Predictors of Early Cardiac Implantable Electronic Device Lead Dislodgement in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Frailty Evaluation
- Very fit—Robust active, energetic, well-motivated, and fit; these people commonly exercise regularly and are in the fittest group for their age.
- Well—Without any active disease, but less fit than people in category 1.
- Well, with treated comorbid disease—Disease symptoms are well controlled compared to those in category 4.
- Apparently vulnerable—Although not clinically dependent, these people commonly complain of being “slowed down” or having disease symptoms.
- Mildly frail—With limited dependence on others for the instrumental activities of daily living.
- Moderately frail—Help is needed with both the instrumental and non-instrumental activities of daily living.
- Severely frail—Completely dependent on others for the activities of daily living or terminally ill.
2.2. Statistical Analysis
2.3. Bioethical Consideration
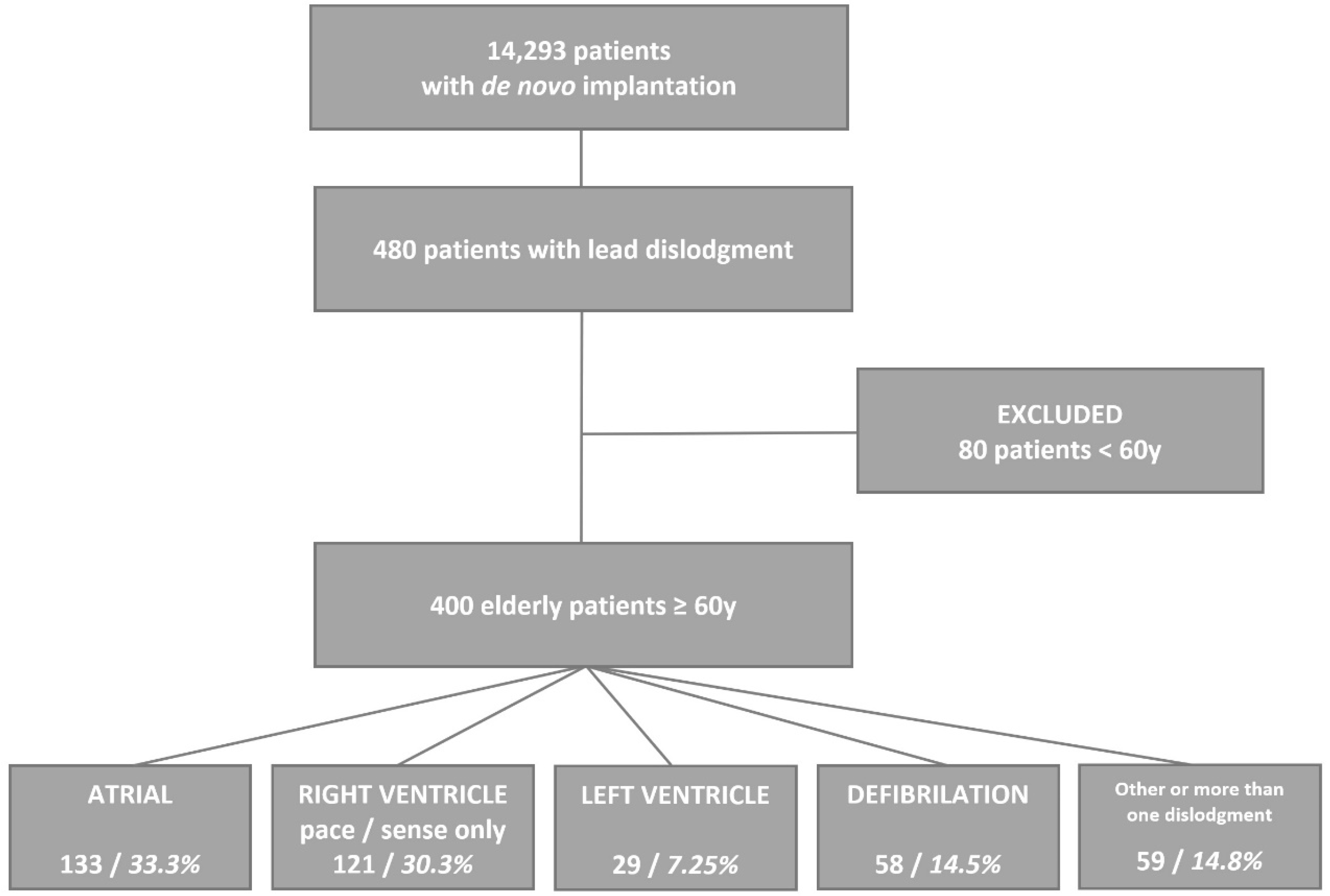
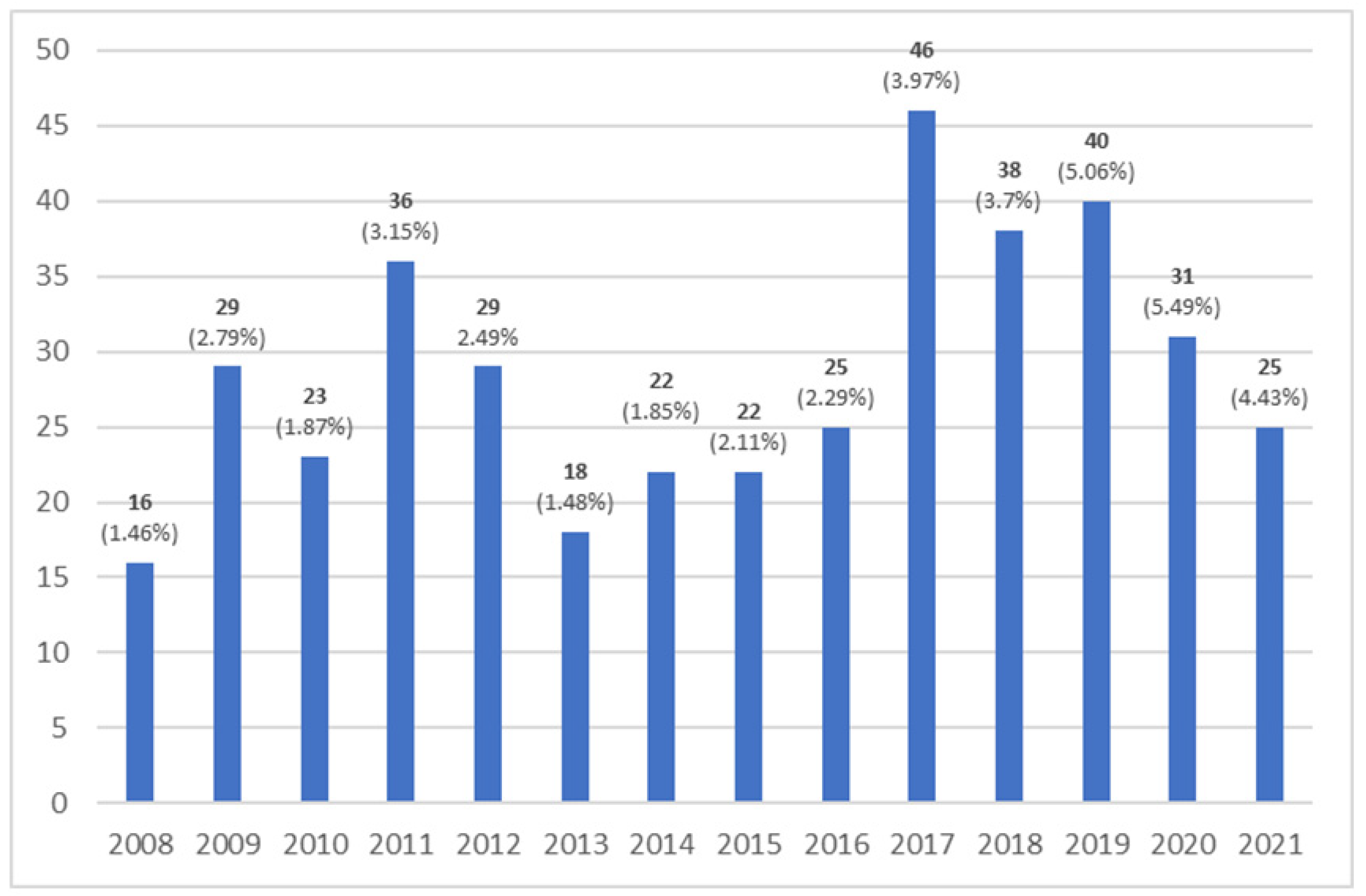
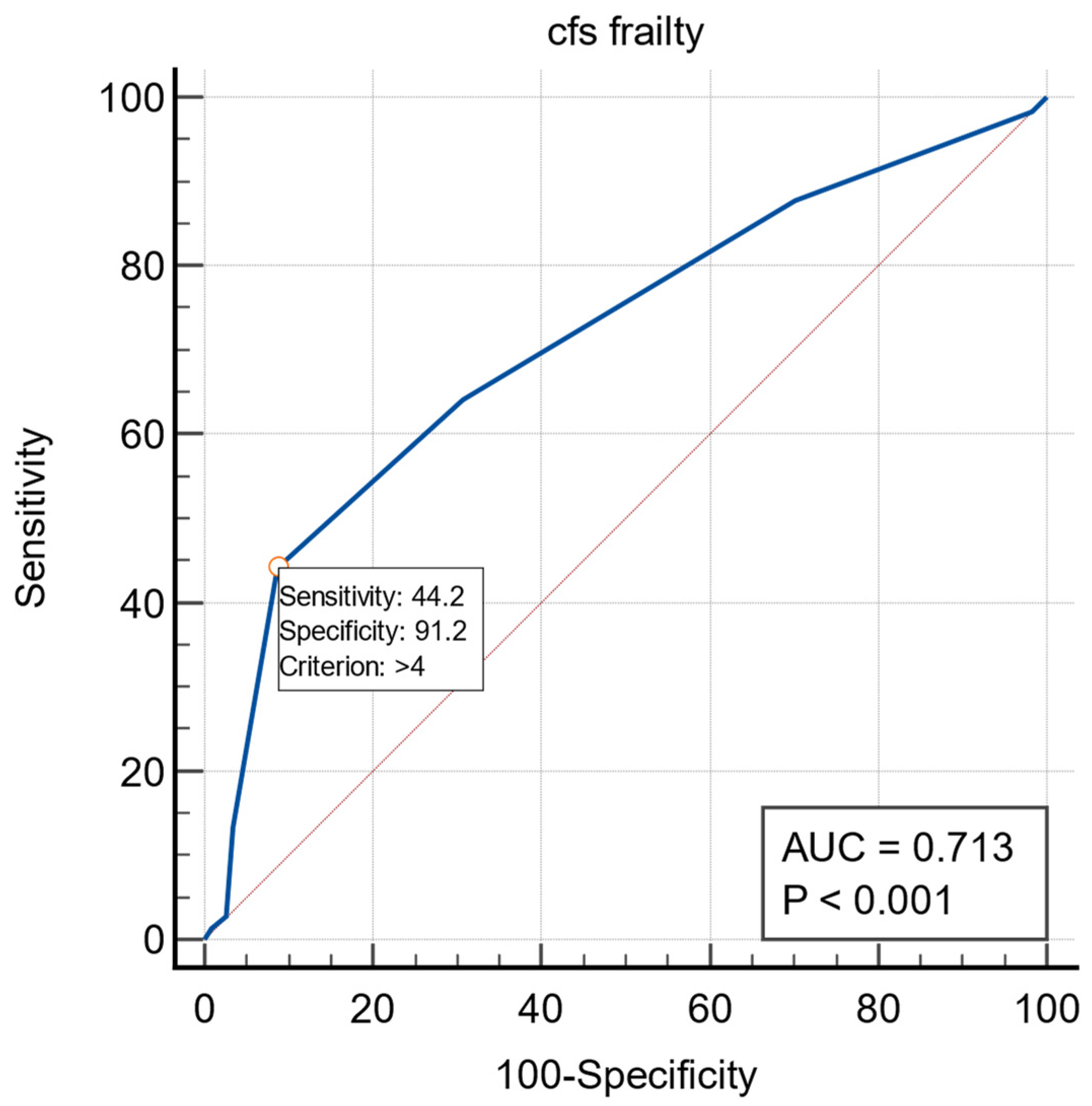
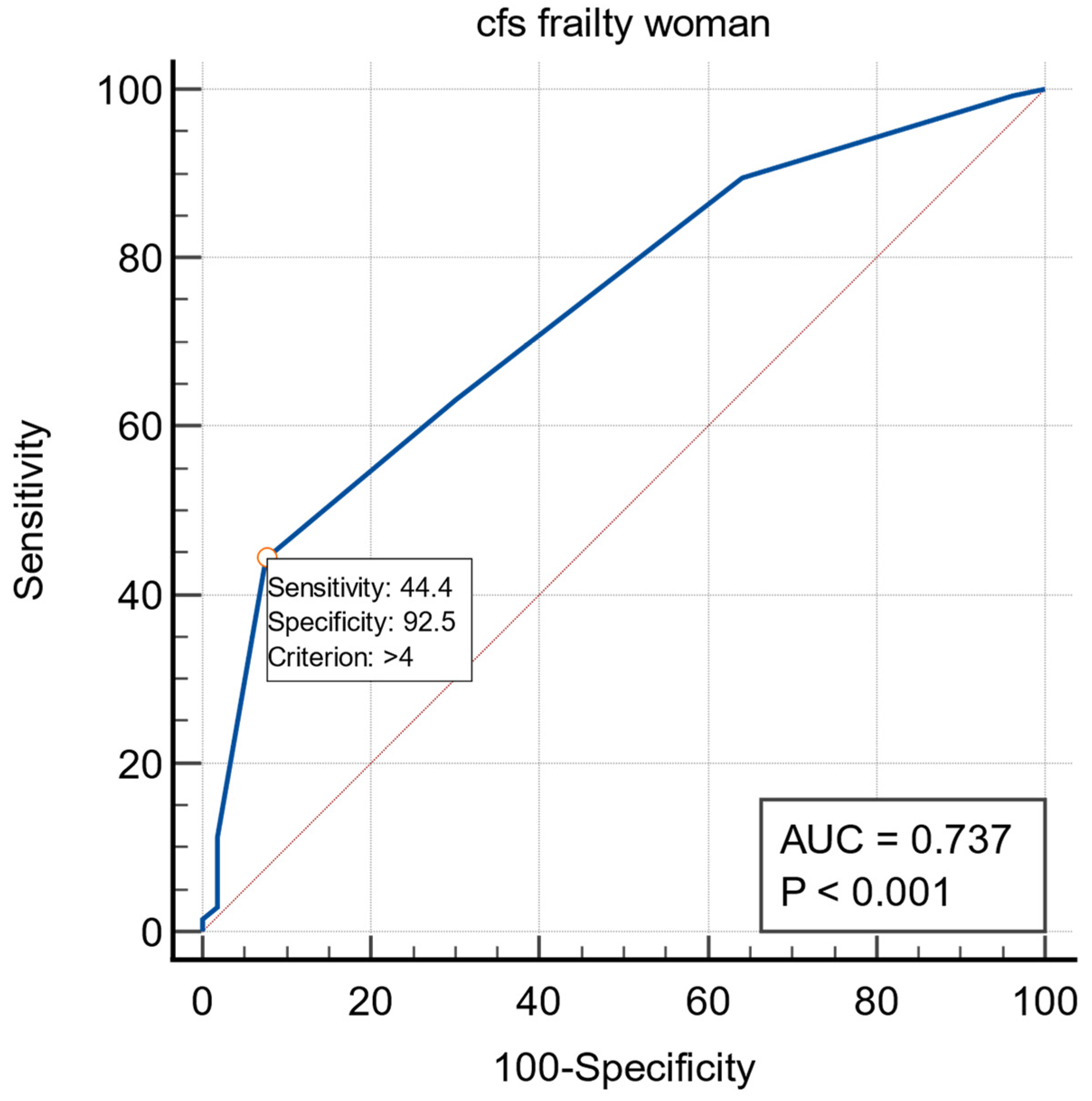
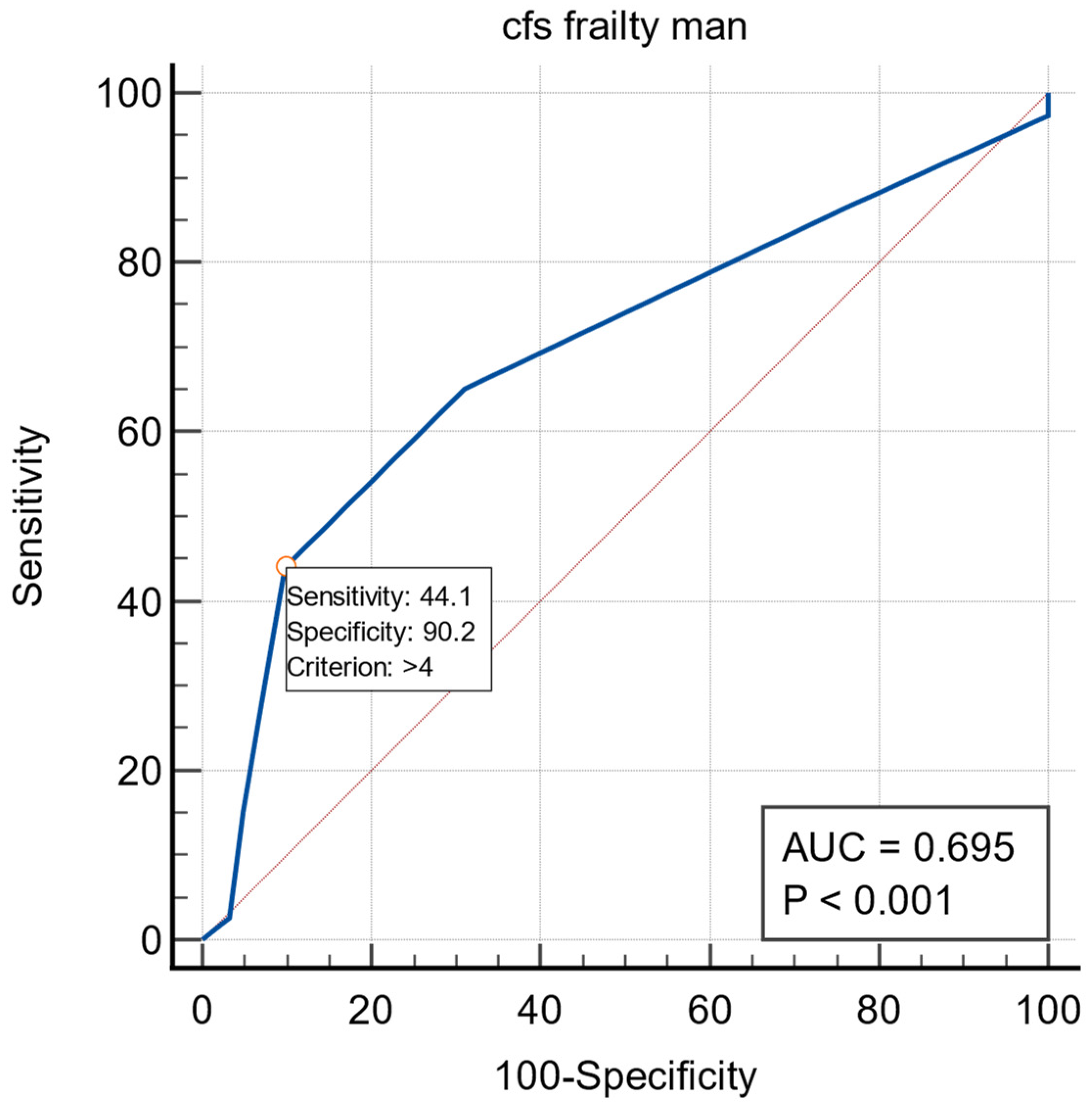
