregiver Quality of Life. Mov. Disord. 2018, 33, 742–749. [Google Scholar] [CrossRef]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to Treatment Adherence in Physiotherapy Outpatient Clinics: A Systematic Review. Man. Ther. 2010, 15, 220. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The Effects of Physical Activity and Exercise on Brain-Derived Neurotrophic Factor in Healthy Humans: A Review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Maltese, M.; Stanic, J.; Tassone, A.; Sciamanna, G.; Ponterio, G.; Vanni, V.; Martella, G.; Imbriani, P.; Bonsi, P.; Mercuri, N.B.; et al. Early Structural and Functional Plasticity Alterations in a Susceptibility Period of DYT1 Dystonia Mouse Striatum. eLife 2018, 7, e33331. [Google Scholar] [CrossRef]
- Pietrelli, A.; Matković, L.; Vacotto, M.; Lopez-Costa, J.J.; Basso, N.; Brusco, A. Aerobic Exercise Upregulates the BDNF-Serotonin Systems and Improves the Cognitive Function in Rats. Neurobiol. Learn. Mem. 2018, 155, 528–542. [Google Scholar] [CrossRef]
- Schirinzi, T.; Di Lazzaro, G.; Sancesario, G.M.; Colona, V.L.; Scaricamazza, E.; Mercuri, N.B.; Martorana, A.; Sancesario, G. Levels of Amyloid-Beta-42 and CSF Pressure Are Directly Related in Patients with Alzheimer’s Disease. J. Neural Transm. 2017, 124, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
| Study | PEDro Total Score | Participants, Demographical, and Clinical Data | Outcome Measurements | Type and Duration of Intervention | Main Results | Adherence Rate |
|---|---|---|---|---|---|---|
| Khalil et al. 2013 | 5 | N = 25 Age = 52.5 ± 13.5 Disease stage = Mild to moderate HD | UHDRS-MS; Gait speed using GaitRite, BBS, Chair sit to stand test, average of daily steps and SF-36. | Exp: supervised home exercise program using a DVD and a walking program. One home visit to teach the program and then weekly follow-up phone calls. CG: continued usual care. 75 min × 3 times a week × 8 weeks | Exp group had significant improvements in gait speed, balance sit-to-stand test, average daily steps, and UHDRS-mMS. No significant changes in the SF-36. | 80% |
| Kloos et al. 2013 | 3 | N = 24 Age = 50.7 ± 14.7 Disease stage = NR | ABC scale; WHOQOL-Brief | Exp: participants played the video game Dance Revolution with therapist supervision in homes. CG: played a handheld video game unsupervised. 45 min × 2 times a week × 6 weeks | No significant changes in the ABC scale and WHOQOL Brief. | NR |
| Reyes et al. 2015 | 7 | N = 18 Age = 56 ± 10.2 Disease stage = NR | Swallowing QOL questionnaire | Exp: home-based resistive inspiratory and expiratory muscle training progressively increased resistance from 30% to 75% of each patient’s maximum respiratory pressure; CG: fixed resistance of 9 cmH2O. 5 sets of 5 reps × 6 days per week × 4 months | Exp group had no significant improvement in swallowing the QOL questionnaire compared to CG. | 100% |
| Quinn et al. 2014 | 7 | N = 30 Age = 57 ± 10.1 Disease stage = middle stage | UHDRS-ms; UHDRS cognitive; BBS, 10 MWT (SS and FS), chair sit-to-stand test; HDQOL, EQ5D Health Index | Exp: home-based task-specific intervention program was individualized to participants’ specific activity limitations in walking, sit-to-stand transfers, and standing ability and modified to their home environments. CG: usual care. 60 min × 2 times a week × 8 weeks | Exp group showed a small effect size in all measures. | 96.9% |
| Busse et al. 2013 | 7 | N = 31 Age = 53.3 ± 12.5 Disease stage = early to middle | UHDRS-ms, UHDRS cognitive 10 MWT (SS and FS), Chair sit-to-stand test, Romberg test, average daily step, SF-36. | Exp: Supervised gym sessions of stationary cycling and resistance exercises and unsupervised home-based walking program consisting of (1) aerobic training at 55%–75% age-predicted maximal HR & moderate to hard levels of exertion on Borg RPE (4–6); (2) strength training for trunk/LE muscles progressed to 2 sets of 8–12 reps at 60–70% of participant’s 1 repetition max and (3) walking at moderate to somewhat hard (3–4 Borg scale) intensities; CG: usual care. 30 min × 1 time per week (gym); 2 times per week (home) × 12 Weeks. | Exp group had a significant improvement in SF-36 Mental Component Summary score and non-significant improvements in UHDRS cognitive scores and Chair sit-to-stand test. | 82% |
| Cruickshank et al. 2018 | 7 | N = 18 Age = 52.5 ± 5.4 Disease stage = middle stage | 10-MWT (SS and FS), BBS, Chair sit-to-stand test. | Exp: supervised exercise aerobic (cycle ergometer) and resistance (machines) strengthening exercises, walking, balance, and fine motor exercises. Cognitive therapy (paper and pencil and cognitive exercises) and ADL. Gym: 60 m. once/week followed by home: 60 m. session per 3 times/week for 36 weeks. CG: Usual care medication | Exp group had no significant improvement in gait speed, BBS, and sit-to-stand test. | 56% |
| Thompson et al., 2012 | 6 | N = 20 Age = 53.05 ± 2.75 Disease stage = Early to middle stage | UHDRS-ms, ABC, SDMT, HVLT-R, SF-36, and HDQOL | Exp: the gym exercise comprised of supervised group sessions 5 min. warm-up, 10 min. aerobic exercise, 40 min. resistance exercise, 5 min. cool-down, once/week for 36 weeks; A tailored, self-monitored home-based exercise 3 times /week for 24 weeks and OT 1 h CG: Usual care | Exp group has significant improvement in motor function, balance, depression, cognition, and QoL | 56% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wardat, M.; Schirinzi, T.; Hadoush, H.; Kassab, M.; Yabroudi, M.A.; Opara, J.; Nawrat-Szołtysik, A.; Khalil, H.; Etoom, M. Home-Based Exercise to Improve Motor Functions, Cognitive Functions, and Quality of Life in People with Huntington’s Disease: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14915. https://doi.org/10.3390/ijerph192214915
Al-Wardat M, Schirinzi T, Hadoush H, Kassab M, Yabroudi MA, Opara J, Nawrat-Szołtysik A, Khalil H, Etoom M. Home-Based Exercise to Improve Motor Functions, Cognitive Functions, and Quality of Life in People with Huntington’s Disease: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(22):14915. https://doi.org/10.3390/ijerph192214915
Chicago/Turabian StyleAl-Wardat, Mohammad, Tommaso Schirinzi, Hikmat Hadoush, Manal Kassab, Mohammad A. Yabroudi, Józef Opara, Agnieszka Nawrat-Szołtysik, Hanan Khalil, and Mohammad Etoom. 2022. "Home-Based Exercise to Improve Motor Functions, Cognitive Functions, and Quality of Life in People with Huntington’s Disease: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 22: 14915. https://doi.org/10.3390/ijerph192214915
APA StyleAl-Wardat, M., Schirinzi, T., Hadoush, H., Kassab, M., Yabroudi, M. A., Opara, J., Nawrat-Szołtysik, A., Khalil, H., & Etoom, M. (2022). Home-Based Exercise to Improve Motor Functions, Cognitive Functions, and Quality of Life in People with Huntington’s Disease: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(22), 14915. https://doi.org/10.3390/ijerph192214915








