Effects of Photodegradation on the Optical Indices of Chromophoric Dissolved Organic Matter from Typical Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Photodegradation Experiment
2.2. Sample Measurements and Analysis
3. Results
3.1. Absorption Results
3.2. Fluorescence Results
3.3. The Degradation Model Results
4. Discussion
4.1. Variability of Photodegradation of CDOM and Different Fluorescent Components from Typical Sources
4.2. Effects of Photodegradation on the Optical Indices for the DOM Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coble, P.G. Marine optical biogeochemistry: The chemistry of ocean color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhou, L.; Zhou, Y.Q.; Zhang, L.Q.; Yao, X.L.; Shi, K.; Jeppesen, E.; Yu, Q.; Zhu, W.N. Chromophoric dissolved organic matter in inland waters: Present knowledge and future challenges. Sci. Total Environ. 2021, 759, 143550. [Google Scholar] [CrossRef] [PubMed]
- Zepp, R.G.; Shank, G.C.; Stabenau, E.; Patterson, K.W.; Cyterski, M.; Fisher, W.; Bartels, E.; Anderson, S.L. Spatial and temporal variability of solar ultraviolet exposure of coral assemblages in the Florida Keys: Importance of colored dissolved organic matter. Limnol. Oceanogr. 2008, 53, 1909–1922. [Google Scholar] [CrossRef] [Green Version]
- D’Sa, E.J.; Kim, H.C.; Ha, S.Y.; Joshi, I. Ross Sea dissolved organic matter optical properties during an austral summer: Biophysical influences. Front. Mar. Sci. 2021, 8, 749096. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Tranvik, L.; Kronberg, L.; Slatis, T.; Martinsen, W. Photochemical production of ammonium and transformation of dissolved organic matter in the Baltic Sea. Mar. Chem. 2007, 104, 227–240. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanoue, E. Production of bio-refractory fluorescent dissolved organic matter in the ocean interior. Nat. Geosci. 2008, 1, 579–582. [Google Scholar] [CrossRef]
