Improved Dewaterability of Waste Activated Sludge by Fe(II)-Activated Potassium Periodate Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Analyses
2.3.1. Determination of Sludge Dewaterability
2.3.2. Sludge Properties
3. Results and Discussion
3.1. Optimization of Sludge Dewatering Conditions
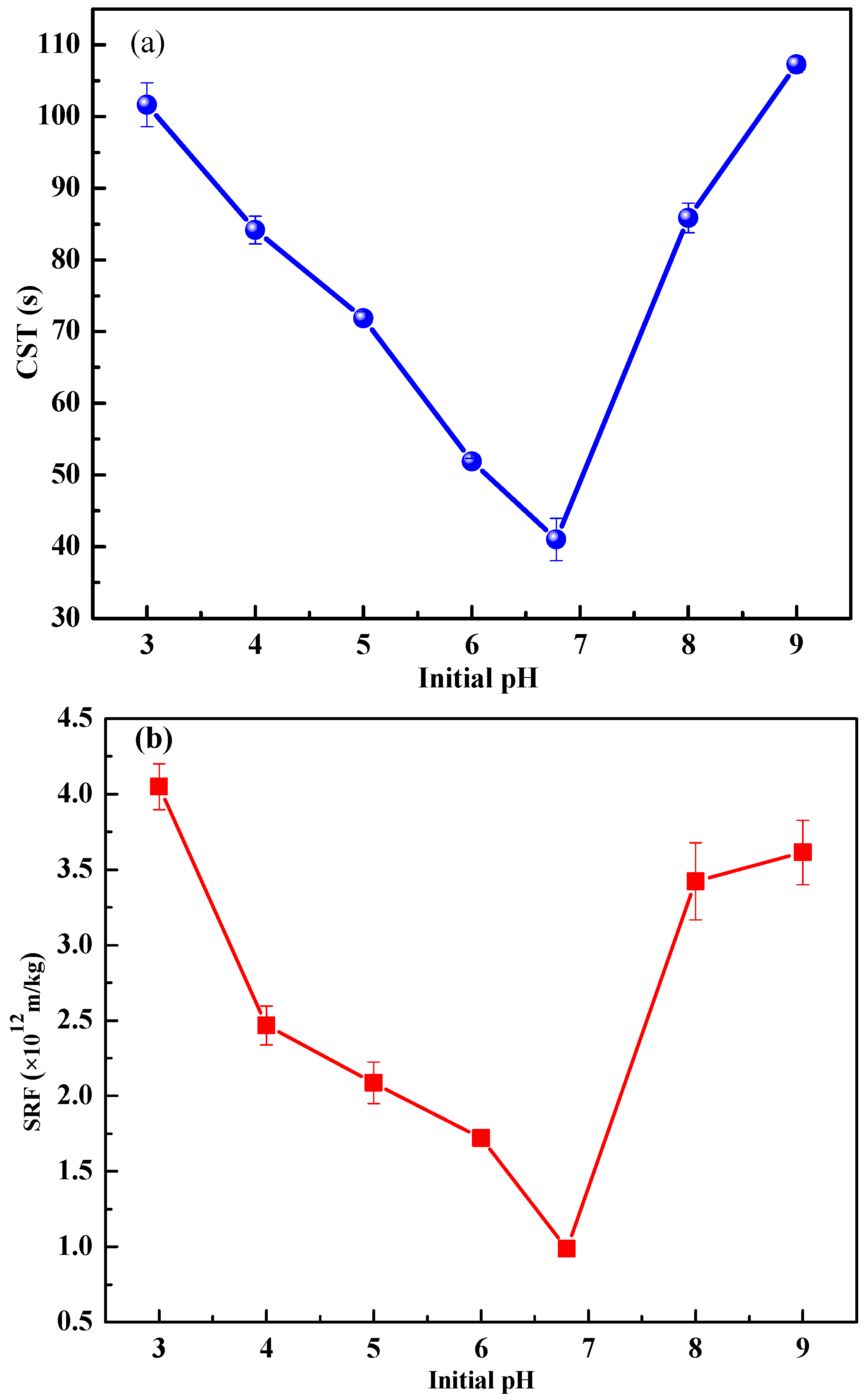
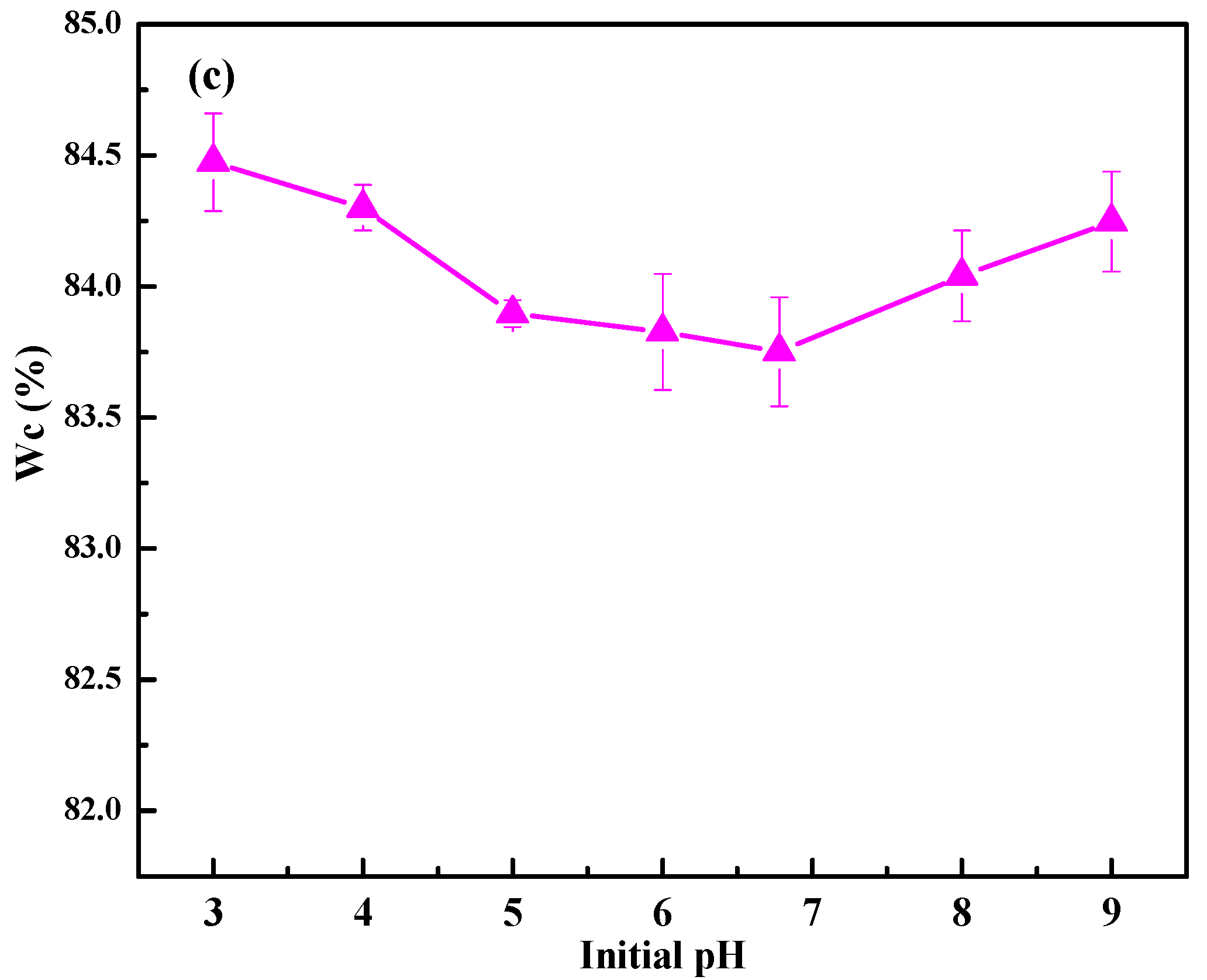
3.1.1. Effects of Initial pH on Sludge Dewaterability
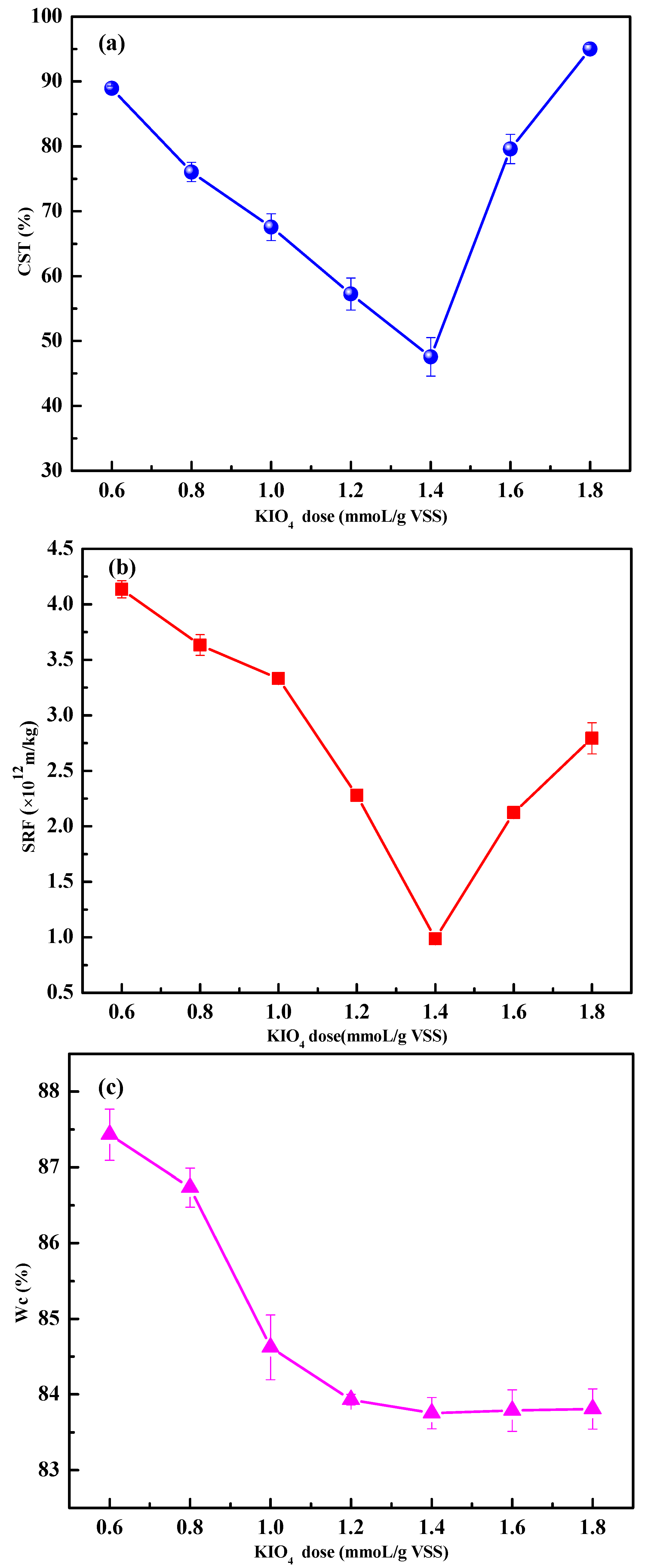
3.1.2. Effects of KIO4 Dose on Sludge Dewaterability
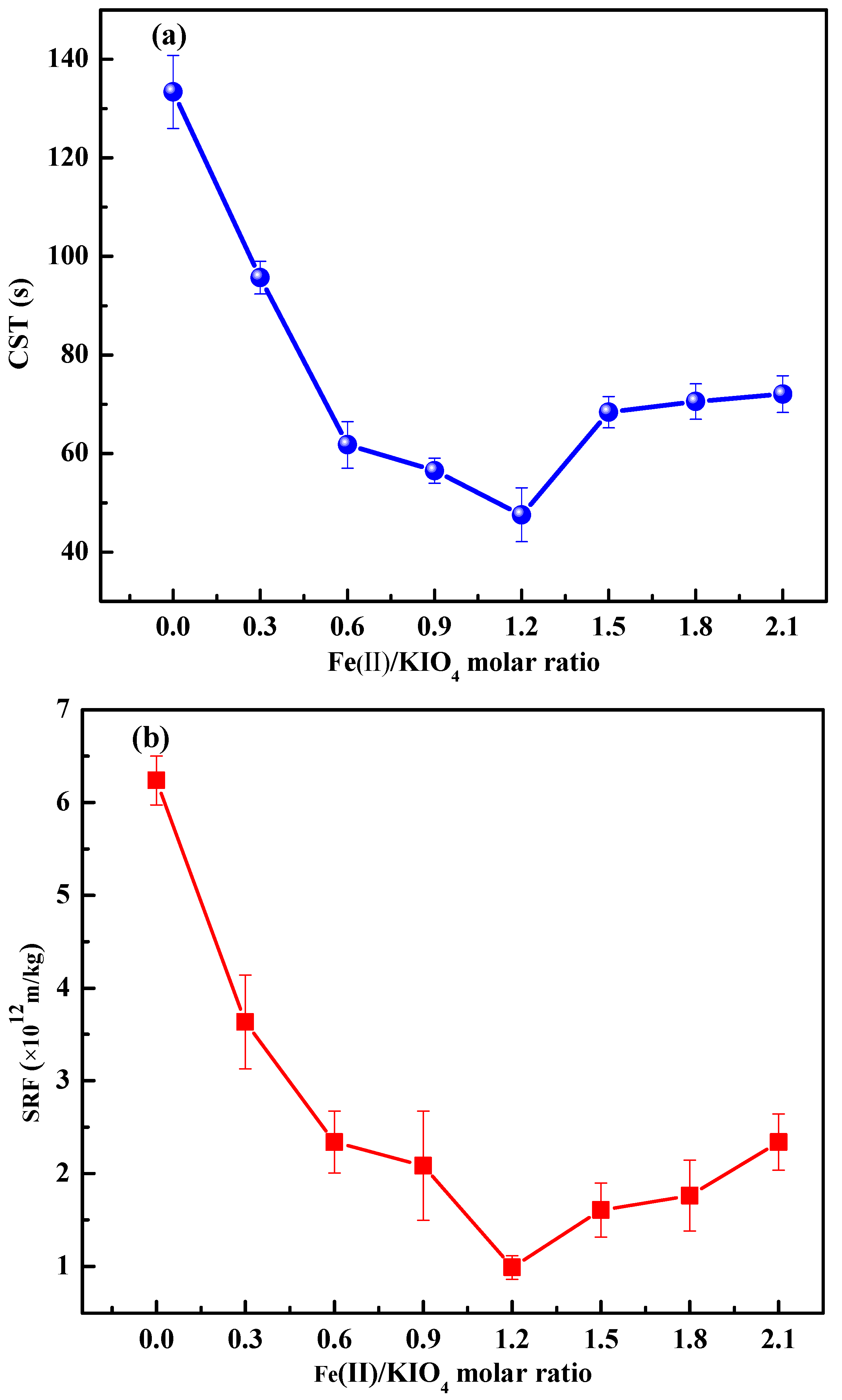
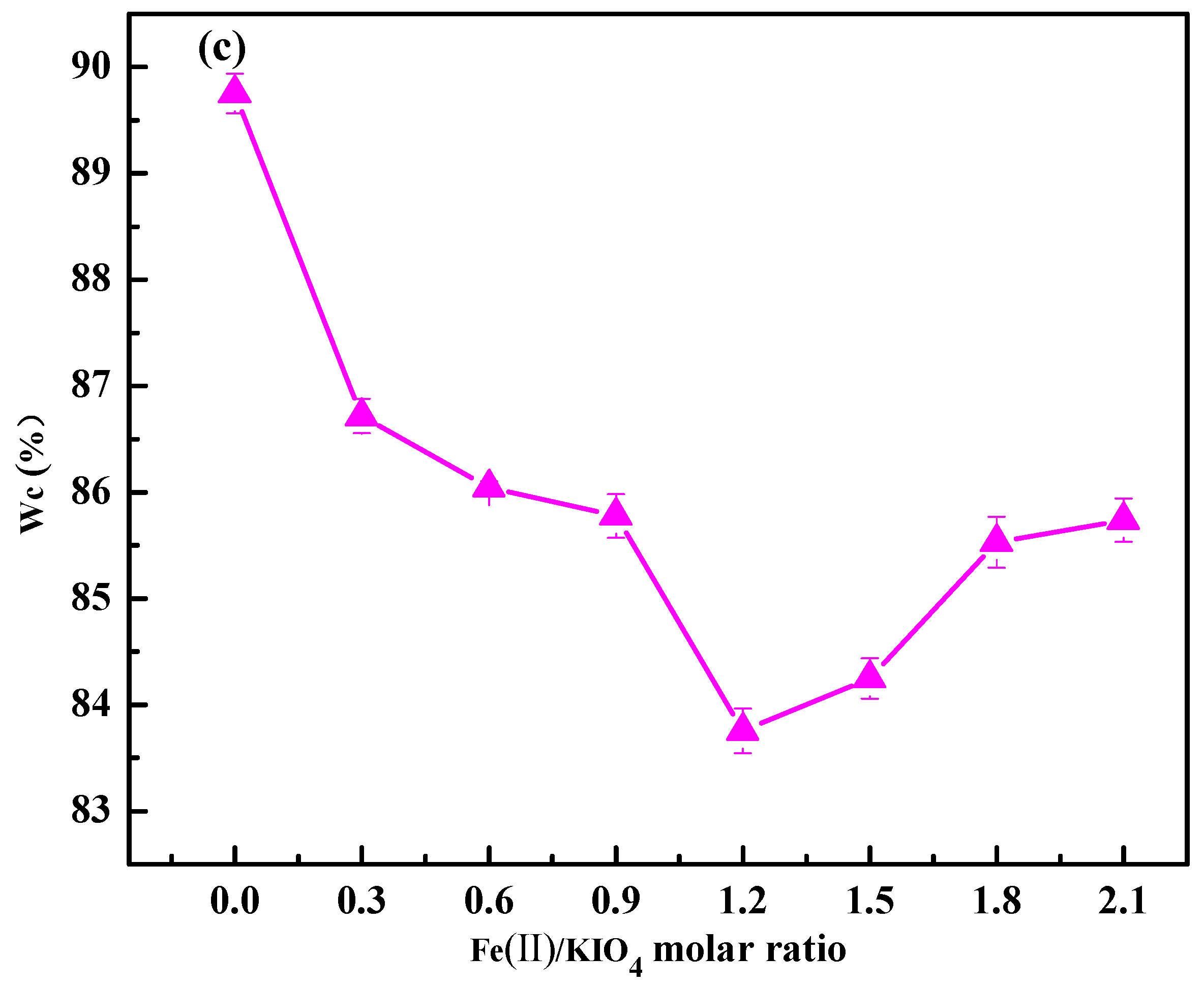
3.1.3. Effects of Fe(II)/KIO4 Molar Ratio on Sludge Dewaterability
3.2. Effects of Fe(II)/KIO4 Process on Sludge Properties
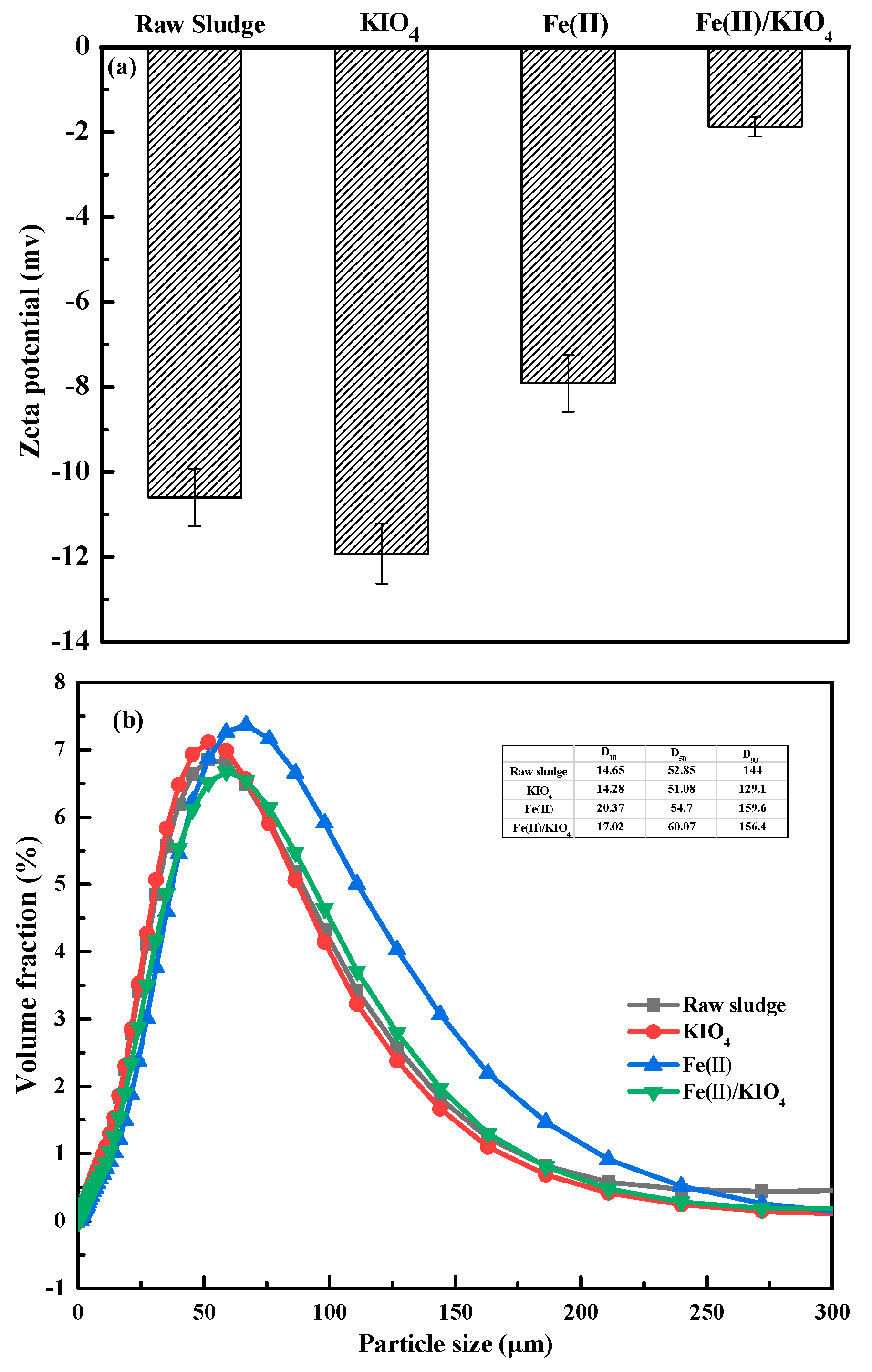
3.2.1. Zeta Potential and Particle Size Distribution
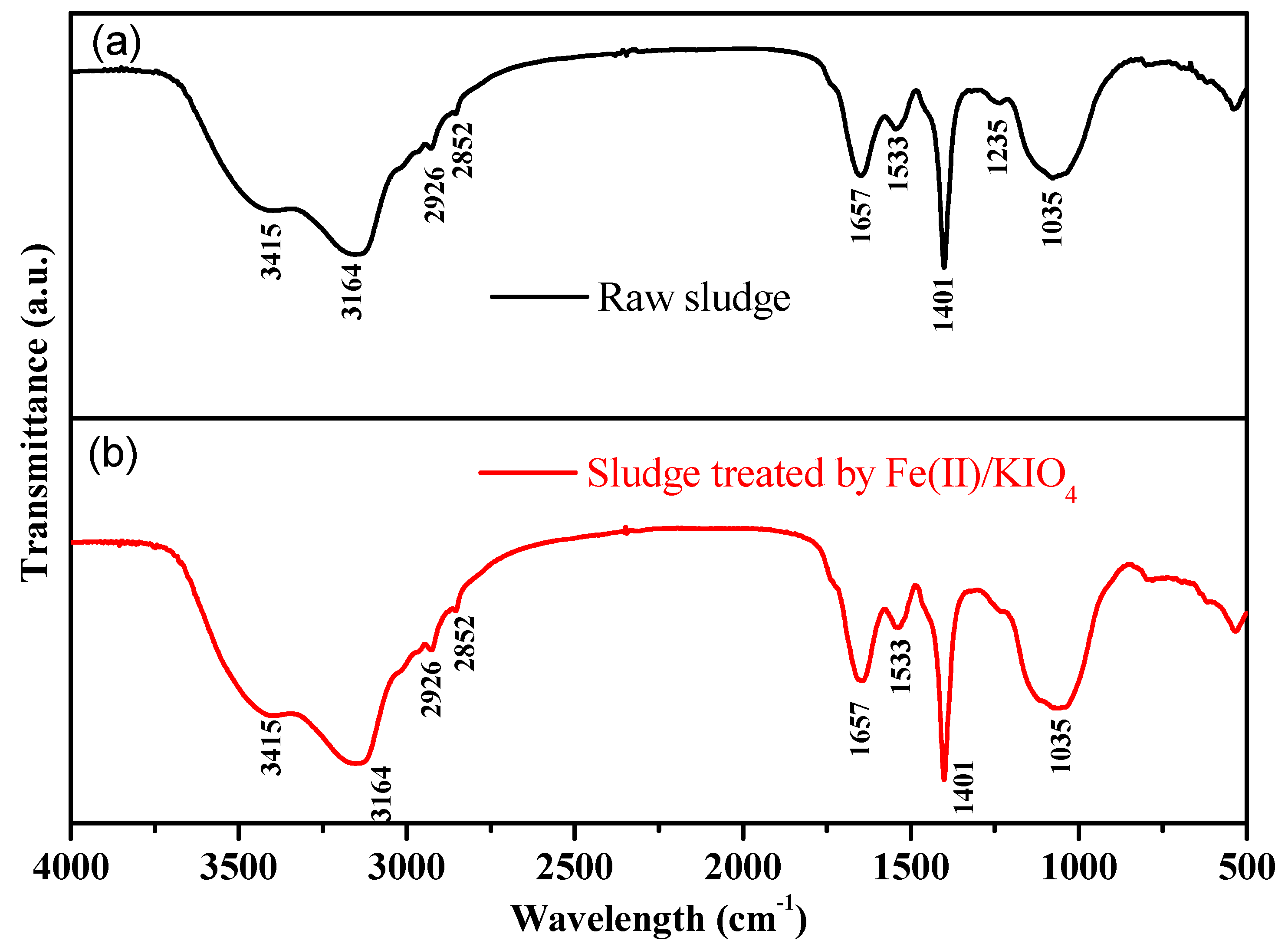
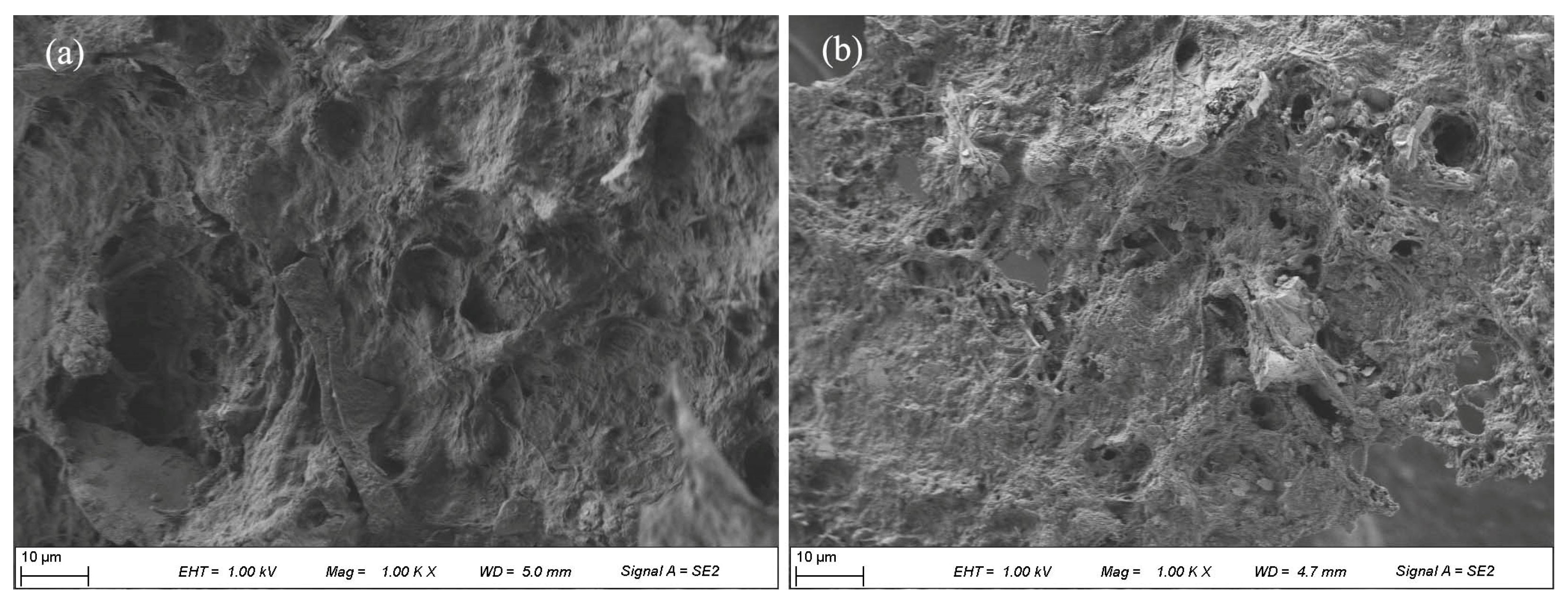
3.2.2. FT-IR Spectra and SEM Analyses
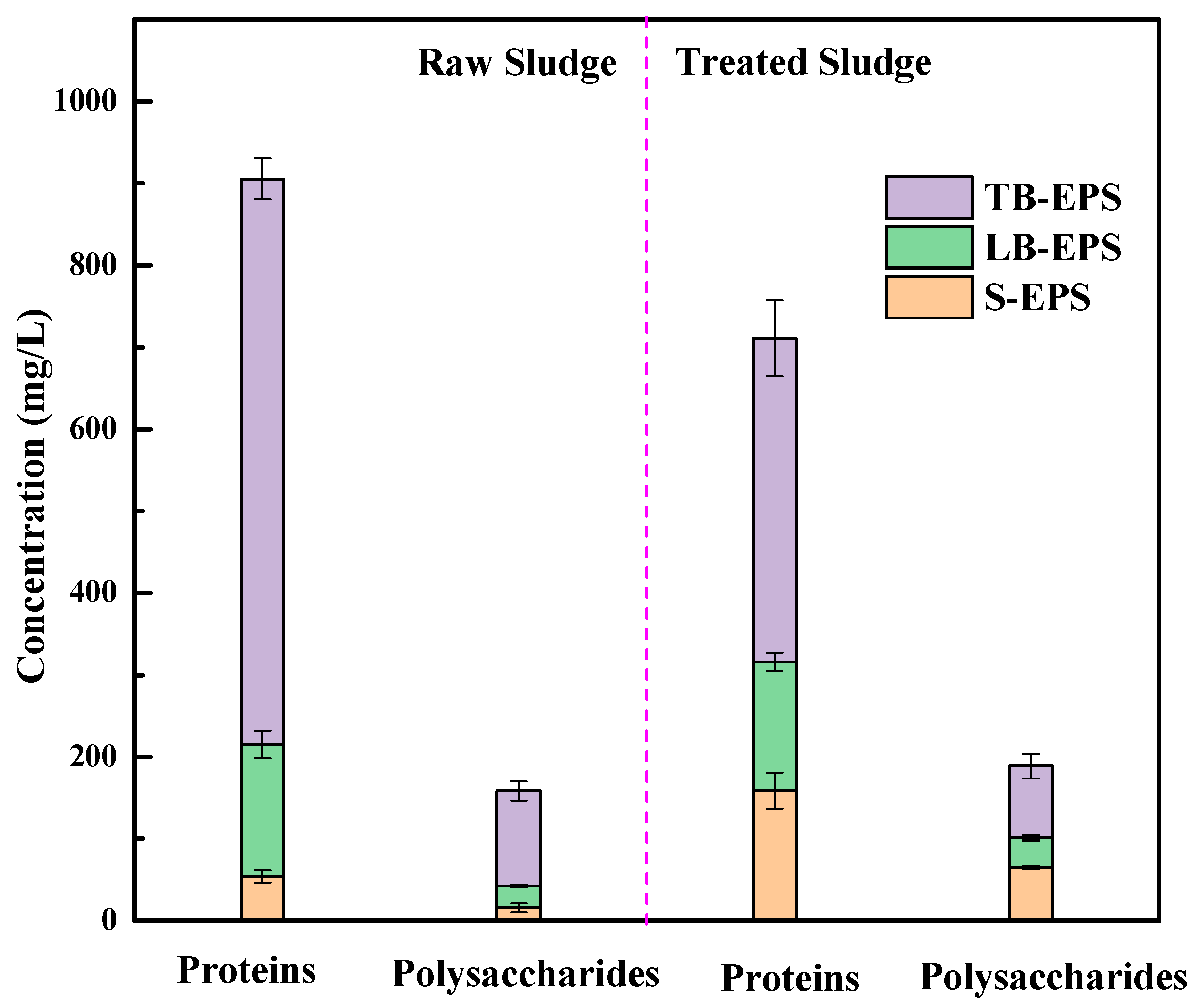
3.2.3. Polysaccharide and Protein in EPS
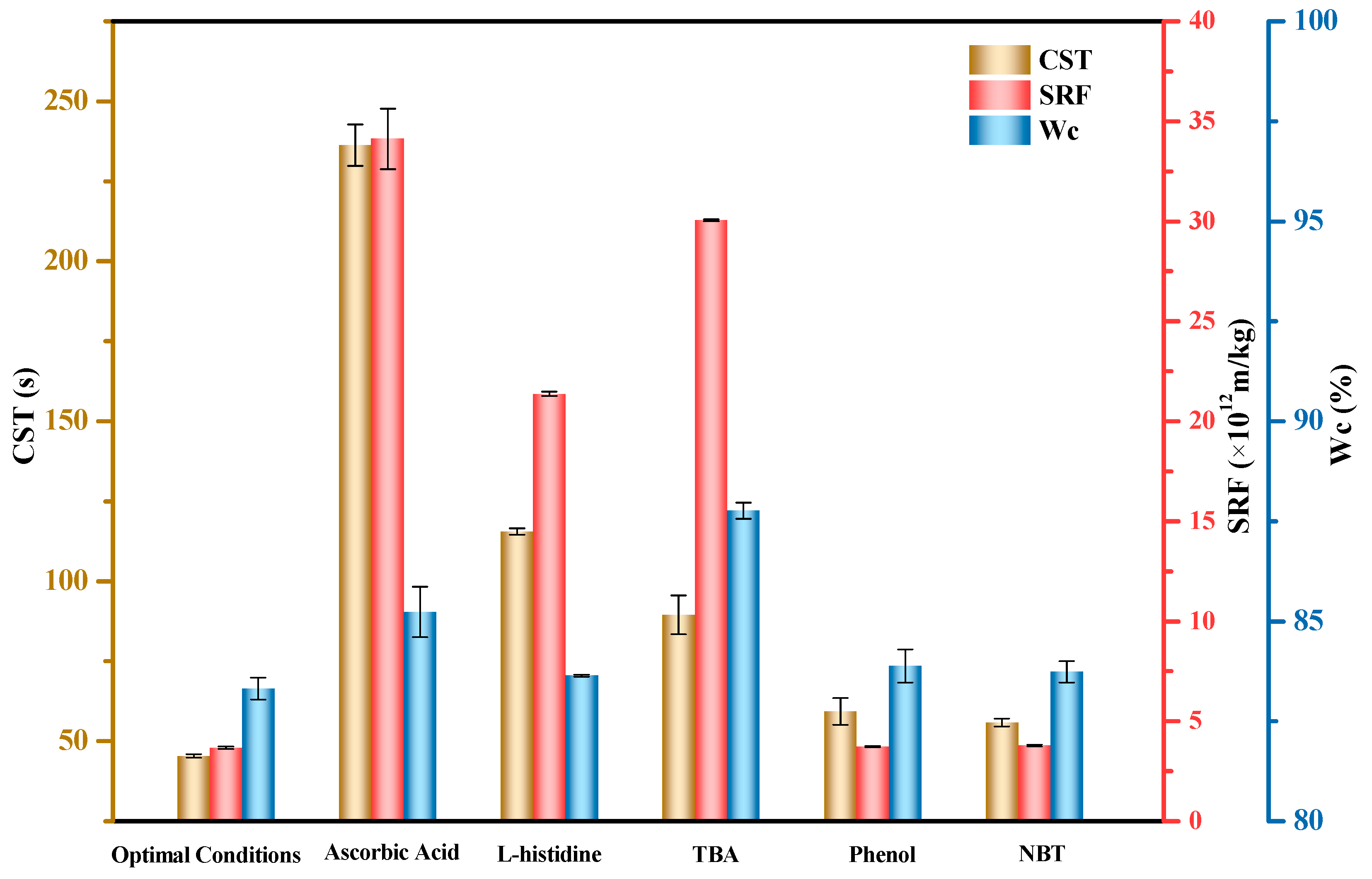
3.3. Reactive Species
3.4. Environmental Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, B.; Zhang, W.; Du, Y.; Wang, R.; Usher, S.P.; Scales, P.J.; Wang, D. Compartmentalization of extracellular polymeric substances (EPS) solubilization and cake microstructure in relation to wastewater sludge dewatering behavior assisted by horizontal electric field: Effect of operating conditions. Water Res. 2018, 130, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhu, X.; Li, J.; Yu, P.; Luo, Y.; Liu, M. Effect of extracellular polymeric substances (EPS) conditioned by combined lysozyme and cationic polyacrylamide on the dewatering performance of activated sludge. Chemosphere 2019, 235, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Jin, R.; Liu, G.; Dong, B.; Zhou, J.; Xing, D. Improving waste activated sludge dewaterability with sodium periodate pre-oxidation on extracellular polymeric substances. Water Environ. Res. 2021, 93, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, H.H.P. Influences of extracellular polymeric substances (EPS) on flocculation, settling, and dewatering of activated sludge. Crit. Rev. Environ. Sci. Technol. 