Effectiveness of a 6-Month Nutrition Intervention in People Living with HIV and Prediabetes Progressing through Stages of Change towards Positive Health Behavior
Abstract
1. Introduction
1.1. Study Population
1.2. Behavioral Change Models
1.3. Nutrition Interventions
2. Material and Methods
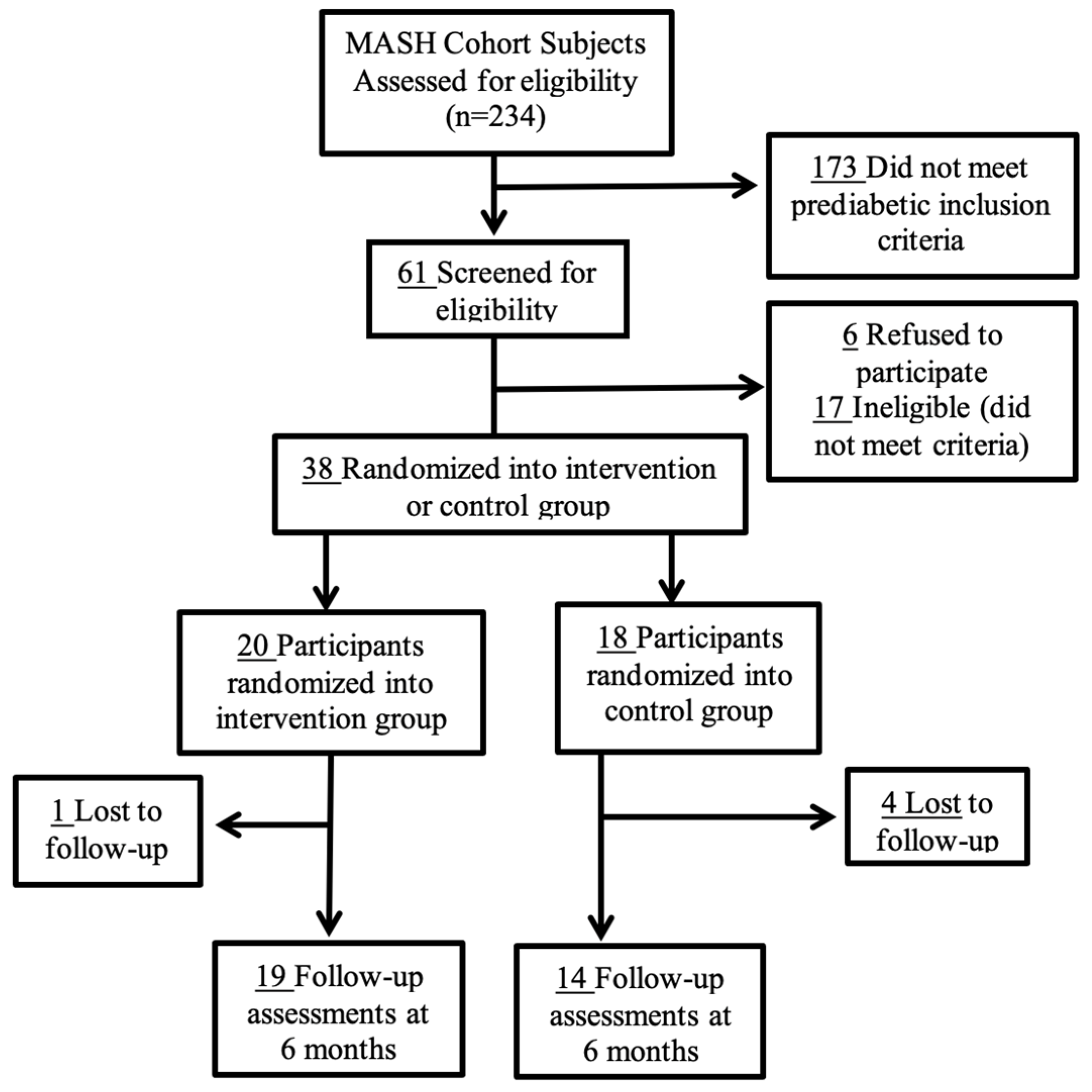
2.1. Study Design and Recruitment
2.2. Intervention
2.3. Questionnaires and Assessment Tools
2.3.1. Stage of Change Tool
2.3.2. Nutrition Knowledge Assessment Tool
2.3.3. Self-Efficacy Tool
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Progression of Stages of Change
4.2. Nutrition Knowledge and Self-Efficacy
4.3. Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Arafath, S.; Campbell, T.; Yusuff, J.; Sharma, R. Prevalence of and Risk Factors for Prediabetes in Patients Infected With HIV. Diabetes Spectr. 2018, 31, 139–143. [Google Scholar] [CrossRef]
- Hernandez-Romieu, A.C.; Garg, S.; Rosenberg, E.S.; Thompson-Paul, A.M.; Skarbinski, J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res. Care 2017, 5, e000304. [Google Scholar] [CrossRef]
- Colagiuri, S. Epidemiology of prediabetes. Med. Clin. N. Am. 2011, 95, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; Cole, S.R.; Li, X.; Kingsley, L.A.; Palella, F.J.; Riddler, S.A.; Visscher, B.R.; Margolick, J.B.; Dobs, A.S. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch. Intern. Med. 2005, 165, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Hadigan, C.; Meigs, J.B.; Corcoran, C.; Rietschel, P.; Piecuch, S.; Basgoz, N.; Davis, B.; Sax, P.; Stanley, T.; Wilson, P.W.; et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin. Infect. Dis. 2001, 32, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Fields-Gardner, C.; Campa, A. Position of the American Dietetic Association: Nutrition Intervention and Human Immunodeficiency Virus Infection. J. Am. Diet. Assoc. 2010, 110, 1105–1119. [Google Scholar] [CrossRef]
- Stanley, T.L.; Grinspoon, S.K. Body composition and metabolic changes in HIV-infected patients. J. Infect. Dis. 2012, 205 (Suppl. 3), S383–S390. [Google Scholar] [CrossRef]
- Willig, A.; Wright, L.; Galvin, T.A. Practice Paper of the Academy of Nutrition and Dietetics: Nutrition Intervention and Human Immunodeficiency Virus Infection. J. Acad. Nutr. Diet. 2018, 118, 486–498. [Google Scholar] [CrossRef]
- The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care 2002, 25, 2165–2171. [CrossRef]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Pellowski, J.A.; Kalichman, S.C.; Matthews, K.A.; Adler, N. A pandemic of the poor: Social disadvantage and the U.S. HIV epidemic. Am. Psychol. 2013, 68, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.L.; Jensen, J.D.; Scherr, C.L.; Brown, N.R.; Christy, K.; Weaver, J. The Health Belief Model as an explanatory framework in communication research: Exploring parallel, serial, and moderated mediation. Health Commun. 2015, 30, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Shabibi, P.; Zavareh, M.S.A.; Sayehmiri, K.; Qorbani, M.; Safari, O.; Rastegarimehr, B.; Mansourian, M. Effect of educational intervention based on the Health Belief Model on promoting self-care behaviors of type-2 diabetes patients. Electron. Physician 2017, 9, 5960–5968. [Google Scholar] [CrossRef] [PubMed]