- Guo, W.; Yang, L.; Yu, X.; Zhai, W.; Hong, H. Photo-production of dissolved inorganic carbon from dissolved organic matter in contrasting coastal waters in the southwestern Taiwan Strait, China. J. Environ. Sci. 2012, 24, 1181–1188. [Google Scholar] [CrossRef]
- Al-Reasi, H.A.; Wood, C.M.; Smith, D.S. Physicochemical and spectroscopic properties of natural organic matter (NOM) from various sources and implications for ameliorative effects on metal toxicity to aquatic biota. Aqua. Tox. 2011, 103, 179–190. [Google Scholar] [CrossRef]
- Xu, H.; Guan, D.-X.; Zou, L.; Lin, H.; Guo, L. Contrasting effects of photochemical and microbial degradation on Cu(II) binding with fluorescent DOM from different origins. Environ. Pollut. 2018, 239, 205–214. [Google Scholar] [CrossRef]
- Chen, L.W.; Zhuang, W.-E.; Yang, L.Y. Critical evaluation of the interaction between fluorescent dissolved organic matter and Pb(II) under variable environmental conditions. Chemosphere 2022, 307, 135875. [Google Scholar] [CrossRef]
- Yang, L.; Kim, D.; Uzun, H.; Karanfil, T.; Hur, J. Assessing trihalomethanes (THMs) and N-nitrosodimethylamine (NDMA) formation potentials in drinking water treatment plants using fluorescence spectroscopy and parallel factor analysis. Chemosphere 2015, 121, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Fichot, C.G.; Miller, W.L. An approach to quantify depth-resolved marine photochemical fluxes using remote sensing: Application to carbon monoxide (CO) photoproduction. Remote Sens. Environ. 2010, 114, 1363–1377. [Google Scholar] [CrossRef]
- Bertilsson, S.; Tranvik, L.J. Photochemical transformation of dissolved organic matter in lakes. Limnol. Oceanogr. 2000, 45, 753–762. [Google Scholar] [CrossRef]
- Moran, M.A.; Sheldon, W.M.; Zepp, R.G. Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter. Limnol. Oceanogr. 2000, 45, 1254–1264. [Google Scholar] [CrossRef]
