High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
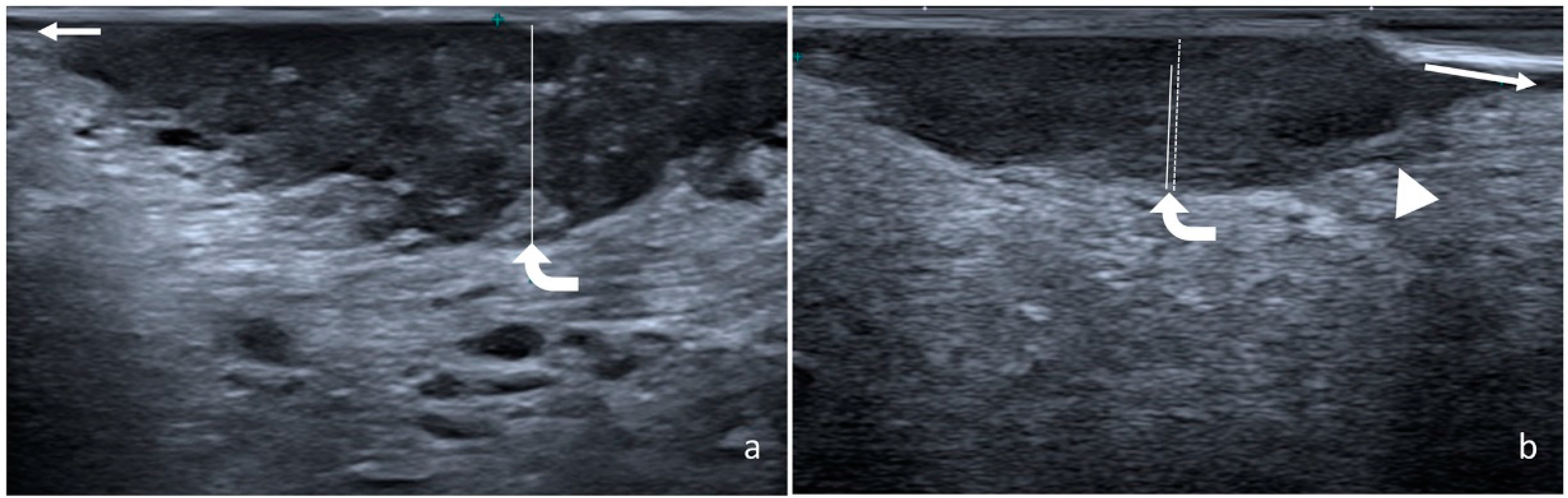
2.2. Measurement of Radiological and Pathological DOI
2.3. Statistical Analyses
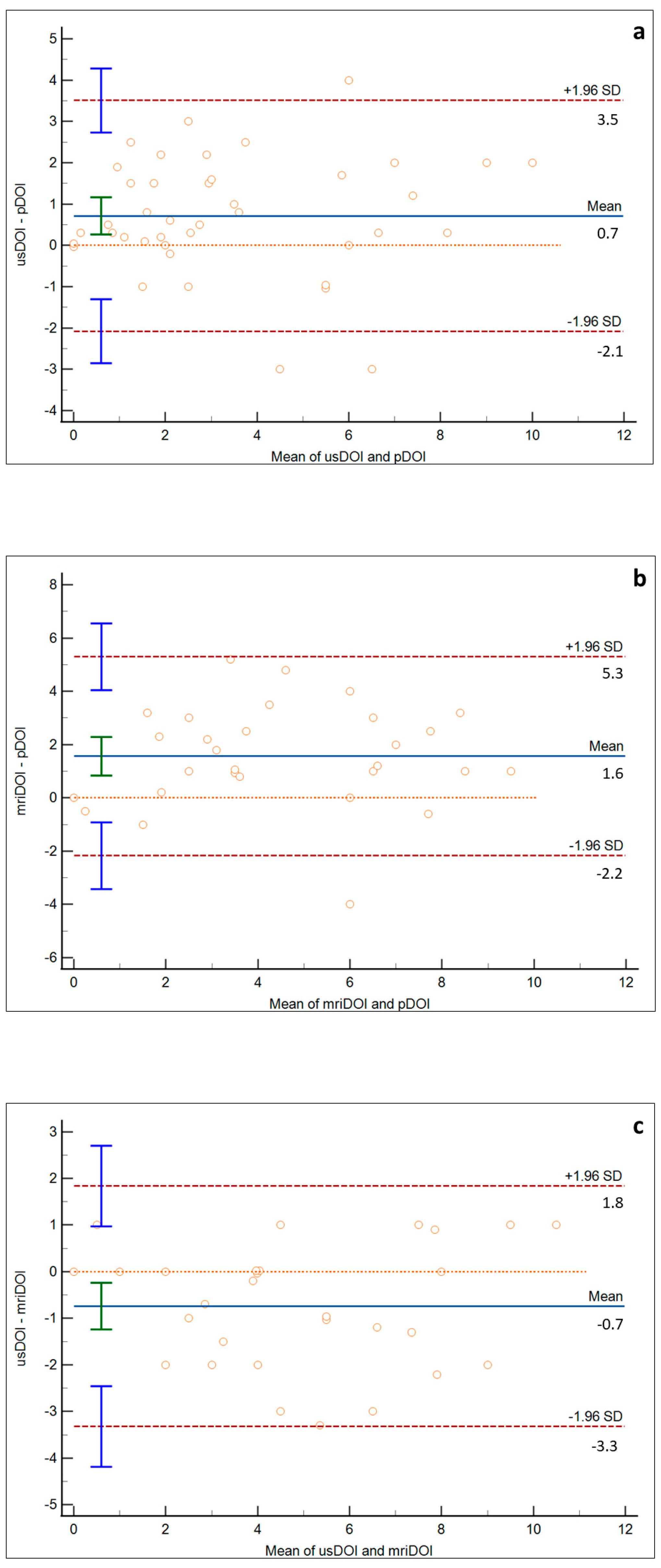
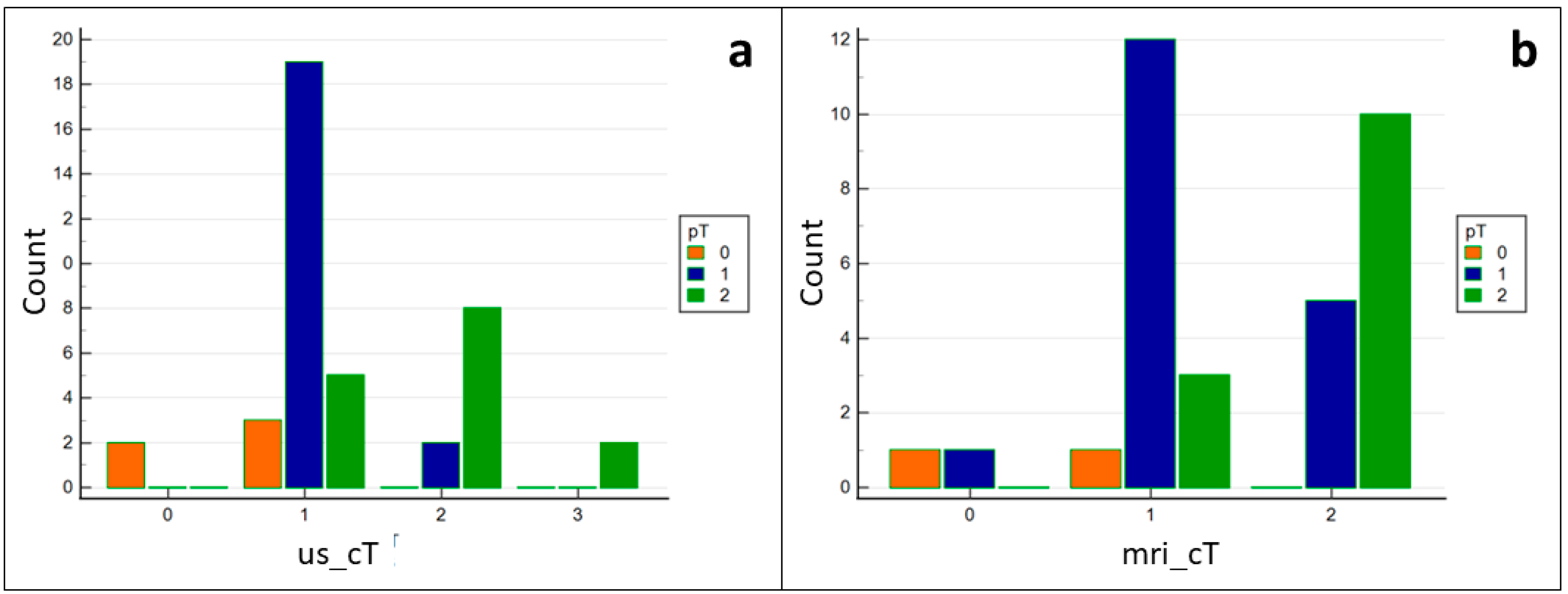
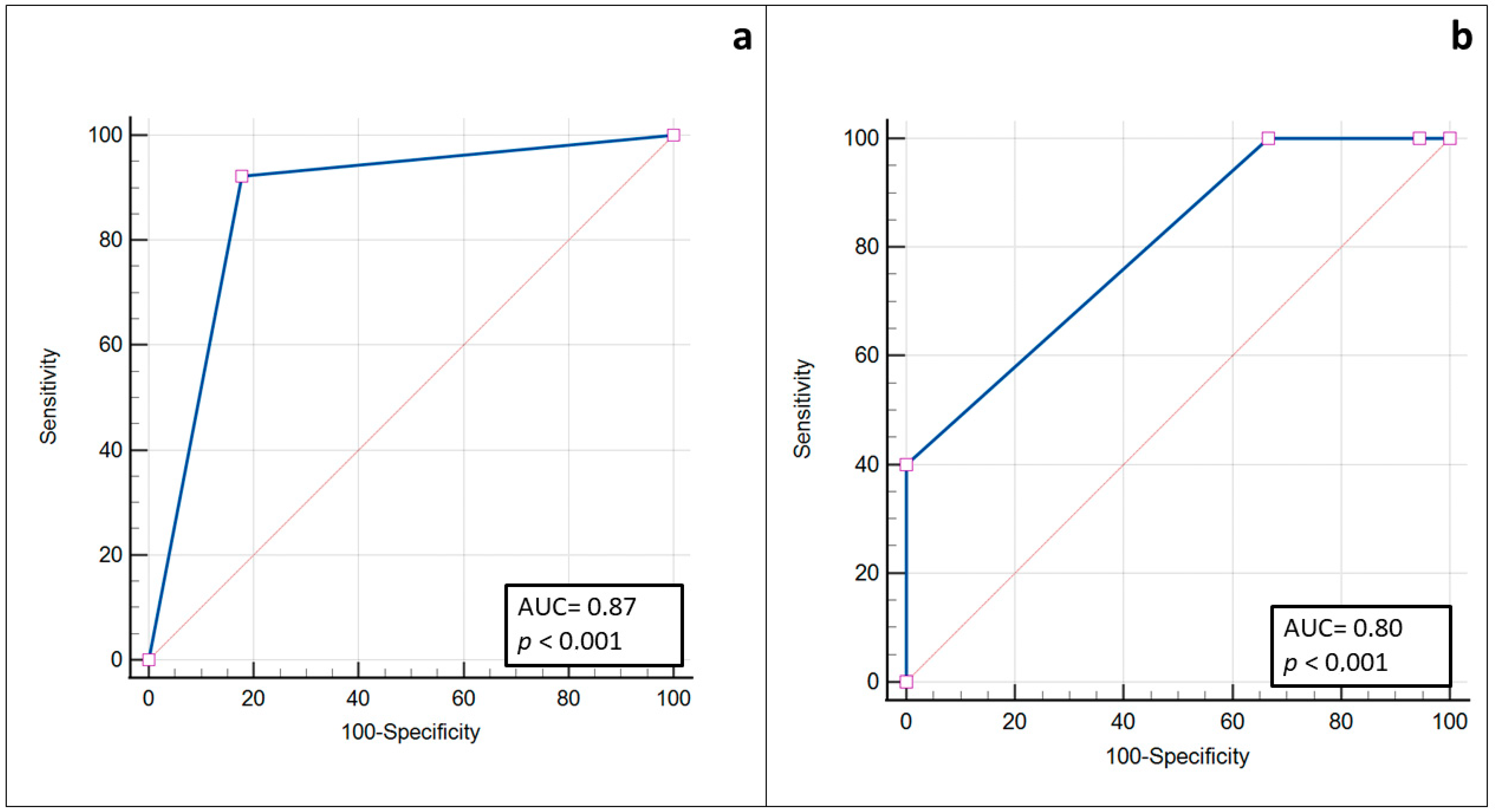
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ganly, I.; Patel, S.; Shah, J. Early Stage Squamous Cell Cancer of the Oral Tongue-Clinicopathologic Features Affecting Outcome. Cancer 2012, 118, 101–111. [Google Scholar] [CrossRef]
- Shinn, J.R.; Wood, C.B.; Colazo, J.M.; Harrell, F.E.; Rohde, S.L.; Mannion, K. Cumulative Incidence of Neck Recurrence with Increasing Depth of Invasion. Oral. Oncol. 2018, 87, 36–42. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Yehuda, M.; Carmel, N.N.; Leshno, M.; Abergel, A.; Gutfeld, O.; Fliss, D.M. Elective Neck Dissection vs. Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue with No Clinically Apparent Lymph Node Metastasis in the Neck a Systematic Review and Meta-Analysis. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 857–865. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Subramaniam, N.; Missale, F.; Marchi, F.; Dokhe, Y.; Vijayan, S.; Nambiar, A.; Mattavelli, D.; Calza, S.; Bresciani, L.; et al. Predictive Nomograms for Oral Tongue Squamous Cell Carcinoma Applying the American Joint Committee on Cancer/Union Internationale Contre Le Cancer 8th Edition Staging System. Head Neck 2021, 43, 1043–1055. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Berdugo, J.; Thompson, L.D.R.; Purgina, B.; Sturgis, C.D.; Tuluc, M.; Seethala, R.; Chiosea, S.I. Measuring Depth of Invasion in Early Squamous Cell Carcinoma of the Oral Tongue: Positive Deep Margin, Extratumoral Perineural Invasion, and Other Challenges. Head Neck Pathol. 2019, 13, 154–161. [Google Scholar] [CrossRef]