- Holmen, H.; Wahl, A.; Torbjørnsen, A.; Jenum, A.K.; Småstuen, M.C.; Ribu, L. Stages of change for physical activity and dietary habits in persons with type 2 diabetes included in a mobile health intervention: The Norwegian study in RENEWING HEALTH. BMJ Open Diabetes Res. Care 2016, 4, e000193. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, L. Helping people with diabetes change behavior: From theory to practice. Diabetes Spectr. 2000, 13, 125. [Google Scholar]
- Narayan, K.M.; Imperatore, G.; Benjamin, S.M.; Engelgau, M.M. Targeting people with pre-diabetes. BMJ 2002, 325, 403–404. [Google Scholar] [CrossRef]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Kolb, J.M.; Kitos, N.R.; Ramachandran, A.; Lin, J.J.; Mann, D.M. What do primary care prediabetes patients need? A baseline assessment of patients engaging in a technology-enhanced lifestyle intervention. J. Bioinform. Diabetes 2014, 1, 4. [Google Scholar]
- Greene, G.W.; Rossi, S.R. Stages of change for reducing dietary fat intake over 18 months. J. Am. Diet. Assoc. 1998, 98, 529–534, quiz 535–526. [Google Scholar] [CrossRef] [PubMed]
- McKee, G.; Bannon, J.; Kerins, M.; FitzGerald, G. Changes in diet, exercise and stress behaviours using the stages of change model in cardiac rehabilitation patients. Eur. J. Cardiovasc. Nurs. 2007, 6, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Sneij, A.; Campa, A.; Baum, M.; Huffman, F.; George Fl Trepka, M.J.; Sales-Martinez, S.; Seminario, L.; Hernandez-Boyer, J. The Effectiveness of a 6-Month Nutrition Intervention on Lowering Fasting Glucose and 8OHdG Levels in Prediabetic People Living with HIV (PLWH) in the MASH Cohort. Am. J. Prev. Med. Public Health 2020, 7, 147–154. [Google Scholar]
- Sneij, A.; Campa, A.; Martinez, S.S.; Stewart, T.; Baum, M. Lower Plasma Zinc Levels in Hyperglycemic People Living with HIV in the MASH cohort. J. AIDS Clin. Res. 2016, 7, 542. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes; American Diabetes Association: Arlington County, VA, USA, 2018. [Google Scholar]
- Robinson, A.H.; Norman, G.J.; Sallis, J.F.; Calfas, K.J.; Rock, C.L.; Patrick, K. Validating stage of change measures for physical activity and dietary behaviors for overweight women. Int. J. Obes. 2008, 32, 1137–1144. [Google Scholar] [CrossRef]
- Pawlak, R.; Colby, S. Benefits, barriers, self-efficacy and knowledge regarding healthy foods; perception of African Americans living in eastern North Carolina. Nutr. Res. Pract. 2009, 3, 56–63. [Google Scholar] [CrossRef]
