Overweight and Obese Adult Patients Show Larger Benefits from Concurrent Training Compared with Pharmacological Metformin Treatment on Insulin Resistance and Fat Oxidation
Abstract
1. Introduction
2. Materials and Methods
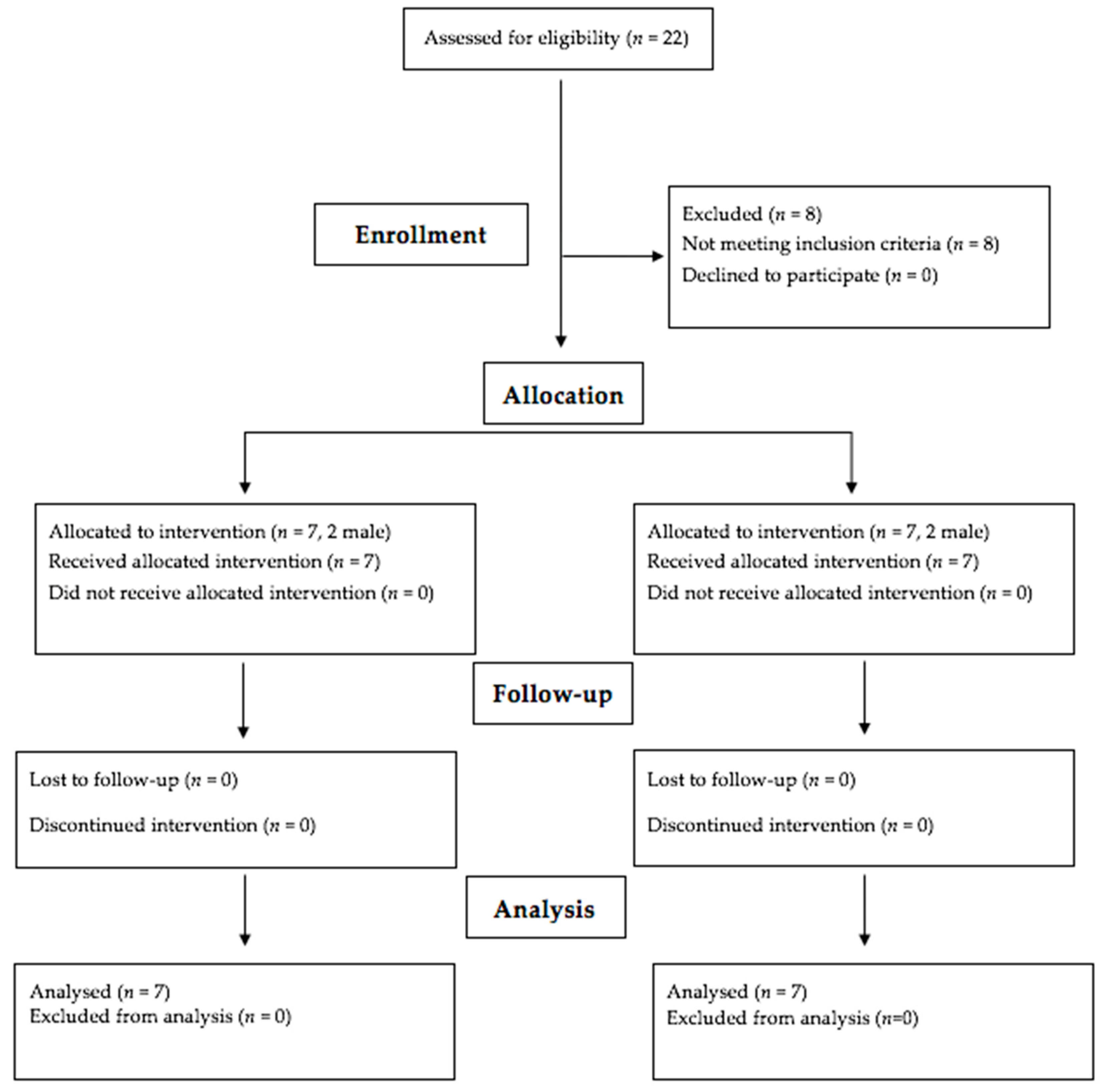
2.1. Participants
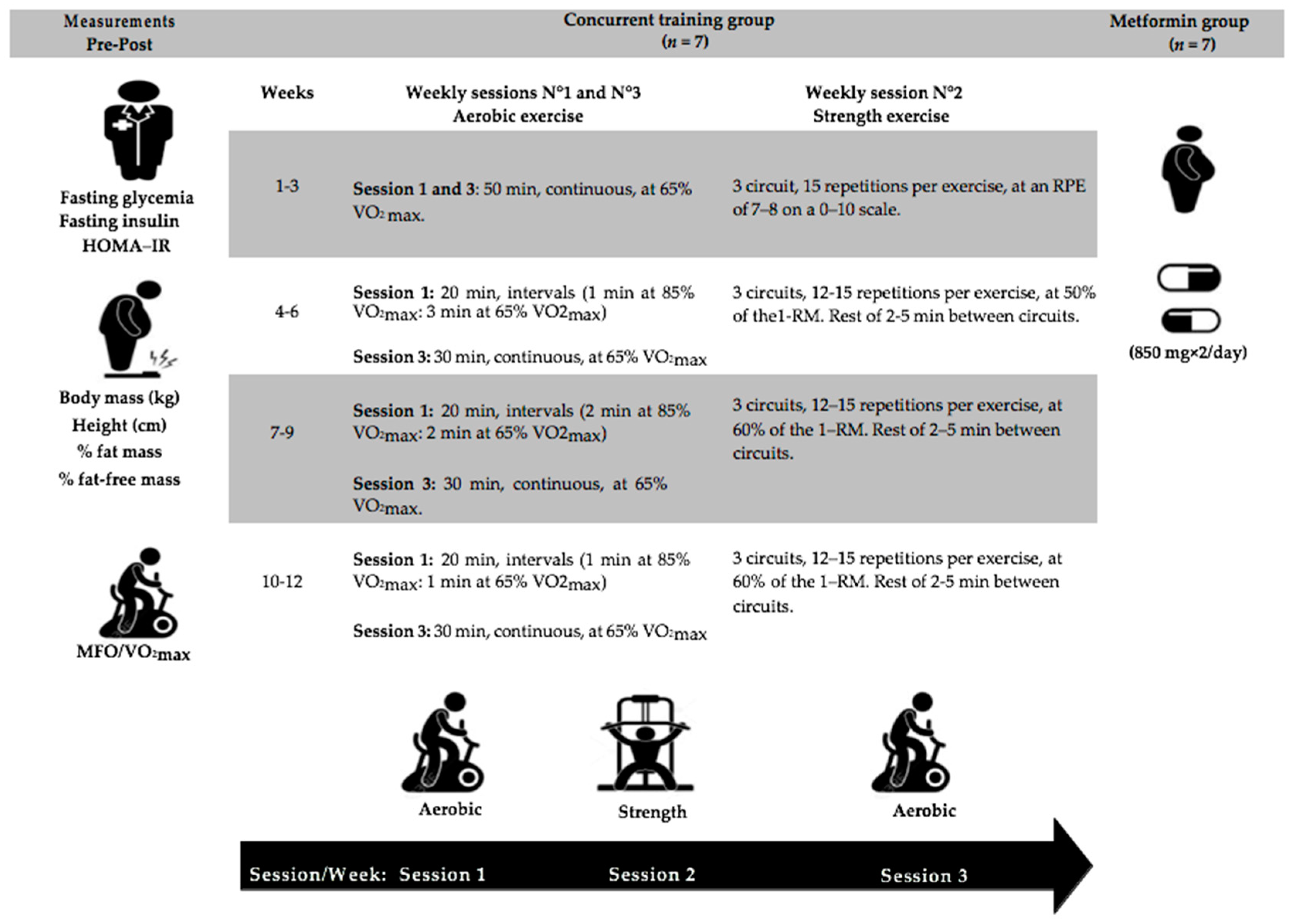
2.2. Experimental Design
2.3. Measurements
2.3.1. Body Composition
2.3.2. Insulin Sensitivity
2.3.3. Cardiorespiratory Fitness
2.3.4. Maximal Fat Oxidation
2.3.5. Concurrent Training
2.3.6. Statistical Analyses
3. Results
4. Discussion
4.1. Clinical Application
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Gutierrez, R.; Gonzalez-Gonzalez, J.G.; Zuñiga-Hernandez, J.A.; McCoy, R.G. Benefits and harms of intensive glycemic control in patients with type 2 diabetes. BMJ 2019, 367, l5887. [Google Scholar] [CrossRef] [PubMed]
- Rogowicz-Frontczak, A.; Majchrzak, A.; Zozulińska-Ziółkiewicz, D. Insulin resistance in endocrine disorders—Treatment options. Endokrynol. Polska 2017, 68, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Shulman, G.I. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2005, 2, e233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Zhang, X.; Yi, Z.; Cichello, S. Skeletal intramyocellular lipid metabolism and insulin resistance. Biophys. Rep. 2015, 1, 90–98. [Google Scholar] [CrossRef]
- Cadeddu, C.; Nocco, S.; Cugusi, L.; Deidda, M.; Bina, A.; Fabio, O.; Bandinu, S.; Cossu, E.; Baroni, M.G.; Mercuro, G. Effects of metformin and exercise training, alone or in association, on cardio-pulmonary performance and quality of life in insulin resistance patients. Cardiovasc. Diabetol. 2014, 13, 93. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Nkambule, B.B.; Obonye, N.; Louw, J. The role of glucose and fatty acid metabolism in the development of insulin resistance in skeletal muscle. In Muscle Cell and Tissue—Current Status of Research Field; Sakuma, K., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef]
- Gonzalez-Franquesa, A.; Patti, M.E. Insulin resistance and mitochondrial dysfunction. Adv. Exp. Med. Biol. 2017, 982, 465–520. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Konopka, A.R.; Laurin, J.L.; Schoenberg, H.M.; Reid, J.J.; Castor, W.M.; Wolff, C.A.; Musci, R.V.; Safairad, O.D.; Linden, M.A.; Biela, L.M.; et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 2019, 18, e12880. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Rodríguez-Moctezuma, J.R.; Robles-López, G.; López-Carmona, J.M.; Gutiérrez-Rosas, M.J. Effects of metformin on the body composition in subjects with risk factors for type 2 diabetes. Diabetes Obes. Metab. 2005, 7, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Stephens, B.R.; Sharoff, C.G.; Hagobian, T.A.; Chipkin, S.R.; Braun, B. Metformin's effect on exercise and postexercise substrate oxidation. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Sigal, R.J.; Kenny, G.P.; Boulé, N.G.; Wells, G.A.; Prud’homme, D.; Fortier, M.; Reid, R.D.; Tulloch, H.; Coyle, D.; Phillips, P.; et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 357–369. [Google Scholar] [CrossRef]
- Sousa, A.; Neiva, H.; Izquierdo, M.; Alves, A.; Duarte-Mendes, P.; Ramalho, A.; Marques, M.; Marinho, D. Concurrent training intensities: A practical approach for program design. Strength Cond. J. 2020, 42, 38–44. [Google Scholar] [CrossRef]
- Johannsen, N.M.; Swift, D.L.; Lavie, C.J.; Earnest, C.P.; Blair, S.N.; Church, T.S. Combined aerobic and resistance training effects on glucose homeostasis, fitness, and other major health indices: A review of current guidelines. Sports Med. 2016, 46, 1809–1818. [Google Scholar] [CrossRef]
- Braun, B.; Sharoff, C.; Chipkin, S.R.; Beaudoin, F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J. Appl. Physiol. 2004, 97, 991–997. [Google Scholar] [CrossRef]
- Cuff, D.J.; Meneilly, G.S.; Martin, A.; Ignaszewski, A.; Tildesley, H.D.; Frohlich, J.J. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003, 26, 2977–2982. [Google Scholar] [CrossRef]
- Malin, S.K.; Gerber, R.; Chipkin, S.R.; Braun, B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 2012, 35, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Boulé, N.G.; Kenny, G.P.; Larose, J.; Khandwala, F.; Kuzik, N.; Sigal, R.J. Does metformin modify the effect on glycaemic control of aerobic exercise, resistance exercise or both? Diabetologia 2013, 56, 2378–2382. [Google Scholar] [CrossRef] [PubMed]
- Sharoff, C.G.; Hagobian, T.A.; Malin, S.K.; Chipkin, S.R.; Yu, H.; Hirshman, M.F.; Goodyear, L.J.; Braun, B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E815–E823. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Braun, B. Effect of metformin on substrate utilization after exercise training in adults with impaired glucose tolerance. Appl. Physiol. Nutr. Metab. 2013, 38, 427–430. [Google Scholar] [CrossRef]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The disease burden associated with overweight and obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef]