- The International Consortium for Outcome Research (ICOR) in Head and Neck Cancer. Primary Tumor Staging for Oral Cancer and a Proposed Modification Incorporating Depth of Invasion: An International Multicenter Retrospective Study. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 1138–1148. [Google Scholar] [CrossRef]
- Chang, B.; He, W.; Ouyang, H.; Peng, J.; Shen, L.; Wang, A.; Wu, P. A Prognostic Nomogram Incorporating Depth of Tumor Invasion to Predict Long-Term Overall Survival for Tongue Squamous Cell Carcinoma with R0 Resection. J. Cancer 2018, 9, 2107–2115. [Google Scholar] [CrossRef]
- Huang, S.H.; Hwang, D.; Lockwood, G.; Goldstein, D.P.; O’Sullivan, B. Predictive Value of Tumor Thickness for Cervical Lymph-Node Involvement in Squamous Cell Carcinoma of the Oral Cavity: A Meta-Analysis of Reported Studies. Cancer 2009, 115, 1489–1497. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Feng, Z.; Cheng, A.; Alzahrani, S.; Li, B.; Han, Z.; Ward, B.B. Elective Neck Dissection in T1N0M0 Oral Squamous Cell Carcinoma: When Is It Necessary? J. Oral Maxillofac. Surg. 2020, 78, 2306–2315. [Google Scholar] [CrossRef]
- van Lanschot, C.G.F.; Klazen, Y.P.; de Ridder, M.A.J.; Mast, H.; ten Hove, I.; Hardillo, J.A.; Monserez, D.A.; Sewnaik, A.; Meeuwis, C.A.; Keereweer, S.; et al. Depth of Invasion in Early Stage Oral Cavity Squamous Cell Carcinoma: The Optimal Cut-off Value for Elective Neck Dissection. Oral Oncol. 2020, 111, 104940. [Google Scholar] [CrossRef]
- Aaboubout, Y.; van der Toom, Q.M.; de Ridder, M.A.J.; de Herdt, M.J.; van der Steen, B.; van Lanschot, C.G.F.; Barroso, E.M.; Nunes Soares, M.R.; ten Hove, I.; Mast, H.; et al. Is the Depth of Invasion a Marker for Elective Neck Dissection in Early Oral Squamous Cell Carcinoma? Front. Oncol. 2021, 11, 628320. [Google Scholar] [CrossRef]
- Piazza, C.; Montalto, N.; Paderno, A.; Taglietti, V.; Nicolai, P. Is It Time to Incorporate ‘Depth of Infiltration’ in the T Staging of Oral Tongue and Floor of Mouth Cancer? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 81–89. [Google Scholar] [CrossRef]