- Sallis, J.F.; Grossman, R.M.; Pinski, R.B.; Patterson, T.L.; Nader, P.R. The development of scales to measure social support for diet and exercise behaviors. Prev. Med. 1987, 16, 825–836. [Google Scholar] [CrossRef]
- Cabral, R.J.; Cotton, D.; Semaan, S.; Gielen, A.C. Application of the transtheoretical model for HIV prevention in a facility-based and a community-level behavioral intervention research study. Health Promot. Pract. 2004, 5, 199–207. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Dietary Guidelines for Americans; United States Department of Agriculture: Washington, DC, USA, 2015.
- Scherr, R.E.; Linnell, J.D.; Dharmar, M.; Beccarelli, L.M.; Bergman, J.J.; Briggs, M.; Brian, K.M.; Feenstra, G.; Hillhouse, J.C.; Keen, C.L.; et al. A Multicomponent, School-Based Intervention, the Shaping Healthy Choices Program, Improves Nutrition-Related Outcomes. J. Nutr. Educ. Behav. 2017, 49, 368–379.e1. [Google Scholar] [CrossRef]
- Mohammadi, S.; Karim, N.A.; Talib, R.A.; Amani, R. The impact of self-efficacy education based on the health belief model in Iranian patients with type 2 diabetes: A randomised controlled intervention study. Asia Pac. J. Clin. Nutr. 2018, 27, 546–555. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows; Version 23.0; IBM Corp.: Armonk, NY, USA, 2015.
- Vancampfort, D.; Byansi, P.K.; Namutebi, H.; Lillian, N.; Kinyanda, E.; Bbosa, R.S.; Ward, P.B.; Lukwata, H.; Mugisha, J. Correlates of physical activity stages of change in people living with HIV in a Ugandan community. Disabil. Rehabil. 2020, 44, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.J.; Sanchez, L.; Hubbard, J.; Lo, J.; Grinspoon, S.K.; Fitch, K.V. Diet Quality Is Low and Differs by Sex in People with HIV. J. Nutr. 2019, 149, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Galvan, F.H.; Bing, E.G.; Fleishman, J.A.; London, A.S.; Caetano, R.; Burnam, M.A.; Longshore, D.; Morton, S.C.; Orlando, M.; Shapiro, M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: Results from the HIV Cost and Services Utilization Study. J. Stud. Alcohol. 2002, 63, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.D.; Peters, B.S.; Rivas, C.; Goff, L.M. Reducing risk of Type 2 diabetes in HIV: A mixed-methods investigation of the STOP-Diabetes diet and physical activity intervention. Diabet. Med. 2020, 37, 1705–1714. [Google Scholar] [CrossRef]
- Bello, T.K.; Gericke, G.J.; MacIntyre, U.E. Development, Implementation, and Process Evaluation of a Theory-Based Nutrition Education Programme for Adults Living With HIV in Abeokuta, Nigeria. Front. Public Health 2019, 7, 30. [Google Scholar] [CrossRef]
- Muthamia, O.G.; Mwangi, A.M.; Mbugua, S.K. The Effects of Nutrition Knowledge on the Dietary Practices of People Living with HIV in Kayole Division, Nairobi-Kenya. Int. J. Nutr. Food Sci. 2014, 3, 597–601. [Google Scholar]