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sterliński, M.; Przybylski, A.; Maciąg, A.; Syska, P.; Pytkowski, M.; Lewandowski, M.; Kowalik, I.; Firek, B.; Kołsut, P.; Religa, G.; et al. Subacute cardiac perforations associated with active fixation leads. Europace 2008, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Bybee, K.A.; Bunch, T.J.; Espinosa, R.E.; Sinak, L.J.; McGoon, M.D.; Hayes, D.L. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005, 2, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Ohlow, M.A.; Lauer, B.; Brunelli, M.; Geller, J.C. Incidence and predictors of pericardial effusion after permanent heart rhythm device implantation: Prospective evaluation of 968 consecutive patients. Circ. J. 2013, 77, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.M.; Epstein, A.E.; Darbar, D.; Dimarco, J.P.; Dunbar, S.B.; Estes, N.A.M.; Ferguson, T.B.; Hammill, S.C.; Karasik, P.E.; Link, M.S.; et al. 2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Heart Rhythm 2012, 9, 1737–1753. [Google Scholar] [CrossRef] [PubMed]
- van Rees, J.B.; de Bie, M.K.; Thijssen, J.; Borleffs, C.J.W.; Schalij, M.J.; van Erven, L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: A systematic review of randomized clinical trials. J. Am. Coll Cardiol. 2011, 58, 995–1000. [Google Scholar] [CrossRef]