- Stubbins, A.; Hubbard, V.; Uher, G.; Law, C.S.; Upstill-Goddard, R.C.; Aiken, G.R.; Mopper, K. Relating carbon monoxide photoproduction to dissolved organic matter functionality. Environ. Sci. Technol. 2008, 42, 3271–3276. [Google Scholar] [CrossRef]
- Lu, Y.; Bauer, J.E.; Canuel, E.A.; Yamashita, Y.; Chambers, R.M.; Jaffé, R. Photochemical and microbial alteration of dissolved organic matter in temperate headwater streams associated with different land use. J. Geophys. Res. Biogeo. 2013, 118, 566–580. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.-Z.; Zhang, J.; Yang, G.-P. Mixing behavior and photobleaching of chromophoric dissolved organic matter in the Changjiang River estuary and the adjacent East China Sea. Estuar. Coast. Shelf S. 2018, 207, 422–434. [Google Scholar] [CrossRef]
- Chen, M.; Jaffé, R. Photo- and bio-reactivity patterns of dissolved organic matter from biomass and soil leachates and surface waters in a subtropical wetland. Water Res. 2014, 61, 181–190. [Google Scholar] [CrossRef]
- Timko, S.A.; Romera-Castillo, C.; Jaffe, R.; Cooper, W.J. Photo-reactivity of natural dissolved organic matter from fresh to marine waters in the Florida Everglades, USA. Environ. Sci. Proc. Imp. 2014, 16, 866–878. [Google Scholar] [CrossRef]
- Zhuang, W.-E.; Chen, W.; Cheng, Q.; Yang, L. Assessing the priming effect of dissolved organic matter from typical sources using fluorescence EEMs-PARAFAC. Chemosphere 2021, 264, 128600. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Limnol. Oceanogr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Ishii, S.K.L.; Boyer, T.H. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: A critical review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Wünsch, U.J.; Bro, R.; Stedmon, C.A.; Wenig, P.; Murphy, K.R. Emerging patterns in the global distribution of dissolved organic matter fluorescence. Anal. Methods 2019, 11, 888–893. [Google Scholar] [CrossRef] [Green Version]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Perdue, E.M.; Green, N.W.; Chen, H.; Mopper, K. Photochemical bleaching of oceanic dissolved organic matter and its effect on absorption spectral slope and fluorescence. Mar. Chem. 2013, 155, 81–91. [Google Scholar] [CrossRef]