- Kelly, T.A.; Kim, S.; Jemmott, L.S.; Icard, L.D.; Chittamuru, D.; Jemmott, J.B., 3rd. Reasoned Action Approach Correlates of Fruit and Vegetable Diet Among African American Men Living With HIV: A Cross-Sectional Study. Health Educ. Behav. 2021, 48, 852–859. [Google Scholar] [CrossRef]

| Behavior | Guideline/Recommendation |
|---|---|
| Physical Activity | ≥30 min of purposeful physical activity regularly, preferably daily |
| Fruit and Vegetable Intake | ≥2 servings of fruits and ≥3 servings of vegetables per day |
| Dietary Fiber Intake | ≥6 servings of grain products, with ≥3 being whole grains |
| Dietary Fat Intake | <30% of energy intake from total fat |
| Alcohol Consumption | ≤1 alcoholic beverage per day (women) ≤2 alcoholic beverages per day (men) |
| Baseline Characteristic | Mean ± SD (N = 38) | Intervention Group (n = 20) Mean ± SD | Control Group (n = 18) Mean ± SD | p Value |
|---|---|---|---|---|
| Birth Sex * | Female: n = 15 (39%) Male: n = 23 ** (61%) | Female: n = 7 (35%) Male: n = 13 (65%) | Female: n = 8 (44%) Male: n = 10 ** (56%) | 0.55 *** |
| Age (years) | 56.70 ± 7.40 | 55.55 ± 6.07 | 58.00 ± 8.72 | 0.40 |
| Annual Income (USD/year) | $13,325.03 ± $11,495.00 | $13,072.21 ± $8577.63 | $13,605.94 ± $14,323.91 | 0.51 |
| Body Mass Index (kg/m2) | 30.10 ± 5.50 | 29.44 ± 5.55 | 30.74 ± 5.50 | 0.40 |
| Waist Circumference (inches) | 40 ± 4.70 | Female: 40.46 ± 6.30 | Female: 40.59 ± 5.17 | Female: 0.73 |
| Male: 39.60 ± 3.86 | Male: 39.28 ± 4.85 | Male: 0.80 | ||
| Total: 39.90 ± 4.72 | Total: 40.07 ± 4.80 | Total: 0.97 | ||
| Hip Circumference (inches) | 42 ± 4.70 | Female: 44.53 ± 6.00 | Female:43.81 ± 5.20 | Female: 0.56 |
| Male: 41.14 ± 3.76 | Male: 41.09 ± 4.46 | Male:0.89 | ||
| Total: 42.32 ± 4.80 | Total: 42.41 ± 4.73 | Total: 0.84 | ||
| Waist-to-hip ratio | 0.95 ± 0.06 | Female: 0.91 ± 0.05 | Female:0.93 ± 0.06 | Female: 0.27 |
| Male: 0.96 ± 0.06 | Male:0.96 ± 0.05 | Male: 0.80 | ||
| Total: 0.94 ± 0.06 | Total: 0.95 ± 0.06 | Total: 0.84 |
| Stages of Change | Physical Activity | Fruits and Vegetables | Fiber | Fat | Alcohol |
|---|---|---|---|---|---|
| Baseline/6-Month | |||||
| Intervention Group (n = 20/n = 19) | |||||
| Inactive Stages of Change | 5/1 | 13/5 | 9/4 | 8/5 | 6/3 |
| Active Stages of Change | 15/18 | 7/14 | 11/15 | 12/14 | 14/16 |
| McNemar’s Test Pre/Post Comparison (p-value) | 0.110 | 0.011 * | 0.063 | 0.227 | 0.250 |
| Control Group (n = 18/n = 14) | |||||
| Inactive Stages of Change | 9/6 | 11/11 | 8/8 | 10/8 | 4/2 |
| Active Stages of Change | 9/8 | 7/3 | 10/6 | 8/6 | 14/12 |
| McNemar’s Test Pre/Post Comparison (p-value) | 0.344 | 0.188 | 0.313 | 0.500 | 0.500 |
| Fisher’s Exact Test Comparison | |||||
| Baseline | 0.184 | 0.535 | 0.615 | 0.258 | 0.360 |
| 6-Month | 0.007 * | 0.002 * | 0.033 * | 0.074 | 0.649 |
| Parameter | Intervention (Mean ± SD) | Pre/ Post (p-Value) | Control (Mean ± SD) | Pre/ Post (p-Value) | Comparison Between Groups (p-Value) | Parameter Changes Comparison (p-Value) | |||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Month | Baseline | 6-Month | Baseline | 6-Month | ||||