- Ghani, A.; Delnoy, P.P.H.M.; Misier, A.R.R.; Smit, J.J.J.; Adiyaman, A.; Ottervanger, J.P.; Elvan, A. Incidence of lead dislodgement, malfunction and perforation during the first year following device implantation. Neth. Heart J. 2014, 22, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, K.; Konstantelias, A.A.; Falagas, M.E. Risk factors for cardiac implantable electronic device infection: A systematic review and meta-analysis. Europace 2015, 17, 767–777. [Google Scholar] [CrossRef]
- Prutkin, J.M.; Reynolds, M.R.; Bao, H.; Curtis, J.P.; Al-Khatib, S.M.; Aggarwal, S.; Uslan, D.Z. Response to Letter Regarding Article, “Rates of and Factors Associated With Infection in 200 909 Medicare Implantable Cardioverter-Defibrillator Implants: Results From the National Cardiovascular Data Registry”. Circulation 2015, 131, e518. [Google Scholar] [CrossRef][Green Version]
- Traykov, V.; Bongiorni, M.G.; Boriani, G.; Burri, H.; Costa, R.; Dagres, N.; Deharo, J.-C.; Epstein, L.; Erba, P.A.; Snygg-Martin, U.; et al. Clinical practice and implementation of guidelines for the prevention, diagnosis and management of cardiac implantable electronic device infections: Results of a worldwide survey under the auspices of the European Heart Rhythm Association. Europace 2019, 21, 1270–1279. [Google Scholar] [CrossRef]
- Poole, J.E.; Gleva, M.J.; Mela, T.; Chung, M.K.; Uslan, D.Z.; Borge, R.; Gottipaty, V.; Shinn, T.; Dan, D.; Feldman, L.A.; et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: Results from the REPLACE registry. Circulation 2010, 122, 1553–1561. [Google Scholar] [CrossRef]