2003, 33, 237–273. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Q.; Chen, Y.; He, Q.; Chen, W. Insight into sludge dewatering by advanced oxidation using persulfate as oxidant and Fe2+ as activator: Performance, mechanism and extracellular polymers and heavy metals behaviors. J. Environ. Manag. 2021, 288, 112476. [Google Scholar] [CrossRef]
- Yu, W.; Wen, Q.; Yang, J.; Xiao, K.; Zhu, Y.; Tao, S.; Liang, S.; Hu, S.; Wu, Q.; Hou, H.; et al. Novel insights into extracellular polymeric substance degradation, hydrophilic/hydrophobic characteristics, and dewaterability of waste activated sludge pretreated by hydroxylamine enhanced Fenton oxidation. ACS EST Eng. 2021, 1, 385–392. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, J.; Yu, W.; Zhang, S.; Liang, S.; Song, J.; Xu, Q.; Ye, N.; He, S.; Yang, C.; et al. Synergetic conditioning of sewage sludge via Fe2+/persulfate and skeleton builder: Effect on sludge characteristics and dewaterability. Chem. Eng. J. 2015, 270, 572–581. [Google Scholar] [CrossRef]
- Ismail, L.; Ferronato, C.; Fine, L.; Jaber, F.; Chovelon, J.-M. Elimination of sulfaclozine from water with SO4•− radicals: Evaluation of different persulfate activation methods. Appl. Catal. B Environ. 2017, 201, 573–581. [Google Scholar] [CrossRef]
- Wei, X.; Gao, N.; Li, C.; Deng, Y.; Zhou, S.; Li, L. Zero-valent iron (ZVI) activation of persulfate (PS) for oxidation of bentazon in water. Chem. Eng. J. 2016, 285, 660–670. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Liang, H.-y.; Zhang, Y.-q.; Huang, S.-b.; Hussain, I. Oxidative degradation of p-chloroaniline by copper oxidate activated persulfate. Chem. Eng. J. 2013, 218, 384–391. [Google Scholar] [CrossRef]
- Djaballah, M.L.; Merouani, S.; Bendjama, H.; Hamdaoui, O. Development of a free radical-based kinetics model for the oxidative degradation of chlorazol black in aqueous solution using periodate photoactivated process. J. Photochem. Photobiol. A Chem. 2020, 408, 113102. [Google Scholar] [CrossRef]