| Nutrition knowledge score (0–25) | 17.74 ± 2.3 | 20.47 ± 2.5 | 0.001 * | 17.64 ± 2.5 | 17.64 ± 2.7 | 0.99 | 0.60 | 0.003 * | 0.006 * |
| Nutrition knowledge (%) | 70.95 ± 9.0 | 81.89 ± 9.8 | 0.001 * | 70.57 ± 10.1 | 67.21 ± 16.1 | 0.29 | 0.60 | 0.003 * | 0.002 * |
| Self-efficacy score (0–75) | 63.32 ± 10.21 | 67.37 ± 6.3 | 0.000 * | 59.50 ± 9.2 | 61.43 ± 5.2 | 0.36 | 0.49 | 0.007 * | 0.014 * |
| Self-efficacy percentage (%) | 83.95 ± 13.8 | 89.79 ± 8.5 | 0.000 * | 79.36 ± 12.3 | 82.07 ± 6.8 | 0.33 | 0.55 | 0.009 * | 0.014 * |
| Stages of Change | Physical Activity | Fruits and Vegetables | Fiber | Fat | Alcohol |
|---|---|---|---|---|---|
| Baseline/6 Months | |||||
| Intervention Group (n = 20/n = 19) | |||||
| Precontemplation | 1/0 | 1/0 | 3/1 | 1/1 | 3/1 |
| Contemplation | 1/0 | 1/0 | 0/0 | 0/0 | 1/1 |
| Preparation | 3/1 | 11/5 | 6/3 | 7/4 | 2/1 |
| Action | 4/1 | 3/3 | 5/3 | 3/3 | 1/1 |
| Maintenance | 11/17 | 4/11 | 6/12 | 9/11 | 13/15 |
| Control Group (n = 18/n = 14) | |||||
| Precontemplation | 3/2 | 2/3 | 0/2 | 1/3 | 1/1 |
| Contemplation | 0/0 | 0/1 | 0/1 | 0/1 | 0/0 |
| Preparation | 6/4 | 9/7 | 8/5 | 9/4 | 3/1 |
| Action | 2/0 | 2/0 | 1/0 | 1/1 | 2/2 |
| Maintenance | 7/8 | 5/3 | 9/6 | 7/5 | 12/10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sneij, A.; Campa, A.; Huffman, F.; George, F.; Trepka, M.J.; Sales Martinez, S.; Baum, M. Effectiveness of a 6-Month Nutrition Intervention in People Living with HIV and Prediabetes Progressing through Stages of Change towards Positive Health Behavior. Int. J. Environ. Res. Public Health 2022, 19, 14675. https://doi.org/10.3390/ijerph192214675
Sneij A, Campa A, Huffman F, George F, Trepka MJ, Sales Martinez S, Baum M. Effectiveness of a 6-Month Nutrition Intervention in People Living with HIV and Prediabetes Progressing through Stages of Change towards Positive Health Behavior. International Journal of Environmental Research and Public Health. 2022; 19(22):14675. https://doi.org/10.3390/ijerph192214675
Chicago/Turabian StyleSneij, Alicia, Adriana Campa, Fatma Huffman, Florence George, Mary Jo Trepka, Sabrina Sales Martinez, and Marianna Baum. 2022. "Effectiveness of a 6-Month Nutrition Intervention in People Living with HIV and Prediabetes Progressing through Stages of Change towards Positive Health Behavior" International Journal of Environmental Research and Public Health 19, no. 22: 14675. https://doi.org/10.3390/ijerph192214675
APA StyleSneij, A., Campa, A., Huffman, F., George, F., Trepka, M. J., Sales Martinez, S., & Baum, M. (2022). Effectiveness of a 6-Month Nutrition Intervention in People Living with HIV and Prediabetes Progressing through Stages of Change towards Positive Health Behavior. International Journal of Environmental Research and Public Health, 19(22), 14675. https://doi.org/10.3390/ijerph192214675