- Afzal, M.R.; Horner, S.; Matre, N.B.; Blake, P.; Dunham, K.; Pinkhas, D.; Okabe, T.; Tyler, J.; Houmsse, M.; Kalbfleisch, S.J.; et al. Comprehensive strategy to reduce the incidence of lead dislodgement for cardiac implantable electronic devices. Pacing Clin. Electrophysiol. 2018, 42, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Migliore, F.; Zorzi, A.; Bertaglia, E.; Leoni, L.; Siciliano, M.; de Lazzari, M.; Ignatiuk, B.; Veronese, M.; Verlato, R.; Tarantini, G.; et al. Incidence, Management, and Prevention of Right Ventricular Perforation by Pacemaker and Implantable Cardioverter Defibrillator Leads. Pacing Clin. Electrophysiol. 2014, 37, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, Y.; Curtis, J.P.; Varosy, P.D. Acute Lead Dislodgements and In-Hospital Mortality in Patients Enrolled in the National Cardiovascular Data Registry Implantable Cardioverter Defibrillator Registry. J. Am. Coll. Cardiol. 2010, 56, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Manzano, L.; Babalis, D.; Roughton, M.; Shibata, M.; Anker, S.D.; Ghio, S.; van Veldhuisen, D.J.; Cohen-Solal, A.; Coats, A.J.; Poole-Wilson, P.P.; et al. Predictors of clinical outcomes in elderly patients with heart failure. Eur. J. Heart Fail. 2011, 13, 528–536. [Google Scholar] [CrossRef]
