Association of Self-Reported Depression Symptoms with Physical Activity Levels in Czechia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sampling
2.3. Data Collection
2.4. Variable Definitions
2.5. Outcomes of Depression
2.6. Data Analysis
3. Results
3.1. Subject Characteristics
3.2. Adjusted Association between Physical Activity Level and Depression
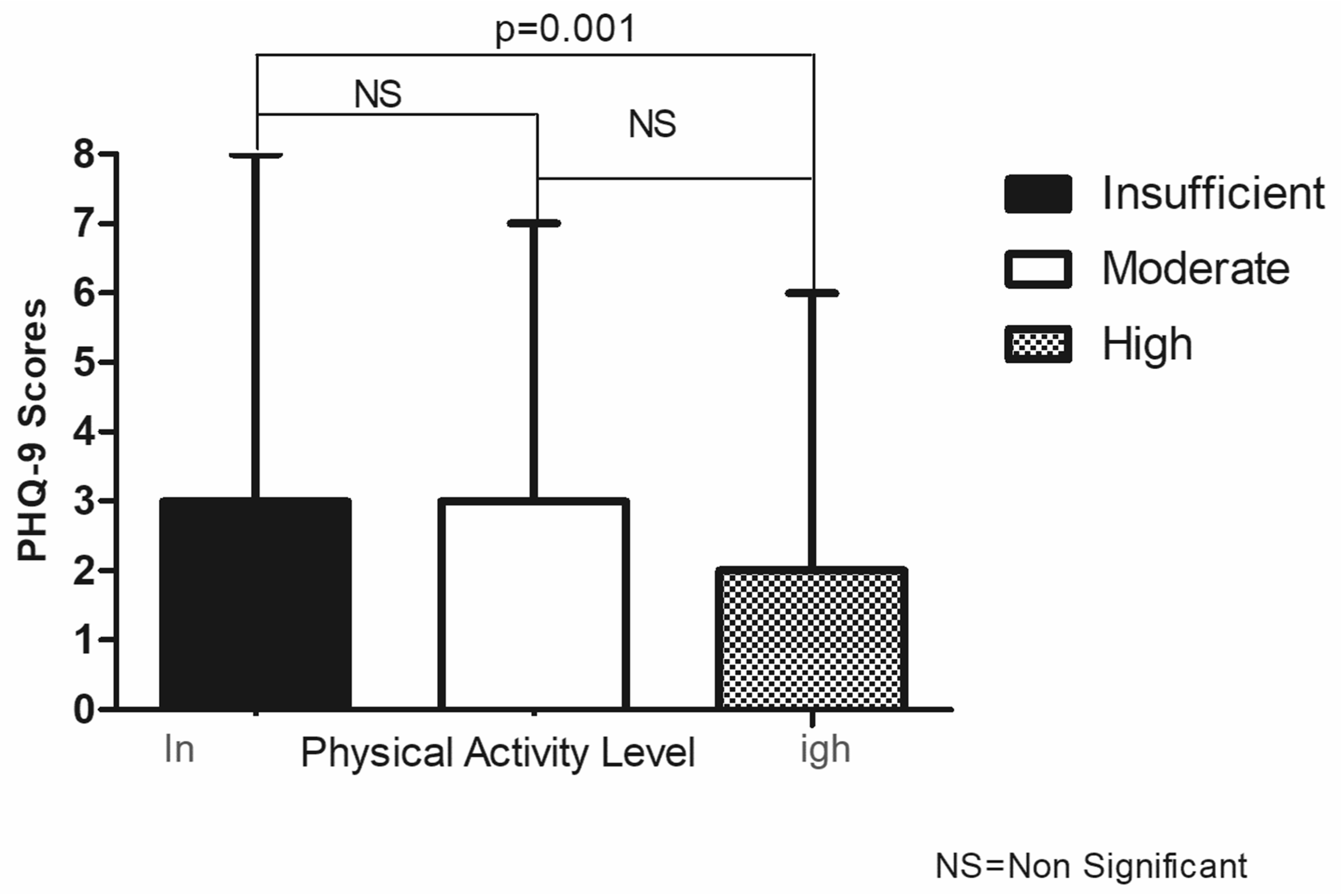
3.3. Association between Physical Activity Level and PHQ-9 Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association, D.; Association, A.P. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Formanek, T.; Kagstrom, A.; Cermakova, P.; Csemy, L.; Mlada, K.; Winkler, P. Prevalence of mental disorders and associated disability: Results from the cross-sectional CZEch mental health Study (CZEMS). Eur. Psychiatr. 2019, 60, 1–6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Cermakova, P.; Pikhart, H.; Kubinova, R.; Bobak, M. Education as inefficient resource against depressive symptoms in the Czech Republic: Cross-sectional analysis of the HAPIEE study. Eur. J. Public Health 2020, 30, 948–952. [Google Scholar] [CrossRef]
- Pavlovska, I.; Polcrova, A.; Mechanick, J.I.; Broz, J.; Infante-Garcia, M.M.; Nieto-Martinez, R.; Maranhao Neto, G.A.; Kunzova, S.; Skladana, M.; Novotny, J.S.; et al. Dysglycemia and Abnormal Adiposity Drivers of Cardiometabolic-Based Chronic Disease in the Czech Population: Biological, Behavioral, and Cultural/Social Determinants of Health. Nutrients 2021, 13, 2338. [Google Scholar] [CrossRef]
- Pec, O. Mental health reforms in the Czech Republic. BJPsych Int. 2019, 16, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, M. Mental health policy in Eastern Europe: A comparative analysis of seven mental health systems. BMC Health Serv. Res. 2014, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Hoschl, C.; Winkler, P.; Pec, O. The state of psychiatry in the Czech Republic. Int. Rev. Psychiatr. 2012, 24, 278–285. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Phillips, C. Brain-Derived Neurotrophic Factor, Depression, and Physical Activity: Making the Neuroplastic Connection. Neural. Plast 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Stanton, R.; Reaburn, P. Exercise and the treatment of depression: A review of the exercise program variables. J. Sci. Med. Sport 2014, 17, 177–182. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Dinas, P.C.; Koutedakis, Y.; Flouris, A.D. Effects of exercise and physical activity on depression. Ir. J. Med. Sci. 2011, 180, 319–325. [Google Scholar] [CrossRef]
- Hamrik, Z.; Sigmundova, D.; Kalman, M.; Pavelka, J.; Sigmund, E. Physical activity and sedentary behaviour in Czech adults: Results from the GPAQ study. Eur. J. Sport Sci. 2014, 14, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Mayo, X.; Liguori, G.; Iglesias-Soler, E.; Copeland, R.J.; Clavel San Emeterio, I.; Lowe, A.; Del Villar, F.; Jimenez, A. The active living gender’s gap challenge: 2013–2017 Eurobarometers physical inactivity data show constant higher prevalence in women with no progress towards global reduction goals. BMC Public Health 2019, 19, 1677. [Google Scholar] [CrossRef]