- Lee, M.-H.; Osburn, C.L.; Shin, K.-H.; Hur, J. New insight into the applicability of spectroscopic indices for dissolved organic matter (DOM) source discrimination in aquatic systems affected by biogeochemical processes. Water Res. 2018, 147, 164–176. [Google Scholar] [CrossRef]
- Derrien, M.; Choi, H.; Jardé, E.; Shin, K.-H.; Hur, J. Do early diagenetic processes affect the applicability of commonly-used organic matter source tracking tools? An assessment through controlled degradation end-member mixing experiments. Water Res. 2020, 173, 115588. [Google Scholar] [CrossRef]
- Yang, L.Y.; Cheng, Q.; Zhuang, W.E.; Wang, H.; Chen, W. Seasonal changes in the chemical composition and reactivity of dissolved organic matter at the land-ocean interface of a subtropical river. Environ. Sci. Pollut. R. 2019, 26, 24595–24608. [Google Scholar] [CrossRef]
- Kothawala, D.N.; Murphy, K.R.; Stedmon, C.A.; Weyhenmeyer, G.A.; Tranvik, L.J. Inner filter correction of dissolved organic matter fluorescence. Limnol. Oceanogr. Methods 2013, 11, 616–630. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Cory, R.M.; Miller, M.P.; McKnight, D.M.; Guerard, J.J.; Miller, P.L. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol. Oceanogr. Methods 2010, 8, 67–78. [Google Scholar]
- Spencer, R.G.M.; Stubbins, A.; Hernes, P.J.; Baker, A.; Mopper, K.; Aufdenkampe, A.K.; Dyda, R.Y.; Mwamba, V.L.; Mangangu, A.M.; Wabakanghanzi, J.N.; et al. Photochemical degradation of dissolved organic matter and dissolved lignin phenols from the Congo River. J. Geophys. Res. Biogeo. 2009, 114, G03010. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef] [Green Version]