- Trout, K.K.; Homko, C.; Tkacs, N.C. Methods of measuring insulin sensitivity. Biol. Res. Nurs. 2007, 8, 305–318. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription; American College of Sports Medicine, Ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018; ISBN 9781609139551. [Google Scholar]
- Faria, S.L.; Faria, O.P.; Cardeal, M.D.; Ito, M.K. Validation study of multi-frequency bioelectrical impedance with dual-energy X-ray absorptiometry among obese patients. Obes. Surg. 2014, 24, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Emoto, M.; Nishizawa, Y.; Maekawa, K.; Hiura, Y.; Kanda, H.; Kawagishi, T.; Shoji, T.; Okuno, Y.; Morii, H. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care 1999, 22, 818–822. [Google Scholar] [CrossRef]
- Acosta, A.M.; Escalona, M.; Maiz, A.; Pollak, F.; Leighton, F. Determinación del índice de resistencia insulínica mediante HOMA en una población de la región metropolitana de Chile [Determination of the insulin resistance index by the Homeostasis Model Assessment in a population of Metropolitan Region in Chile]. Rev. Med. Chil. 2002, 130, 1227–1231. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Brun, J.F.; Romain, A.J.; Mercier, J. Maximal lipid oxidation during exercise (Lipoxmax): From physiological measurements to clinical applications. Facts and uncertainties. Sci. Sports 2011, 26, 57–71. [Google Scholar] [CrossRef]
- Jones, N.L.; Makrides, L.; Hitchcock, C.; Chypchar, T.; McCartney, N. Normal standards for an incremental progressive cycle ergometer test. Am. Rev. Respir. Dis. 1985, 131, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Grada, C.O.; Ryan, M.; Roche, H.M.; De Vito, G.; Gibney, M.J.; Gibney, E.R.; Brennan, L. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol. Nutr. Food Res. 2013, 57, 1246–1254. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Sweet, T.W.; Foster, C.; McGuigan, M.R.; Brice, G. Quantitation of resistance training using the session rating of perceived exertion method. J. Strength Cond. Res. 2004, 18, 796–802. [Google Scholar] [CrossRef]