- Stubbs, B.; Koyanagi, A.; Schuch, F.B.; Firth, J.; Rosenbaum, S.; Veronese, N.; Solmi, M.; Mugisha, J.; Vancampfort, D. Physical activity and depression: A large cross-sectional, population-based study across 36 low- and middle-income countries. Acta Psychiatr. Scand. 2016, 134, 546–556. [Google Scholar] [CrossRef]
- Fromel, K.; Jakubec, L.; Groffik, D.; ChmelIk, F.; Svozil, Z.; Safar, M. Physical Activity of Secondary School Adolescents at Risk of Depressive Symptoms. J. Sch. Health 2020, 90, 641–650. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Marques, A.; Bordado, J.; Peralta, M.; Gouveia, E.R.; Tesler, R.; Demetriou, Y.; Gomez Baya, D. Cross-sectional and prospective relationship between physical activity and depression symptoms. Sci. Rep. 2020, 10, 16114. [Google Scholar] [CrossRef]
- Currier, D.; Lindner, R.; Spittal, M.J.; Cvetkovski, S.; Pirkis, J.; English, D.R. Physical activity and depression in men: Increased activity duration and intensity associated with lower likelihood of current depression. J. Affect. Disord. 2020, 260, 426–431. [Google Scholar] [CrossRef]
- Jokela, M.; Virtanen, M.; Batty, G.D.; Kivimäki, M. Inflammation and Specific Symptoms of Depression. JAMA Psychiatr. 2016, 73, 87–88. [Google Scholar] [CrossRef]
- Mossaheb, N.; Zehetmayer, S.; Jungwirth, S.; Weissgram, S.; Rainer, M.; Tragl, K.H.; Fischer, P. Are specific symptoms of depression predictive of Alzheimer’s dementia? J. Clin. Psychiatr. 2012, 73, 1009–1015. [Google Scholar] [CrossRef]
- Luepker, R.V. WHO MONICA Project: What Have We Learned and Where to Go from Here? Public Health Rev. 2011, 33, 373–396. [Google Scholar] [CrossRef]
- Movsisyan, N.K.; Vinciguerra, M.; Lopez-Jimenez, F.; Kunzova, S.; Homolka, M.; Jaresova, J.; Cifkova, R.; Sochor, O. Kardiovize Brno 2030, a prospective cardiovascular health study in Central Europe: Methods, baseline findings and future directions. Eur. J. Prev. Cardiol. 2018, 25, 54–64. [Google Scholar] [CrossRef]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Benzon Larsen, S.; Vogel, U.; Christensen, J.; Hansen, R.D.; Wallin, H.; Overvad, K.; Tjønneland, A.; Tolstrup, J. Interaction between ADH1C Arg272Gln and alcohol intake in relation to breast cancer risk suggests that ethanol is the causal factor in alcohol related breast cancer. Cancer Lett. 2010, 295, 191–197. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Int. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Epel, E.S.; Reus, V.I.; Mellon, S.H. Depression gets old fast: Do stress and depression accelerate cell aging? Depress. Anxiety 2010, 27, 327–338. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Bjelland, I.; Krokstad, S.; Mykletun, A.; Dahl, A.A.; Tell, G.S.; Tambs, K. Does a higher educational level protect against anxiety and depression? The HUNT study. Soc. Sci. Med. 2008, 66, 1334–1345. [Google Scholar] [CrossRef]