- Wünsch, U.J.; Murphy, K.R.; Stedmon, C.A. Fluorescence quantum yields of natural organic matter and organic compounds: Implications for the fluorescence-based interpretation of organic matter composition. Front. Mar. Sci. 2015, 2, 98. [Google Scholar] [CrossRef] [Green Version]
- D’Andrilli, J.; McConnell, J.R. Polar ice core organic matter signatures reveal past atmospheric carbon composition and spatial trends across ancient and modern timescales. J. Glaciol. 2021, 67, 1028–1042. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.; Khan, S.J. Organic matter fluorescence in municipal water recycling schemes: Toward a unified PARAFAC model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef]
- Yamashita, Y.; Panton, A.; Mahaffey, C.; Jaffé, R. Assessing the spatial and temporal variability of dissolved organic matter in Liverpool Bay using excitation–emission matrix fluorescence and parallel factor analysis. Ocean Dyn. 2011, 61, 569–579. [Google Scholar] [CrossRef]
- Dainard, P.G.; Guéguen, C. Distribution of PARAFAC modeled CDOM components in the North Pacific Ocean, Bering, Chukchi and Beaufort Seas. Mar. Chem. 2013, 157, 216–223. [Google Scholar] [CrossRef]
- Walker, S.A.; Amon, R.M.W.; Stedmon, C.A. Variations in high-latitude riverine fluorescent dissolved organic matter: A comparison of large Arctic rivers. J. Geophys. Res. Biogeo. 2013, 118, 1689–1702. [Google Scholar] [CrossRef]
- Amaral, V.; Ortega, T.; Romera-Castillo, C.; Forja, J. Linkages between greenhouse gases (CO2, CH4, and N2O) and dissolved organic matter composition in a shallow estuary. Sci. Total Environ. 2021, 788, 147863. [Google Scholar] [CrossRef] [PubMed]
- Gonsior, M.; Peake, B.M.; Cooper, W.T.; Podgorski, D.; D’Andrilli, J.; Cooper, W.J. Photochemically induced changes in dissolved organic matter identified by ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Environ. Sci. Technol. 2009, 43, 698–703. [Google Scholar] [CrossRef]