- Grégoire, V.; Lefebvre, J.-L.; Licitra, L.; Felip, E. Squamous Cell Carcinoma of the Head and Neck: EHNS–ESMO–ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2010, 21, v184–v186. [Google Scholar] [CrossRef]
- Marchi, F.; Filauro, M.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Santori, G.; Parrinello, G.; Canevari, F.R.M.; Piazza, C.; Peretti, G. Magnetic Resonance vs. Intraoral Ultrasonography in the Preoperative Assessment of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 9, 1571. [Google Scholar] [CrossRef]
- Shintani, S.; Yoshihama, Y.; Ueyama, Y.; Terakado, N.; Kamei, S.; Fijimoto, Y.; Hasegawa, Y.; Matsuura, H.; Matsumura, T. The Usefulness of Intraoral Ultrasonography in the Evaluation of Oral Cancer. Int. J. Oral. Maxillofac. Surg. 2001, 30, 139–143. [Google Scholar] [CrossRef]
- Yoon, B.C.; Bulbul, M.D.; Sadow, P.M.; Faquin, W.C.; Curtin, H.D.; Varvares, M.A.; Juliano, A.F. Comparison of Intraoperative Sonography and Histopathologic Evaluation of Tumor Thickness and Depth of Invasion in Oral Tongue Cancer: A Pilot Study. Am. J. Neuroradiol. 2020, 41, 1245–1250. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Tarabichi, O.; Parikh, A.S.; Yoon, B.C.; Juliano, A.; Sadow, P.M.; Faquin, W.; Gropler, M.; Walker, R.; Puram, S.V.; et al. The Utility of Intra-Oral Ultrasound in Improving Deep Margin Clearance of Oral Tongue Cancer Resections. Oral. Oncol. 2021, 122, 105512. [Google Scholar] [CrossRef]
- Songra, A.K.; Ng, S.Y.; Farthing, P.; Hutchison, I.L.; Bradley, P.F. Observation of Tumour Thickness and Resection Margin at Surgical Excision of Primary Oral Squamous Cell Carcinoma—Assessment by Ultrasound. Int. J. Oral. Maxillofac. Surg. 2006, 35, 324–331. [Google Scholar] [CrossRef]
- Yamane, M.; Ishii, J.; Izumo, T.; Nagasawa, T.; Amagasa, T. Noninvasive Quantitative Assessment of Oral Tongue Cancer by Intraoral Ultrasonography. Head Neck 2007, 29, 307–314. [Google Scholar] [CrossRef]