- Du, J.; Xiao, G.; Xi, Y.; Zhu, X.; Su, F.; Kim, S.H. Periodate activation with manganese oxides for sulfanilamide degradation. Water Res. 2020, 169, 115278. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, F.; Choi, W. Production of reactive oxygen species by the reaction of periodate and hydroxylamine for rapid removal of organic pollutants and waterborne bacteria. Environ. Sci. Technol. 2020, 54, 6427–6437. [Google Scholar] [CrossRef]
- He, L.; Lv, L.; Pillai, S.C.; Wang, H.; Yang, L. Efficient degradation of diclofenac sodium by periodate activation using Fe/cu bimetallic modified sewage sludge biochar/UV system. Sci. Total Environ. 2021, 783, 146974. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, H.; Liang, Y.; Cao, Y.; Xiao, Y.; Ma, J. Degradation of bisphenol AF in water by periodate activation with FeS (mackinawite) and the role of sulfur species in the generation of sulfate radicals. Chem. Eng. J. 2021, 407, 126738. [Google Scholar] [CrossRef]
- Zong, Y.; Shao, Y.; Zeng, Y.; Shao, B.; Xu, L.; Zhao, Z.; Liu, W.; Wu, D. Enhanced oxidation of organic contaminants by iron(II)-activated periodate: The significance of high-valent iron-oxo species. Environ. Sci. Technol. 2021, 55, 7634–7642. [Google Scholar] [CrossRef]
- Guo, D.; Yao, Y.; You, S.; Jin, L.; Lu, P.; Liu, Y. Ultrafast degradation of micropollutants in water via electro-periodate activation catalyzed by nanoconfined Fe2O3. Appl. Catal. B Environ. 2022, 309, 121289. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Chen, Y.; Shen, S.; Xue, J.; Ma, Y.; Zheng, L.; Wu, L.; Zhang, Z.; Yang, L. Iron-manganese oxide loaded sludge biochar as a novel periodate activator for thiacloprid efficient degradation over a wide pH range. Sep. Purif. Technol. 2022, 288, 120703. [Google Scholar] [CrossRef]
- He, L.; Yang, C.; Ding, J.; Lu, M.-Y.; Chen, C.-X.; Wang, G.-Y.; Jiang, J.-Q.; Ding, L.; Liu, G.-S.; Ren, N.-Q.; et al. Fe, N-doped carbonaceous catalyst activating periodate for micropollutant removal: Significant role of electron transfer. Appl. Catal. B: Environ. 2022, 303, 120880. [Google Scholar] [CrossRef]