- LeSuer, D.; McCormick, J.; Mayhew, J.; Wasserstein, R.; Arnold, M. The accuracy of prediction equations for estimating 1-rm performance in the bench press, squat, and deadlift. J. Strength Cond. Res. 1997, 11, 211–213. [Google Scholar] [CrossRef]
- Jimenez, A.J.; De Paz, J.A. Application of the 1RM estimation formulas from the RM in bench press in a group of physically active middle-aged women. J. Human Sport Exerc. 2008, 3, 10–22. [Google Scholar] [CrossRef]
- Boulé, N.G.; Robert, C.; Bell, G.J.; Johnson, S.T.; Bell, R.C.; Lewanczuk, R.Z.; Gabr, R.Q.; Brocks, D.R. Metformin and exercise in type 2 diabetes: Examining treatment modality interactions. Diabetes Care 2011, 34, 1469–1474. [Google Scholar] [CrossRef]
- Hawley, J.A. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes/Metab. Res. Rev. 2004, 20, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Lessard, S.J. Exercise training-induced improvements in insulin action. Acta Physiol. (Oxf.) 2008, 192, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Cabañas, A.; Morales-Palomo, F.; Alvarez-Jimenez, L.; Ortega, J.F.; Mora-Rodriguez, R. Effects of chronic metformin treatment on training adaptations in men and women with hyperglycemia: A prospective study. Obesity (Silver Spring) 2022, 30, 1219–1230. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Dias, S.; Strasser, B.; Hoffmann, G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: A systematic review and network meta-analysis. PLoS ONE 2013, 8, e82853. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Tokubuchi, I.; Tajiri, Y.; Iwata, S.; Hara, K.; Wada, N.; Hashinaga, T.; Nakayama, H.; Mifune, H.; Yamada, K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 2017, 12, e0171293. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.; Eze, P.; Stephens, B.R.; Hagobian, T.A.; Sharoff, C.G.; Chipkin, S.R.; Goldstein, B. Impact of metformin on peak aerobic capacity. Appl. Physiol. Nutr. Metab. 2008, 33, 61–67. [Google Scholar] [CrossRef]
- Paquin, J.; Lagacé, J.C.; Brochu, M.; Dionne, I.J. Exercising for insulin sensitivity—Is there a mechanistic relationship with quantitative changes in skeletal muscle mass? Front. Physiol. 2021, 12, 656909. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef]