- Baek, C.-H.; Son, Y.-I.; Jeong, H.-S.; Chung, M.K.; Park, K.-N.; Ko, Y.-H.; Kim, H.-J. Intraoral Sonography–Assisted Resection of T1–2 Tongue Cancer for Adequate Deep Resection. Otolaryngol. Head Neck Surg. 2008, 139, 805–810. [Google Scholar] [CrossRef]
- Kaneoya, A.; Hasegawa, S.; Tanaka, Y.; Omura, K. Quantitative Analysis of Invasive Front in Tongue Cancer Using Ultrasonography. J. Oral Maxillofac. Surg. 2009, 67, 40–46. [Google Scholar] [CrossRef]
- Mark Taylor, S.; Drover, C.; MacEachern, R.; Bullock, M.; Hart, R.; Psooy, B.; Trites, J. Is Preoperative Ultrasonography Accurate in Measuring Tumor Thickness and Predicting the Incidence of Cervical Metastasis in Oral Cancer? Oral Oncol. 2010, 46, 38–41. [Google Scholar] [CrossRef]
- Kodama, M.; Khanal, A.; Habu, M.; Iwanaga, K.; Yoshioka, I.; Tanaka, T.; Morimoto, Y.; Tominaga, K. Ultrasonography for Intraoperative Determination of Tumor Thickness and Resection Margin in Tongue Carcinomas. J. Oral Maxillofac. Surg. 2010, 68, 1746–1752. [Google Scholar] [CrossRef]
- Chammas, M.C.; Macedo, T.A.A.; Moyses, R.A.; Gerhard, R.; Durazzo, M.D.; Cernea, C.R.; Cerri, G.G. Relationship between the Appearance of Tongue Carcinoma on Intraoral Ultrasonography and Neck Metastasis. Oral Radiol. 2011, 27, 1–7. [Google Scholar] [CrossRef]
- Lodder, W.L.; Teertstra, H.J.; Tan, I.B.; Pameijer, F.A.; Smeele, L.E.; van Velthuysen, M.L.F.; van den Brekel, M.W.M. Tumour Thickness in Oral Cancer Using an Intra-Oral Ultrasound Probe. Eur. Radiol. 2011, 21, 98–106. [Google Scholar] [CrossRef]
- Joshi, P.; Pol, J.; Sudesh, A. Ultrasonography—A Diagnostic Modality for Oral and Maxillofacial Diseases. Contemp. Clin. Dent. 2014, 5, 345. [Google Scholar] [CrossRef]
- Yesuratnam, A.; Wiesenfeld, D.; Tsui, A.; Iseli, T.A.; Hoorn, S.V.; Ang, M.T.; Guiney, A.; Phal, P.M. Preoperative Evaluation of Oral Tongue Squamous Cell Carcinoma with Intraoral Ultrasound and Magnetic Resonance Imaging—Comparison with Histopathological Tumour Thickness and Accuracy in Guiding Patient Management. Int. J. Oral Maxillofac. Surg. 2014, 43, 787–794. [Google Scholar] [CrossRef]