- Akhtar-Danesh, N.; Landeen, J. Relation between depression and sociodemographic factors. Int. J. Ment. Health Syst. 2007, 1, 4. [Google Scholar] [CrossRef]
- McHugh, R.K.; Weiss, R.D. Alcohol Use Disorder and Depressive Disorders. Alcohol. Res. 2019, 40, arcr.v40.1.01. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, T.; Boone, S.; de Mutsert, R.; Penninx, B.; Rosendaal, F.; le Cessie, S.; Milaneschi, Y.; Mook- Kanamori, D. The association between overall and abdominal adiposity and depressive mood: A cross-sectional analysis in 6459 participants. Psychoneuroendocrinology 2019, 110, 104429. [Google Scholar] [CrossRef]
- Bădescu, S.V.; Tătaru, C.; Kobylinska, L.; Georgescu, E.L.; Zahiu, D.M.; Zăgrean, A.M.; Zăgrean, L. The association between Diabetes mellitus and Depression. J. Med. Life 2016, 9, 120–125. [Google Scholar]
- Asztalos, M.; De Bourdeaudhuij, I.; Cardon, G. The relationship between physical activity and mental health varies across activity intensity levels and dimensions of mental health among women and men. Public Health Nutr. 2010, 13, 1207–1214. [Google Scholar] [CrossRef]
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatr. 2022, 79, 550–559. [Google Scholar] [CrossRef]
- Sun, W.; Yu, M.; Zhou, X. Effects of physical exercise on attention deficit and other major symptoms in children with ADHD: A meta-analysis. Psychiatr. Res. 2022, 311, 114509. [Google Scholar] [CrossRef]
- McKercher, C.; Patton, G.C.; Schmidt, M.D.; Venn, A.J.; Dwyer, T.; Sanderson, K. Physical Activity and Depression Symptom Profiles in Young Men and Women With Major Depression. Psychosom. Med. 2013, 75, 366–374. [Google Scholar] [CrossRef]
- Hollands, L.; Lambert, J.; Price, L.; Powell, D.; Greaves, C. Ecological momentary assessment of mood and physical activity in people with depression. J. Affect. Disord. 2020, 271, 293–299. [Google Scholar] [CrossRef]
- Althumiri, N.A.; Basyouni, M.H.; BinDhim, N.F. Exploring the Association Between Physical Activity and Risk of Mental Health Disorders in Saudi Arabian Adults: Cross-sectional Study. JMIR Public Health Surveill. 2021, 7, e25438. [Google Scholar] [CrossRef]
- Chen, M.J. The neurobiology of depression and physical exercise. In Routledge Handbook of Physical Activity and Mental Health; Routledge: Abingdon, UK, 2013; pp. 191–206. [Google Scholar]
- Puetz, T.W.; Herring, M.P. Physical activity and feelings of fatigue. In Routledge Handbook of Physical Activity and Mental Health; Routledge: Abingdon, UK, 2013; pp. 444–461. [Google Scholar]
- Puetz, T.W. Physical activity and feelings of energy and fatigue. Sports Med. 2006, 36, 767–780. [Google Scholar] [CrossRef]