- Singh, M.; Stewart, R.; White, H. Importance of frailty in patients with cardiovascular disease. Eur. Heart J. 2014, 35, 1726–1731. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Dębski, M.; Ulman, M.; Ząbek, A.; Boczar, K.; Haberka, K.; Kuniewicz, M.; Lelakowski, J.; Małecka, B. Lead-related complications after DDD pacemaker implantation. Kardiol. Polska 2018, 76, 1224–1231. [Google Scholar] [CrossRef]
- Ellenbogen, K.A.; Hellkamp, A.S.; Wilkoff, B.; Camunãs, J.L.; Love, J.C.; Hadjis, T.A.; Lee, K.L.; Lamas, G.A. Complications arising after implantation of DDD pacemakers: The MOST experience. Am. J. Cardiol. 2003, 92, 740–741. [Google Scholar] [CrossRef]
- Swindle, J.P.; Rich, M.W.; McCann, P.; Burroughs, T.E.; Hauptman, P.J. Implantable cardiac device procedures in older pa-tients: Use and in-hospital outcomes. Arch. Intern. Med. 2010, 170, 631–637. [Google Scholar] [CrossRef][Green Version]
- Huang, D.T.; Sesselberg, H.W.; McNitt, S.; Noyes, K.; Andrews, M.L.; Hall, W.J.; Dick, A.; Daubert, J.P.; Zareba, W.; Moss, A.J.; et al. Improved Survival Associated with Prophylactic Implantable Defibrillators in Elderly Patients with Prior Myocardial Infarction and Depressed Ventricular Function: A MADIT-II Substudy. J. Cardiovasc. Electrophysiol. 2007, 18, 833–838. [Google Scholar] [CrossRef]