- Dirven, R.; Ebrahimi, A.; Moeckelmann, N.; Palme, C.E.; Gupta, R.; Clark, J. Tumor Thickness versus Depth of Invasion—Analysis of the 8th Edition American Joint Committee on Cancer Staging for Oral Cancer. Oral Oncol. 2017, 74, 30–33. [Google Scholar] [CrossRef]
- Liu, B.; Amaratunga, R.; Veness, M.; Wong, E.; Abdul-Razak, M.; Coleman, H.; Gebski, V.; Sundaresan, P. Tumor Depth of Invasion versus Tumor Thickness in Guiding Regional Nodal Treatment in Early Oral Tongue Squamous Cell Carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 45–50. [Google Scholar] [CrossRef]
- Iida, Y.; Kamijo, T.; Kusafuka, K.; Omae, K.; Nishiya, Y.; Hamaguchi, N.; Morita, K.; Onitsuka, T. Depth of Invasion in Superficial Oral Tongue Carcinoma Quantified Using Intraoral Ultrasonography. Laryngoscope 2018, 128, 2778–2782. [Google Scholar] [CrossRef]
- Filauro, M.; Missale, F.; Marchi, F.; Iandelli, A.; Carobbio, A.L.C.; Mazzola, F.; Parrinello, G.; Barabino, E.; Cittadini, G.; Farina, D.; et al. Intraoral Ultrasonography in the Assessment of DOI in Oral Cavity Squamous Cell Carcinoma: A Comparison with Magnetic Resonance and Histopathology. Eur. Arch. Oto Rhino Laryngol. 2021, 278, 2943–2952. [Google Scholar] [CrossRef]
- Rocchetti, F.; Tenore, G.; Montori, A.; Cassoni, A.; Cantisani, V.; di Segni, M.; di Gioia, C.R.T.; Carletti, R.; Valentini, V.; Polimeni, A.; et al. Preoperative Evaluation of Tumor Depth of Invasion in Oral Squamous Cell Carcinoma with Intraoral Ultrasonography: A Retrospective Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 130–138. [Google Scholar] [CrossRef]
- Takamura, M.; Kobayashi, T.; Nikkuni, Y.; Katsura, K.; Yamazaki, M.; Maruyama, S.; Tanuma, J.; Hayashi, T. A Comparative Study between CT, MRI, and Intraoral US for the Evaluation of the Depth of Invasion in Early Stage (T1/T2) Tongue Squamous Cell Carcinoma. Oral Radiol. 2022, 38, 114–125. [Google Scholar] [CrossRef]