- Thompson, W.R.; Sallis, R.; Joy, E.; Jaworski, C.A.; Stuhr, R.M.; Trilk, J.L. Exercise Is Medicine. Am. J. Lifestyle Med. 2020, 14, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Albright, A.L.; Blissmer, B.J.; Braun, B.; Chasan-Taber, L.; Fernhall, B.; Regensteiner, J.G.; Rubin, R.R.; Sigal, R.J. Exercise and type 2 diabetes: American College of Sports Medicine and the American Diabetes Association: Joint position statement. Exercise and type 2 diabetes. Med. Sci. Sports Exerc. 2010, 42, 2282–2303. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Blair, S.N.; Jakicic, J.M.; Manore, M.M.; Rankin, J.W.; Smith, B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2009, 41, 459–471. [Google Scholar] [CrossRef] [PubMed]
| Variables | Metformin Group | Concurrent Training Group | p Values |
|---|---|---|---|
| Sex (n = male/n = female) | 2/5 | 2/5 | |
| Age (years) | 34.4 ± 14.0 | 32.9 ± 8.3 | p = 0.802 |
| Body mass (kg) | 94.2 ± 13.9 | 85.3 ± 19.7 | p = 0.345 |
| Height (cm) | 165.8 ± 7.2 | 165.1 ± 10.5 | p = 0.901 |
| Body mass index (kg·m−2) | 34.4 ± 6.0 | 30.8 ± 4.0 | p = 0.217 |
| Fat mass (%) | 42.1 ± 12.9 | 35 ± 8.2 | p = 0.325 |
| Fat-free mass (%) | 53.1 ± 9.1 | 50.1 ± 14.8 | p = 0.143 |
| ANOVA Outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Metformin Group (n = 7) | Concurrent Training Group (n = 7) | Time F(1, 12), p (ηp2) | Group F(1, 12), p (ηp2) | Group × Time F(1, 12), p (ηp2) | ||
| Pre | Post (Δ%, 95% CI) | Pre | Post (Δ%, 95% CI) | ||||
| HOMA-IR | 3.2 ± 1.0 | 3.2 ± 1.3 (−4.0, −23.0 to 19.8) | 3.6 ± 0.7 | 0.6 ± 0.4 (−84.5, −91.9 to −70.3) | F = 43.5, p < 0.001 (0.78) | F = 6.9, p = 0.021 (0.36) | F = 43.0, p < 0.001 (0.78) * |
| Fasting glycemia (mg·dL−1) | 83.4 ± 8.2 | 82.9 ± 6.9 (−0.6, −6.2 to 5.4) | 88.3 ± 4.0 | 88.0 ± 3.4 (−0.3, −3.6 to −3.1) | F = 0.1, p = 0.720 (0.01) | F = 2.9, p = 0.116 (0.19) | F = 0.0, p = 0.904 (0.00) |
| Fasting insulin (mg·dL−1) | 15.6 ± 3.7 | 15.7 ± 3.7 (−3.4, −24.4 to 23.4) | 16.4 ± 3.3 | 2.9 ± 1.9 (−84.6, −92.0 to −70.7) | F = 38.1, p < 0.001 (0.76) | F = 10.9, p < 0.001 (0.47) | F = 39.3, p < 0.001 (0.76) * |
| VO2max (L·min–1) | 2.1 ± 0.6 | 2.1 ± 0.7 (−2.9, −11.3 to 6.4) | 2.1 ± 0.9 | 2.4 ± 1.1 (12.3, 1.3 to 24.5) | F = 3.8, p = 0.073 (0.24) | F = 0.2, p = 0.698 (0.01) | F = 6.5, p = 0.025 (0.35) * |
| Body mass (kg) | 94.2 ± 13.9 | 89.4 ± 14.8 (−5.4, −8.1 to −2.6) | 85.3 ± 19.7 | 77.2 ± 19.1(−9.7, −13.7 to −5.5) | F = 58.6, p < 0.001 (0.83) | F = 1.4, p = 0.270 (0.10) | F = 3.6, p = 0.08 (0.23) * |