- Buckinx, F.; Rolland, Y.; Reginster, J.Y.; Ricour, C.; Petermans, J.; Bruyère, O. Burden of frailty in the elderly population: Per-spectives for a public health challenge. Arch Public Health 2015, 73, 19. [Google Scholar] [CrossRef] [PubMed]
- Kluszczyńska, M.; Młynarska, A.; Mikulakova, W. Influence of Frailty Syndrome on Kinesiophobia According to the Gender of Patients after Coronary Artery Bypass Surgery. Healthcare 2021, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Mlynarska, A.; Mlynarski, R.; Golba, K.S. Influence of frailty on the quality of life patients qualified for pacemaker implantation. J. Clin. Nurs. 2017, 27, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Mlynarska, A.; Mlynarski, R.; Golba, K. Frailty as a predictor of negative outcomes after cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2018, 41, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Mlynarska, A.; Mlynarski, R.; Marcisz, C.; Golba, K.S. Modified frailty as a novel factor in predicting the response to cardiac resynchronization in the elderly population. Clin. Interv. Aging 2019, 14, 437–443. [Google Scholar] [CrossRef]
- Mlynarska, A.; Mlynarski, R.; Golba, K.S. Frailty syndrome in patients with heart rhythm disorders. Geriatr. Gerontol. Int. 2016, 17, 1313–1318. [Google Scholar] [CrossRef]