- Noorlag, R.; Klein Nulent, T.J.W.; Delwel, V.E.J.; Pameijer, F.A.; Willems, S.M.; de Bree, R.; van Es, R.J.J. Assessment of Tumour Depth in Early Tongue Cancer: Accuracy of MRI and Intraoral Ultrasound. Oral Oncol. 2020, 110, 104895. [Google Scholar] [CrossRef]
- Umstattd, L.A.; Mills, J.C.; Critchlow, W.A.; Renner, G.J.; Zitsch, R.P. Shrinkage in Oral Squamous Cell Carcinoma: An Analysis of Tumor and Margin Measurements in Vivo, Post-Resection, and Post-Formalin Fixation. Am. J. Otolaryngol. 2017, 38, 660–662. [Google Scholar] [CrossRef]
- Johnson, R.E.; Sigman, J.D.; Funk, G.F.; Robinson, R.A.; Hoffman, H.T. Quantification of Surgical Margin Shrinkage in the Oral Cavity. Head Neck 1997, 19, 281–286. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, L.; Li, J.; Chen, P.; Shi, W.; Shen, S.; Zhang, C.; Zhang, Z. Assessing the Magnetic Resonance Imaging in Determining the Depth of Invasion of Tongue Cancer. Oral Dis. 2021, 27, 457–463. [Google Scholar] [CrossRef]
- Klein Nulent, T.J.W.; Noorlag, R.; van Cann, E.M.; Pameijer, F.A.; Willems, S.M.; Yesuratnam, A.; Rosenberg, A.J.W.P.; de Bree, R.; van Es, R.J.J. Intraoral Ultrasonography to Measure Tumor Thickness of Oral Cancer: A Systematic Review and Meta-Analysis. Oral Oncol. 2018, 77, 29–36. [Google Scholar] [CrossRef]


| Advantages | Disadvantages | |
|---|---|---|
| CT | Spatial resolution Visualization of bone erosion Panoramic | Low contrast resolution Influenced by metal artifacts Superficial lesions not always visible |
| MRI | Contrast resolution Multiparametric Panoramic | Influenced by metal artifacts Superficial lesions not always visible |
| IOUS | Very high spatial resolution Direct visualization of lesions | Operator dependent: rotational overestimation, compression distortion, need for expert hands Not possible if trismus is present Expensive and not diffuse technology |