- He, L.; Yang, S.; Shen, S.; Ma, Y.; Chen, Y.; Xue, J.; Wang, J.; Zheng, L.; Wu, L.; Zhang, Z.; et al. Novel insights into the mechanism of periodate activation by heterogeneous ultrasonic-enhanced sludge biochar: Relevance for efficient degradation of levofloxacin. J. Hazard. Mater. 2022, 434, 128860. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Wu, H.; Zhu, Z.; Guo, H. Periodate activation for degradation of organic contaminants: Processes, performance and mechanism. Sep. Purif. Technol. 2022, 292, 120928. [Google Scholar] [CrossRef]
- Ling, C.; Wu, S.; Han, J.; Dong, T.; Zhu, C.; Li, X.; Xu, L.; Zhang, Y.; Zhou, M.; Pan, Y. Sulfide-modified zero-valent iron activated periodate for sulfadiazine removal: Performance and dominant routine of reactive species production. Water Res. 2022, 220, 118676. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, L.; Ma, Y.; Wu, L.; Zheng, L.; Wang, J.; Chen, Y.; Li, Y.; Zhang, Z. Periodate-based oxidation focusing on activation, multivariate-controlled performance and mechanisms for water treatment and purification. Sep. Purif. Technol. 2022, 289, 120746. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, H.-Y.; Choi, J.; Nam, I.-H.; Lee, S.; Lee, S.; Kim, J.-H.; Lee, C.; Lee, J. Oxidizing Capacity of Periodate Activated with Iron-Based Bimetallic Nanoparticles. Environ. Sci. Technol. 2014, 48, 8086–8093. [Google Scholar] [CrossRef] [PubMed]
- Bokare, A.D.; Choi, W. Singlet-oxygen generation in alkaline periodate solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qiu, W.; Ma, J.; Xu, H.; Wang, D.; Cheng, H.; Zhang, W.; He, X. The overestimated role of singlet oxygen for pollutants degradation in some non-photochemical systems. Chem. Eng. J. 2020, 401, 126128. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Lin, C.J.; Liao, C.H.; Huang, R.Y.; Ting, W.P. Dewatering of activated sludge by Fenton’s reagent. Adv. Environ. Res. 2003, 7, 667–670. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Zhou, X.; Jiang, G.; Zhang, T.; Wang, Q.; Xie, G.J.; Yuan, Z. Role of extracellular polymeric substances in improvement of sludge dewaterability through peroxidation. Bioresour. Technol. 2015, 192, 817–820. [Google Scholar] [CrossRef]