- Harir, M.; Cawley, K.M.; Hertkorn, N.; Schmitt-Kopplin, P.; Jaffé, R. Molecular and spectroscopic changes of peat-derived organic matter following photo-exposure: Effects on heteroatom composition of DOM. Sci. Total Environ. 2022, 838, 155790. [Google Scholar] [CrossRef]
- Benner, R.; Biddanda, B. Photochemical transformations of surface and deep marine dissolved organic matter: Effects on bacterial growth. Limnol. Oceanogr. 1998, 43, 1373–1378. [Google Scholar] [CrossRef]
- Bittar, T.B.; Vieira, A.A.H.; Stubbins, A.; Mopper, K. Competition between photochemical and biological degradation of dissolved organic matter from the cyanobacteria Microcystis aeruginosa. Limnol. Oceanogr. 2015, 60, 1172–1194. [Google Scholar] [CrossRef]
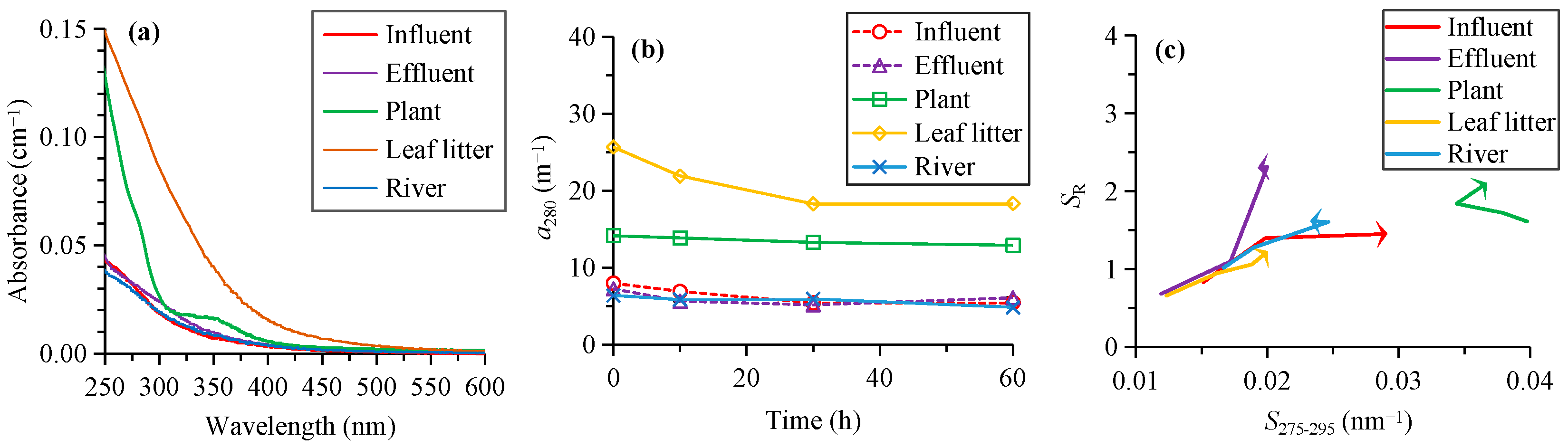
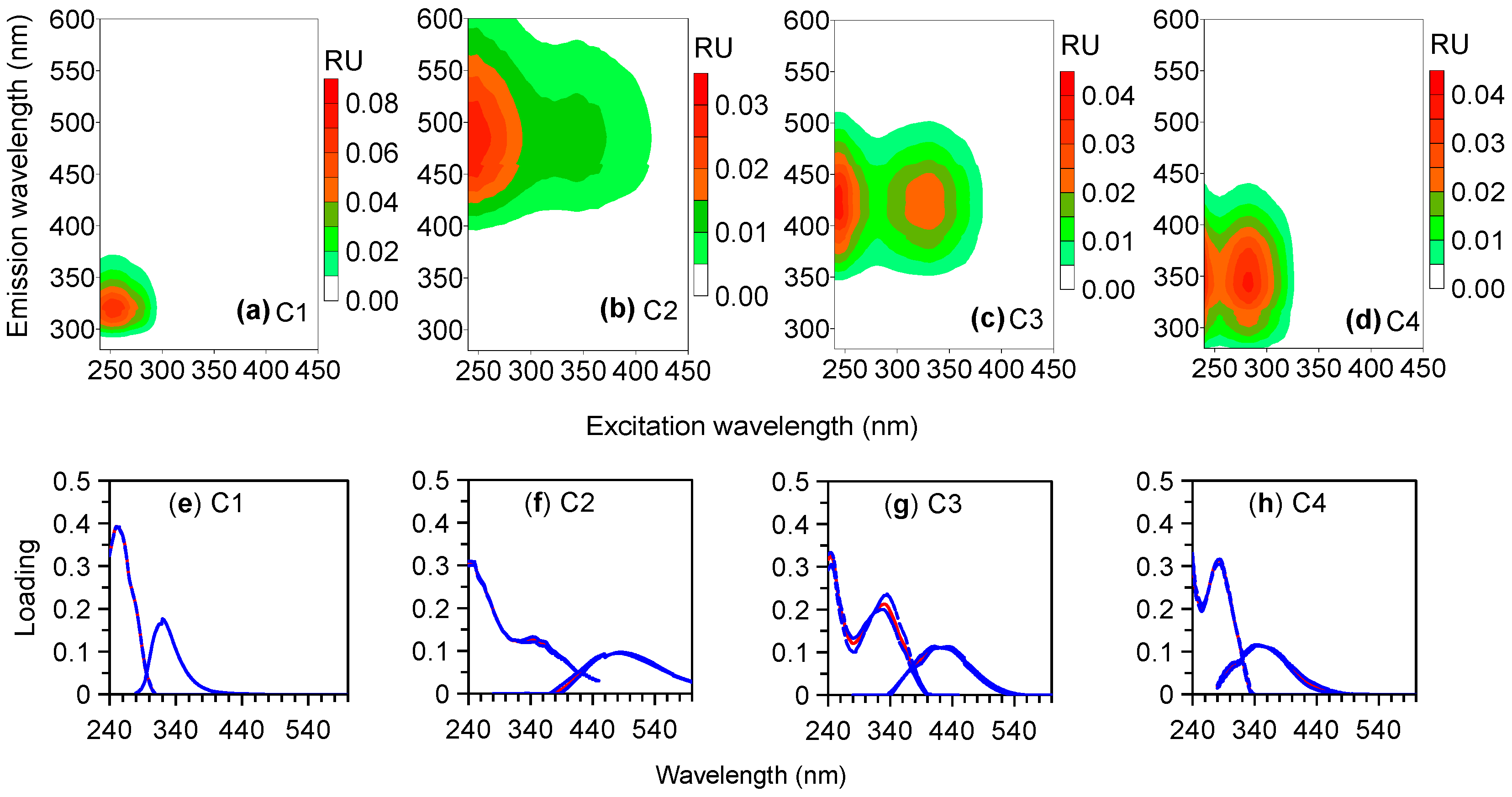
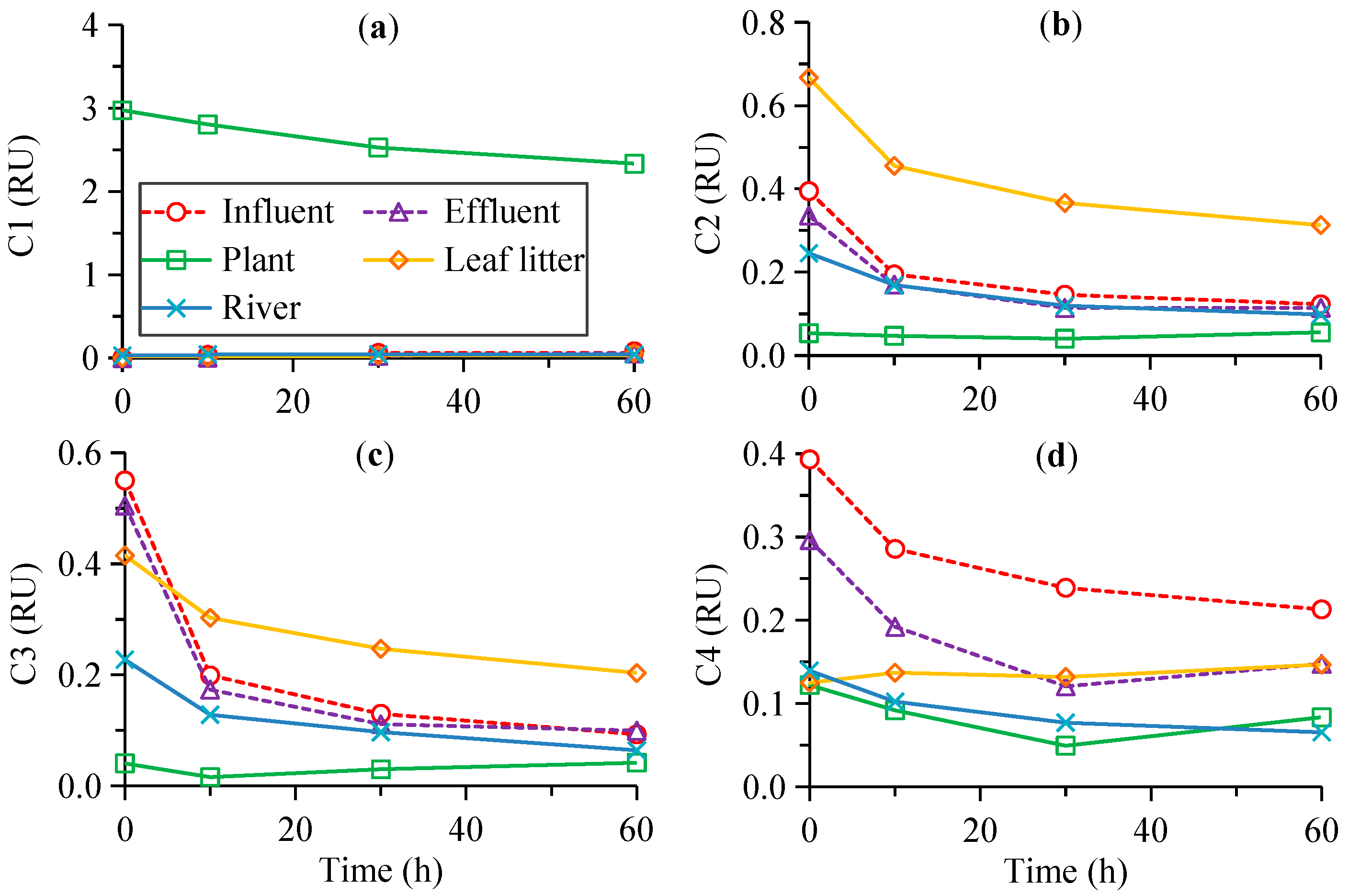
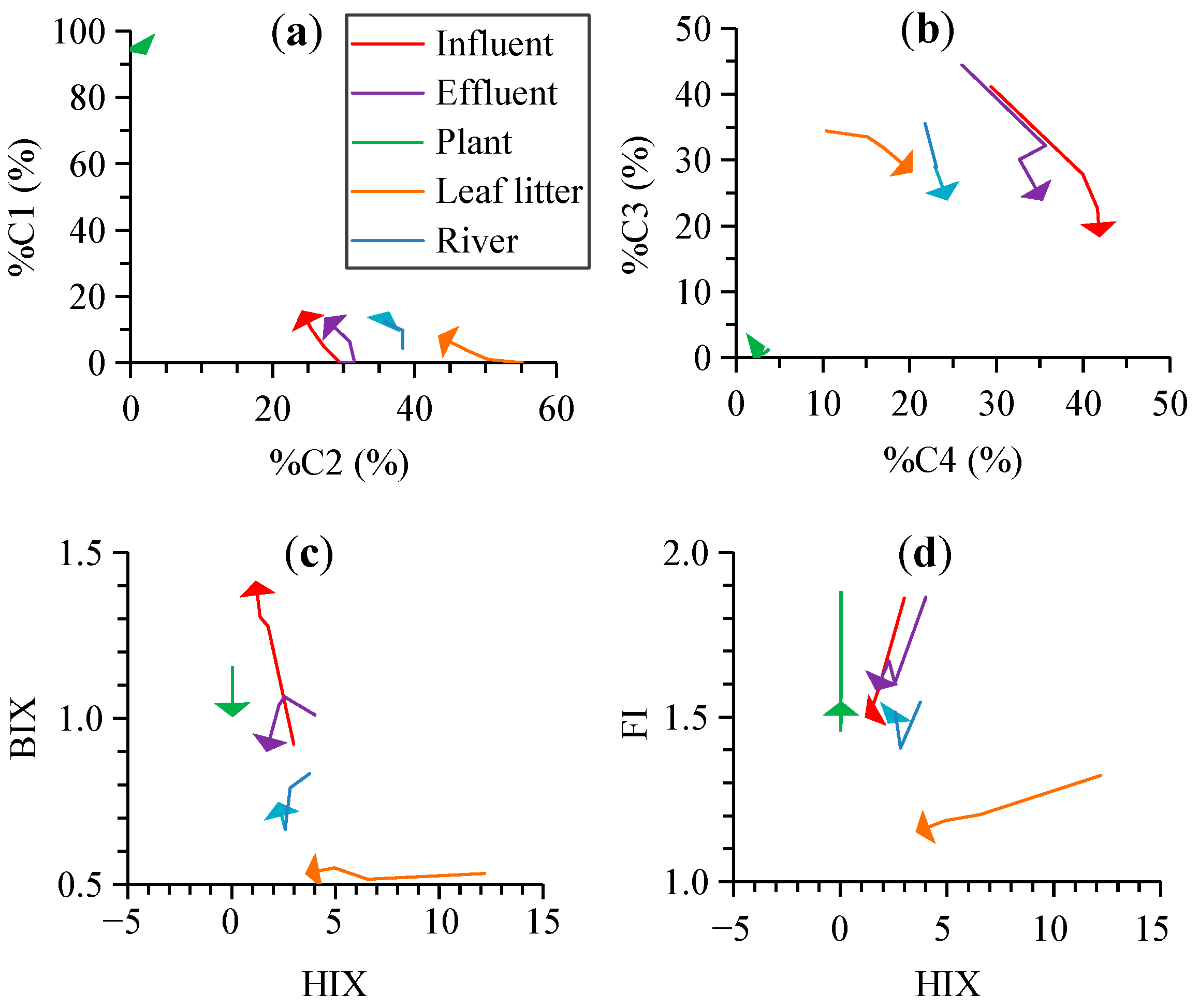

| Source | Time (h) | a280 (m−1) | S275–295 (nm−1) | SR | C1 (RU) | C2 (RU) | C3 (RU) | C4 (RU) | %C1 | %C2 | %C3 | %C4 | HIX | BIX | FI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Influent | 0 | 8.0 | 0.0151 | 0.83 | 0.00 | 0.39 | 0.55 | 0.39 | 0.0 | 29.5 | 41.1 | 29.4 | 3.0 | 0.92 | 1.86 |
| 60 | 5.5 | 0.0291 | 1.45 | 0.08 | 0.12 | 0.09 | 0.21 | 15.7 | 24.1 | 18.3 | 41.9 | 1.2 | 1.41 | 1.50 | |
| %Change | −31 | 92 | 75 | / 1 | −69 | −83 | −46 | / 1 | −18 | −56 | 43 | −61 | 53 | −19 | |
| Effluent | 0 | 7.2 | 0.0119 | 0.68 | 0.00 | 0.34 | 0.51 | 0.30 | 0.0 | 29.5 | 44.4 | 26.0 | 4.0 | 1.01 | 1.86 |
| 60 | 6.1 | 0.0193 | 2.30 | 0.06 | 0.11 | 0.10 | 0.15 | 13.5 | 27.3 | 23.8 | 35.3 | 1.7 | 0.90 | 1.58 | |
| %Change | −16 | 62 | 237 | / 1 | −66 | −80 | −50 | / 1 | −7 | −46 | 36 | −58 | −11 | −15 | |
| Plant | 0 | 14.1 | 0.0398 | 1.61 | 2.97 | 0.05 | 0.04 | 0.12 | 93.2 | 1.7 | 1.3 | 3.8 | 0.04 | 1.16 | 1.56 |
| 60 | 12.9 | 0.0367 | 2.10 | 2.33 | 0.06 | 0.04 | 0.08 | 92.8 | 2.2 | 1.7 | 3.3 | 0.04 | 1.00 | 1.55 | |