- Stillman, C.M.; Esteban-Cornejo, I.; Brown, B.; Bender, C.M.; Erickson, K.I. Effects of Exercise on Brain and Cognition Across Age Groups and Health States. Trends Neurosci. 2020, 43, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Du Rietz, E.; Barker, A.R.; Michelini, G.; Rommel, A.S.; Vainieri, I.; Asherson, P.; Kuntsi, J. Beneficial effects of acute high-intensity exercise on electrophysiological indices of attention processes in young adult men. Behav. Brain Res. 2019, 359, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Schuch, F.B. Chapter 1-Exercise for the Prevention and Treatment of Depression. In Exercise-Based Interventions for Mental Illness; Stubbs, B., Rosenbaum, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–18. [Google Scholar]
- LaCaille, R.; Marshall, E. Psychosocial Benefits and Aspects of Physical Activity. In Encyclopedia of Behavioral Medicine; Gellman, M., Ed.; Springer: New York, NY, USA, 2019; pp. 1–8. [Google Scholar]
- Uher, R.; Muthén, B.; Souery, D.; Mors, O.; Jaracz, J.; Placentino, A.; Petrovic, A.; Zobel, A.; Henigsberg, N.; Rietschel, M.; et al. Trajectories of change in depression severity during treatment with antidepressants. Psychol. Med. 2010, 40, 1367–1377. [Google Scholar] [CrossRef]
- National Mental Health Action Plan 2020–2030 Prague: Ministry of Health. 2020. Available online: https://www.mzcr.cz/wp-content/uploads/2020/01/National-Mental-Health-Action-Plan-2020-2030.pdf (accessed on 10 September 2022).
- McKeon, G.; Curtis, J.; Rosenbaum, S. Promoting physical activity for mental health: An updated evidence review and practical guide. Curr. Opin. Psychiatr. 2022, 35, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Areerob, P.; Hennessy, D.; Goncalves-Bradley, D.C.; Mesagno, C.; Grace, F. Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: A systematic review and network meta-analysis of randomised controlled trials. F1000Research 2020, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Levis, B.; Benedetti, A.; Thombs, B.D. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: Individual participant data meta-analysis. BMJ 2019, 365, l1476. [Google Scholar] [CrossRef]
| PHQ-9 (Score Median Range) | p | |
|---|---|---|
| Gender | ||
| Male | 2.0 (0–20) | |
| Female | 3.0 (0–23) | <0.001 |
| Age Categories | ||
| 25–34 | 3.0 (0–20) | |
| 35–44 | 3.0 (0–19) | |
| 45–54 | 2.5 (0–23) | |
| 55–64 | 2.0 (0–23) | 0.023 |
| Educational Level | ||
| Primary | 3.0 (0–23) | |
| Secondary | 2.0 (0–23) | |
| Higher | 2.0 (0–20) | 0.002 |
| Household income (EUR) | ||
| Low (<1200) | 3.0 (0–23) | |
| Middle (1200–1800) | 2.0 (0–18) | |
| High (>1800) | 2.0 (0–19) | <0.001 |
| Living in Couple | ||
| No | 3.0 (0–23) | |
| Yes | 2.0 (0–19) | 0.002 |
| Alcohol Users | ||
| No | 3.0 (0–18) | |
| Yes | 2.0 (0–23) | 0.006 |
| Smokers | ||
| No | 2.0 (0–23) | |
| Yes | 2.0 (0–23) | 0.591 |
| High Body Fat Percentage | ||
| Absent | 2.0 (0–23) | |
| Present | 3.0 (0–20) | 0.002 |
| Diabetes | ||
| Absent | 2.0 (0–23) | |
| Present | 3.0 (0–14) | 0.370 |
| Hypertension | ||
| Absent | 2.0 (0–23) | |
| Present | 2.0 (0–23) | 0.443 |
| Cardiovascular Disease History | ||
| Absent | 2.0 (0–23) | |
| Present | 3.0 (0–17) | 0.312 |
| The Mann–Whitney U test was used to determine different medians. | ||