- Liu, J.; Qi, Y.; Wang, D.; Li, X.; Yu, Z.; Xin, L.; Deng, Y.; Wang, L.; Yi, K.; Zeng, G. Enhanced dewaterability of waste activated sludge by Fe(II)-activated peroxymonosulfate oxidation. Bioresour. Technol. 2016, 206, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, K.-M.; Kim, H.-E.; Lee, H.-J.; Lee, C.; Lee, C. Disintegration of Waste Activated Sludge by Thermally-Activated Persulfates for Enhanced Dewaterability. Environ. Sci. Technol. 2016, 50, 7106–7115. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, J.; Tao, S.; Shi, Y.; Yu, J.; Lv, Y.; Liang, S.; Xiao, K.; Liu, B.; Hou, H.; et al. A comparatively optimization of dosages of oxidation agents based on volatile solids and dry solids content in dewatering of sewage sludge. Water Res. 2017, 126, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Qi, C.; Lin, C. Activation of periodate by granular activated carbon for acid orange 7 decolorization. J. Taiwan Inst. Chem. Eng. 2016, 68, 211–217. [Google Scholar] [CrossRef]
- Yang, P.; Li, D.; Zhang, W.; Ai, J.; Peng, S.; Wang, D.; Cui, F. Study of sludge conditioning using organic acids chelated ferrous ion catalyzed NaClO oxidation: Evolution of extracellular polymeric substances and floc structure. J. Environ. Manag. 2021, 280, 111757. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, P.; Yang, X.; Chen, Z.; Wang, D. Insights into the respective role of acidification and oxidation for enhancing anaerobic digested sludge dewatering performance with Fenton process. Bioresour. Technol. 2015, 181, 247–253. [Google Scholar] [CrossRef]
- Bo, J.; Wilén, B.; Lant, P. Impacts of morphological, physical and chemical properties of sludge flocs on dewaterability of activated sludge. Chem. Eng. J. 2004, 98, 115–126. [Google Scholar]
- Cai, M.-Q.; Hu, J.-Q.; Wells, G.; Seo, Y.; Spinney, R.; Ho, S.-H.; Dionysiou, D.D.; Su, J.; Xiao, R.; Wei, Z. Understanding mechanisms of synergy between acidification and ultrasound treatments for activated sludge dewatering: From bench to pilot–scale investigation. Environ. Sci. Technol. 2018, 52, 4313–4323. [Google Scholar] [CrossRef]