- Ruiz-Granell, R.; Ferrero, A.; Morell-Cabedo, S.; Martinez-Brotons, A.; Bertomeu, V.; Llacer, A.; García-Civera, R. Implantation of sin-gle-lead atrioventricular permanent pacemakers guided by electroanatomic navigation without the use of fluoroscopy. Europace 2008, 10, 1048–1051. [Google Scholar] [CrossRef]
- Pagan, E.; Gabriels, J.; Khodak, A.; Chang, D.; Beldner, S.; Epstein, L.M.; Willner, J. Safety of leadless pacemaker implantation in the very elderly. Heart Rhythm 2020, 17, 2023–2028. [Google Scholar] [CrossRef]
| Variable | B | SE | Wald | 95% CI | p |
|---|---|---|---|---|---|
| Frailty | 0.5986 | 0.09885 | 36.6727 | 1.4991–2.2086 | <0.0001 |
| Age | 0.0310 | 0.01554 | 3.9775 | 1.0005–1.0634 | 0.0461 |
| Constant | −3.5227 | 1.20654 | 8.5248 |
| Variable | B | SE | Wald | 95% CI | p |
|---|---|---|---|---|---|
| Frailty | 0.75069 | 0.15842 | 22.4536 | 1.5530–2.8899 | <0.0001 |
| Age | 0.040446 | 0.022820 | 3.1414 | 0.9957–1.0889 | 0.0763 |
| Constant | −4.73957 | 1.83476 | 6.6730 |
| Variable | B | SE | Wald | 95% CI | p |
|---|---|---|---|---|---|
| Frailty | 0.48990 | 0.12698 | 14.8846 | 1.2725–2.0934 | 0.0001 |
| Age | 0.02426 | 0.021824 | 1.2357 | 1.0005–1.0634 | 0.2663 |