| Physical Activity Level # and PHQ-9 Scores | |||
|---|---|---|---|
| Physical Activity Level | Model 1 | Model 2 | Model 3 |
| PR (95% CI) | PR (95% CI) | PR (95% CI) | |
| Insufficient | 1 | 1 | 1 |
| Moderate | 0.85 * (0.74–0.97) | 0.81 ** (0.71–0.93) | 0.85 * (0.75–0.97) |
| High | 0.80 ** (0.70–0.91) | 0.78 *** (0.68–0.88) | 0.80 ** (0.70–0.92) |
| Physical Activity Level # | |||
|---|---|---|---|
| Moderate | High | ||
| PR (95% CI) | PR (95% CI) | ||
| “Little interest or pleasure in doing things” | 0.90 (0.73–1.12) | 0.83 (0.67–1.06) | |
| “Feeling down, depressed, or hopeless” | 0.66 (0.48–0.91) | 0.59 (0.44–0.81) | |
| “Trouble falling or staying asleep, or sleeping too much” | 0.96 (0.80–1.16) | 0.97 (0.81–1.16) | |
| “Feeling tired or having little energy” | 0.89 (0.78–1.08) | 0.86 (0.76–0.98) | |
| “Poor appetite or overeating” | 0.80 (0.61–1.06) | 0.75 (0.57–0.98) | |
| “Feeling bad about yourself or that you are a failure or have let yourself or your family down” | 0.74 (0.56–0.98) | 0.66 (0.51–0.86) | |
| “Trouble concentrating on things, such as reading the newspaper or watching television” | 0.84 (0.64–1.10) | 0.71 (0.54–0.94) | |
| Moving or speaking so slowly that other people could have noticed. Or the opposite, being so fidgety or restless that you have been moving around a lot more than usual | 0.68 (0.43–1.08) | 0.69 (0.44–1.08) | |
| “Thoughts that you would be better off dead, or of hurting yourself” | 0.96 (0.36–2.51) | 0.64 (0.24–1.70) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranhao Neto, G.A.; Lattari, E.; Oliveira, B.R.R.; Polcrova, A.B.; Infante-Garcia, M.M.; Kunzova, S.; Stokin, G.B.; Gonzalez-Rivas, J.P. Association of Self-Reported Depression Symptoms with Physical Activity Levels in Czechia. Int. J. Environ. Res. Public Health 2022, 19, 14319. https://doi.org/10.3390/ijerph192114319
Maranhao Neto GA, Lattari E, Oliveira BRR, Polcrova AB, Infante-Garcia MM, Kunzova S, Stokin GB, Gonzalez-Rivas JP. Association of Self-Reported Depression Symptoms with Physical Activity Levels in Czechia. International Journal of Environmental Research and Public Health. 2022; 19(21):14319. https://doi.org/10.3390/ijerph192114319
Chicago/Turabian StyleMaranhao Neto, Geraldo A., Eduardo Lattari, Bruno Ribeiro Ramalho Oliveira, Anna Bartoskova Polcrova, Maria M. Infante-Garcia, Sarka Kunzova, Gorazd B. Stokin, and Juan P. Gonzalez-Rivas. 2022. "Association of Self-Reported Depression Symptoms with Physical Activity Levels in Czechia" International Journal of Environmental Research and Public Health 19, no. 21: 14319. https://doi.org/10.3390/ijerph192114319
APA StyleMaranhao Neto, G. A., Lattari, E., Oliveira, B. R. R., Polcrova, A. B., Infante-Garcia, M. M., Kunzova, S., Stokin, G. B., & Gonzalez-Rivas, J. P. (2022). Association of Self-Reported Depression Symptoms with Physical Activity Levels in Czechia. International Journal of Environmental Research and Public Health, 19(21), 14319. https://doi.org/10.3390/ijerph192114319