- Ywa, B.; Yla, B.; Kza, B.; Lxa, B.; Qiang, R.; Zy, C.; Qi, L.C.; Ywa, B.; Rc, D. The role of extracellular polymeric substances (EPS) in the reduction of Cr(VI) by Pannonibacter phragmitetus BB. J. Environ. Chem. Eng. 2021, 9, 106163. [Google Scholar]
- Xiao, J.; Ge, D.; Yuan, H.; Zhu, N. Waste activated sludge conditioning in a new Fe2+/persulfate/tannic acid process: Effectiveness and optimization study to enhance dewaterability. J. Environ. Chem. Eng. 2020, 8, 103785. [Google Scholar] [CrossRef]
- Wang, Q.; Kim, T.-H.; Reitzel, K.; Almind-Jørgensen, N.; Nielsen, U.G. Quantitative determination of vivianite in sewage sludge by a phosphate extraction protocol validated by PXRD, SEM-EDS, and 31P NMR spectroscopy towards efficient vivianite recovery. Water Res. 2021, 202, 117411. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.; Huang, S.; Cheng, J.; Han, Y.; Wang, Y.; Dong, Y.; Hu, J.; Li, G.; Yuan, H.; Zhu, N. A new environment-friendly polyferric sulfate-catalyzed ozonation process for sludge conditioning to achieve deep dewatering and simultaneous detoxification. J. Clean. Prod. 2022, 359, 132049. [Google Scholar] [CrossRef]
- Xiao, K.; Chen, Y.; Jiang, X.; Yang, Q.; Seow, W.Y.; Zhu, W.; Zhou, Y. Variations in physical, chemical and biological properties in relation to sludge dewaterability under Fe (II)—Oxone conditioning. Water Res. 2017, 109, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Mowla, D.; Tran, H.N.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378. [Google Scholar] [CrossRef]
- Raynaud, M.; Vaxelaire, J.; Olivier, J.; Dieude-Fauvel, E.; Baudez, J.-C. Compression dewatering of municipal activated sludge: Effects of salt and pH. Water Res. 2012, 46, 4448–4456. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, D.; Ma, T.; Bai, R. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Q.; Wang, D.; Li, X.; Zeng, G.; Li, Z.; Deng, Y.; Liu, J.; Yi, K. Advanced landfill leachate treatment using iron-carbon microelectrolysis-Fenton process: Process optimization and column experiments. J. Hazard. Mater. 2016, 318, 460–467. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, S.; Liang, J.; Zhang, S.; Sun, S.; Tang, B.; Xu, Q. Role of organic compounds from different EPS fractions and their effect on sludge dewaterability by combining anaerobically mesophilic digestion pre-treatment and Fenton’s reagent/lime. Chem. Eng. J. 2017, 321, 123–138. [Google Scholar] [CrossRef]
- He, X.S.; Xi, B.D.; Li, X.; Pan, H.W.; An, D.; Bai, S.G.; Li, D.; Cui, D.Y. Fluorescence excitation-emission matrix spectra coupled with parallel factor and regional integration analysis to characterize organic matter humification. Chemosphere 2013, 93, 2208–2215. [Google Scholar] [CrossRef]
- Wu, B.; Ni, B.-J.; Horvat, K.; Song, L.; Chai, X.; Dai, X.; Mahajan, D. Occurrence state and molecular structure analysis of extracellular proteins with implications on the dewaterability of waste-activated sludge. Environ. Sci. Technol. 2017, 51, 9235–9243. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, C.; Yao, Y.; Sun, L.; Gong, F.; Li, D.; Pei, K.; Lu, W.; Chen, W. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: Implications for the removal of organic pollutants. Chem. Eng. J. 2015, 281, 953–960. [Google Scholar] [CrossRef]
- Bu, L.; Shi, Z.; Zhou, S. Modeling of Fe (II)-activated persulfate oxidation using atrazine as a target contaminant. Sep. Purif. Technol. 2016, 169, 59–65. [Google Scholar] [CrossRef]
- Leinisch, F.; Mariotti, M.; Hägglund, P.; Davies, M.J. Structural and functional changes in RNAse A originating from tyrosine and histidine cross-linking and oxidation induced by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 2018, 126, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Dong, H.; Sun, B.; Guan, X. Role of ferrate(IV) and ferrate(V) in activating ferrate(VI) by calcium sulfite for enhanced oxidation of organic contaminants. Environ. Sci. Technol. 2019, 53, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Wang, S.; Ding, Z.; Wang, S.; Zhou, Y.; Huang, L.; Wang, X. Amine-functionalized zirconium metal-organic framework as efficient visible-light photocatalyst for aerobic organic transformations. Chem. Commun. 