| Constant | −2.64838 | 1.64288 | 2.5987 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlynarski, R.; Mlynarska, A.; Joniec, M.; Gladysz-Wanha, S.; Honkowicz, M.; Stachanczyk, J.; Golba, K.S. Predictors of Early Cardiac Implantable Electronic Device Lead Dislodgement in the Elderly. Int. J. Environ. Res. Public Health 2022, 19, 14766. https://doi.org/10.3390/ijerph192214766
Mlynarski R, Mlynarska A, Joniec M, Gladysz-Wanha S, Honkowicz M, Stachanczyk J, Golba KS. Predictors of Early Cardiac Implantable Electronic Device Lead Dislodgement in the Elderly. International Journal of Environmental Research and Public Health. 2022; 19(22):14766. https://doi.org/10.3390/ijerph192214766
Chicago/Turabian StyleMlynarski, Rafal, Agnieszka Mlynarska, Michal Joniec, Sylwia Gladysz-Wanha, Maciej Honkowicz, Joanna Stachanczyk, and Krzysztof S. Golba. 2022. "Predictors of Early Cardiac Implantable Electronic Device Lead Dislodgement in the Elderly" International Journal of Environmental Research and Public Health 19, no. 22: 14766. https://doi.org/10.3390/ijerph192214766
APA StyleMlynarski, R., Mlynarska, A., Joniec, M., Gladysz-Wanha, S., Honkowicz, M., Stachanczyk, J., & Golba, K. S. (2022). Predictors of Early Cardiac Implantable Electronic Device Lead Dislodgement in the Elderly. International Journal of Environmental Research and Public Health, 19(22), 14766. https://doi.org/10.3390/ijerph192214766