| N (%) | |
|---|---|
| Patients | 41 (100) |
| Females | 16 (39) |
| Males | 25 (61) |
| Mean age (st. deviation) | 64.07 (17.67) |
| pTis | 5 (12.20) |
| pT1 | 21 (51.22) |
| pT2 | 15 (36.58) |
| Mean pDOI (st. deviation) | 3.07 (2.65) |
| Mean usDOI (st. deviation) | 3.79 (2.74) |
| Mean mriDOI (st. deviation) | 5.39 (2.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caprioli, S.; Casaleggio, A.; Tagliafico, A.S.; Conforti, C.; Borda, F.; Fiannacca, M.; Filauro, M.; Iandelli, A.; Marchi, F.; Parrinello, G.; et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations. Int. J. Environ. Res. Public Health 2022, 19, 14900. https://doi.org/10.3390/ijerph192214900
Caprioli S, Casaleggio A, Tagliafico AS, Conforti C, Borda F, Fiannacca M, Filauro M, Iandelli A, Marchi F, Parrinello G, et al. High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations. International Journal of Environmental Research and Public Health. 2022; 19(22):14900. https://doi.org/10.3390/ijerph192214900
Chicago/Turabian StyleCaprioli, Simone, Alessandro Casaleggio, Alberto Stefano Tagliafico, Cristina Conforti, Fabio Borda, Martina Fiannacca, Marta Filauro, Andrea Iandelli, Filippo Marchi, Giampiero Parrinello, and et al. 2022. "High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations" International Journal of Environmental Research and Public Health 19, no. 22: 14900. https://doi.org/10.3390/ijerph192214900
APA StyleCaprioli, S., Casaleggio, A., Tagliafico, A. S., Conforti, C., Borda, F., Fiannacca, M., Filauro, M., Iandelli, A., Marchi, F., Parrinello, G., Peretti, G., & Cittadini, G. (2022). High-Frequency Intraoral Ultrasound for Preoperative Assessment of Depth of Invasion for Early Tongue Squamous Cell Carcinoma: Radiological–Pathological Correlations. International Journal of Environmental Research and Public Health, 19(22), 14900. https://doi.org/10.3390/ijerph192214900