2012, 48, 11656–11658. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.-H.; Pignatello, J.J. Oxidation of Organic Compounds in Water by Unactivated Peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Jiang, N.; Wang, Z.; Jiang, J.; Zhang, T. Trace cupric species triggered decomposition of peroxymonosulfate and degradation of organic pollutants: Cu(III) being the primary and selective intermediate oxidant. Environ. Sci. Technol. 2020, 54, 4686–4694. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| pH | 6.8 ± 0.2 |
| Water content of filter cake (Wc, %) | 83.75 ± 0.21 |
| Total suspended solids (TSS, g/L) | 21.25 ± 0.3 |
| Volatile suspended solid (VSS, g/L) | 8.14 ± 0.1 |
| Specific resistance filtration (SRF) (1012 m/kg) | 6.24 ± 0.4 |
| Zeta potential (mV) | −10.60 ± 0.4 |
| Capillary suction time (CST) (s) | 133.35 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Liu, Q.; Wang, Y.; Zhu, Y.; Fang, D.; Wu, G.; Zeng, Z.; Peng, H. Improved Dewaterability of Waste Activated Sludge by Fe(II)-Activated Potassium Periodate Oxidation. Int. J. Environ. Res. Public Health 2022, 19, 14726. https://doi.org/10.3390/ijerph192214726
Xiao H, Liu Q, Wang Y, Zhu Y, Fang D, Wu G, Zeng Z, Peng H. Improved Dewaterability of Waste Activated Sludge by Fe(II)-Activated Potassium Periodate Oxidation. International Journal of Environmental Research and Public Health. 2022; 19(22):14726. https://doi.org/10.3390/ijerph192214726
Chicago/Turabian StyleXiao, Hong, Qing Liu, Yingjun Wang, Ying Zhu, Dexin Fang, Ganxue Wu, Zhenxing Zeng, and Hong Peng. 2022. "Improved Dewaterability of Waste Activated Sludge by Fe(II)-Activated Potassium Periodate Oxidation" International Journal of Environmental Research and Public Health 19, no. 22: 14726. https://doi.org/10.3390/ijerph192214726
APA StyleXiao, H., Liu, Q., Wang, Y., Zhu, Y., Fang, D., Wu, G., Zeng, Z., & Peng, H. (2022). Improved Dewaterability of Waste Activated Sludge by Fe(II)-Activated Potassium Periodate Oxidation. International Journal of Environmental Research and Public Health, 19(22), 14726. https://doi.org/10.3390/ijerph192214726