| %Change | −9 | −8 | 31 | −22 | / 1 | / 1 | −32 | 0 | / 1 | / 1 | −14 | / 1 | −13 | −1 | |
| Leaf litter | 0 | 25.6 | 0.0123 | 0.66 | 0.00 | 0.67 | 0.42 | 0.13 | 0.0 | 55.2 | 34.4 | 10.4 | 12.2 | 0.53 | 1.32 |
| 60 | 18.3 | 0.0200 | 1.23 | 0.06 | 0.31 | 0.20 | 0.15 | 8.2 | 43.3 | 28.2 | 20.3 | 3.5 | 0.53 | 1.15 | |
| %Change | −28 | 63 | 86 | / 1 | −53 | −51 | 17 | / 1 | −22 | −18 | 96 | −71 | 0 | −13 | |
| River | 0 | 6.4 | 0.0167 | 1.02 | 0.03 | 0.25 | 0.23 | 0.14 | 4.4 | 38.3 | 35.5 | 21.8 | 3.8 | 0.83 | 1.55 |
| 60 | 4.8 | 0.0233 | 1.62 | 0.04 | 0.10 | 0.06 | 0.07 | 15.4 | 36.4 | 23.9 | 24.3 | 2.2 | 0.75 | 1.48 | |
| %Change | −24 | 40 | 59 | / 1 | −60 | −72 | −53 | / 1 | −5 | −33 | 12 | −40 | −10 | −4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, W.-E.; Chen, W.; Yang, L. Effects of Photodegradation on the Optical Indices of Chromophoric Dissolved Organic Matter from Typical Sources. Int. J. Environ. Res. Public Health 2022, 19, 14268. https://doi.org/10.3390/ijerph192114268
Zhuang W-E, Chen W, Yang L. Effects of Photodegradation on the Optical Indices of Chromophoric Dissolved Organic Matter from Typical Sources. International Journal of Environmental Research and Public Health. 2022; 19(21):14268. https://doi.org/10.3390/ijerph192114268
Chicago/Turabian StyleZhuang, Wan-E, Wei Chen, and Liyang Yang. 2022. "Effects of Photodegradation on the Optical Indices of Chromophoric Dissolved Organic Matter from Typical Sources" International Journal of Environmental Research and Public Health 19, no. 21: 14268. https://doi.org/10.3390/ijerph192114268
APA StyleZhuang, W.-E., Chen, W., & Yang, L. (2022). Effects of Photodegradation on the Optical Indices of Chromophoric Dissolved Organic Matter from Typical Sources. International Journal of Environmental Research and Public Health, 19(21), 14268. https://doi.org/10.3390/ijerph192114268