| BMI (kg·m−2) | 34.4 ± 6.0 | 32.6± 6.0(−5.3, −8.0 to −2.6) | 30.8 ± 4.0 | 27.9 ± 3.7 (−9.7, −13.8 to −5.4) | F = 49.1, p < 0.001 (0.80) | F = 2.6, p = 0.130 (0.17) | F = 4.5, p = 0.054 (0.27) * |
| Fat mass (%) | 42.1 ± 12.9 | 36.3 ± 13.7 (−13.4, −19.0 to −7.4) | 35.0 ± 8.2 | 27.3 ± 6.5 (−22.8, −27.4 to −17.9) | F = 136.8, p < 0.001 (0.91) | F = 1.7, p = 0.217 (0.12) | F = 7.7, p = 0.016 (0.39) * |
| Fat-free mass (%) | 53.1 ± 9.1 | 53.1 ± 9.7 (−0.1, −2.5 to 2.8) | 50.1 ± 14.8 | 49.9 ± 15.0 (−0.3, −3.2 to −2.7) | F = 0.0, p = 0.927 (0.00) | F = 0.2, p = 0.651 (0.01) | F = 0.0, p = 0.927 (0.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azócar-Gallardo, J.; Ramirez-Campillo, R.; Afonso, J.; Sá, M.; Granacher, U.; González-Rojas, L.; Ojeda-Aravena, A.; García-García, J.M. Overweight and Obese Adult Patients Show Larger Benefits from Concurrent Training Compared with Pharmacological Metformin Treatment on Insulin Resistance and Fat Oxidation. Int. J. Environ. Res. Public Health 2022, 19, 14331. https://doi.org/10.3390/ijerph192114331
Azócar-Gallardo J, Ramirez-Campillo R, Afonso J, Sá M, Granacher U, González-Rojas L, Ojeda-Aravena A, García-García JM. Overweight and Obese Adult Patients Show Larger Benefits from Concurrent Training Compared with Pharmacological Metformin Treatment on Insulin Resistance and Fat Oxidation. International Journal of Environmental Research and Public Health. 2022; 19(21):14331. https://doi.org/10.3390/ijerph192114331
Chicago/Turabian StyleAzócar-Gallardo, Jairo, Rodrigo Ramirez-Campillo, José Afonso, Mário Sá, Urs Granacher, Luis González-Rojas, Alex Ojeda-Aravena, and José Manuel García-García. 2022. "Overweight and Obese Adult Patients Show Larger Benefits from Concurrent Training Compared with Pharmacological Metformin Treatment on Insulin Resistance and Fat Oxidation" International Journal of Environmental Research and Public Health 19, no. 21: 14331. https://doi.org/10.3390/ijerph192114331
APA StyleAzócar-Gallardo, J., Ramirez-Campillo, R., Afonso, J., Sá, M., Granacher, U., González-Rojas, L., Ojeda-Aravena, A., & García-García, J. M. (2022). Overweight and Obese Adult Patients Show Larger Benefits from Concurrent Training Compared with Pharmacological Metformin Treatment on Insulin Resistance and Fat Oxidation. International Journal of Environmental Research and Public Health, 19(21), 14331. https://doi.org/10.3390/ijerph192114331










