Clusters of Pregnant Women with Severe Acute Respiratory Syndrome Due to COVID-19: An Unsupervised Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Variables
- Demographic and obstetric characteristics: maternal age group (10–17, 18–34, 35–42, and 43–49); race/skin color (White, mixed, Black, Asian, and Indigenous); gestational stage (first—1st; second—2nd; and third—3rd trimester, puerperium, or no available data on gestational age). We dichotomized race/skin color into White/Asian and Indigenous/mixed/Black/no available (NA) data;
- Symptoms (yes or no): 1: Ageusia; 2: anosmia; 3: abdominal pain; 4: diarrhea; 5: vomiting; 6: dyspnea; 7: respiratory distress; 8: oxygen saturation less than ; 9: chest pain; 10: fever; 11: cough; 12: sore throat; 13: fatigue; 14: myalgia; 15: inappetence; 16: headache; 17: malaise; 18: nasal congestion;
- Comorbidities (yes or no): 19: Chronic heart disease; 20: asthma; 21: diabetes mellitus; 22: hypertension; 23: immunodeficiency or immunodepression; 24: obesity; 25: eclampsia;
- Hospital characteristics: Use of mechanical ventilation (MV) (26a: yes, invasive MV; 26b: yes, non-invasive MV, or no); 27: admission to the intensive care unit (ICU) (yes or no); 28: hospital outcome (discharge or death).
2.3. Statistical Analysis
3. Results
3.1. Multiple Correspondence Analysis
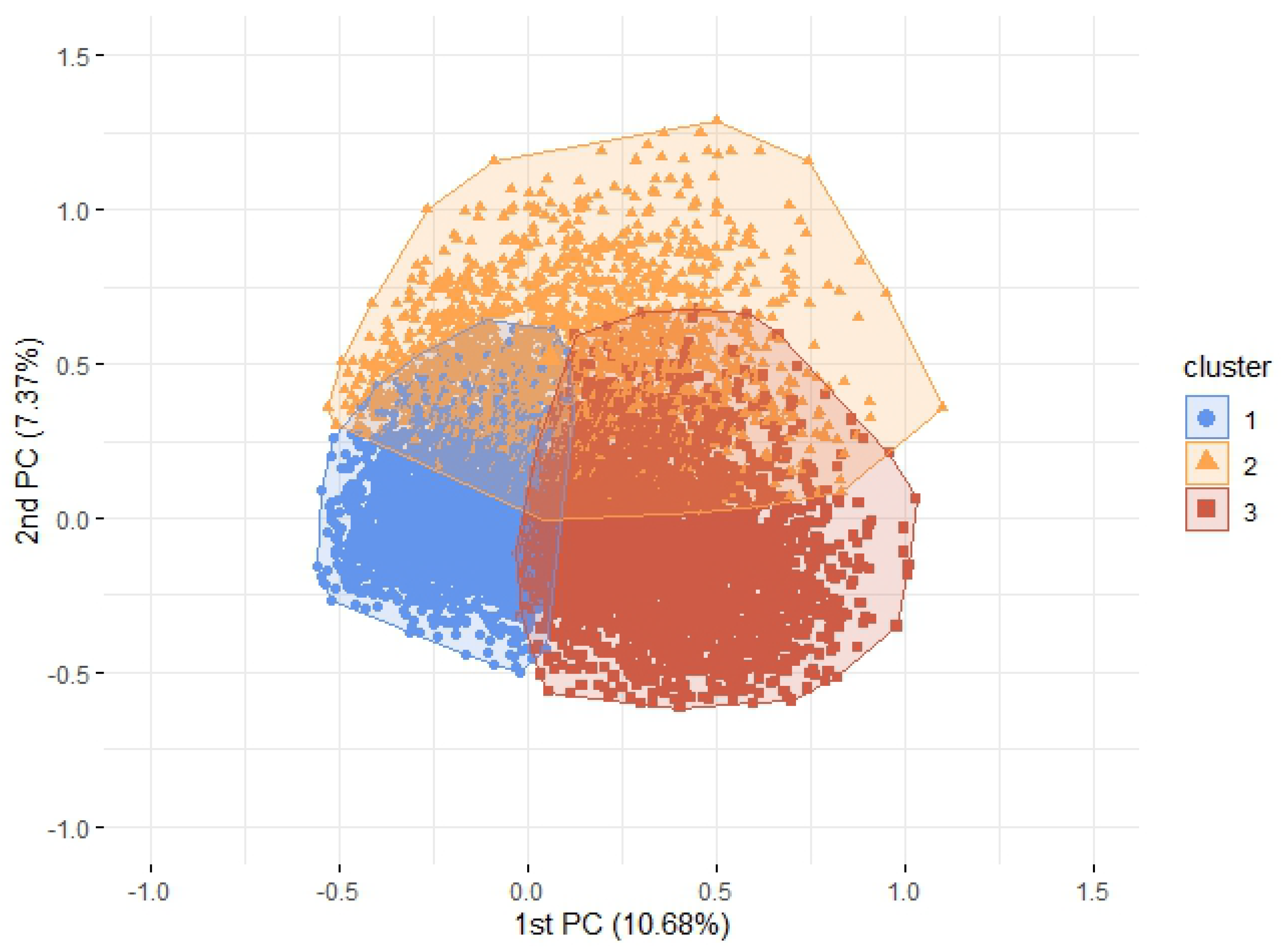
3.2. Agglomerative Hierarchical Clustering
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS | Severe acute respiratory syndrome |
| SIVEP-Gripe | Influenza Epidemiological Surveillance Information System of Brazil |
| PC | Principal Component |
| MV | Mechanical ventilation |
| ICU | Intensive care unit |
| MCA | Multiple Correspondence Analysis |
| RR | Relative Risk |
| CI | Confidence Interval |
References
- Huang, C.; Wang, Y.; Liu, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Orientações Sobre a Otimização do uso de Oxigênio e Suporte Ventilatório em Pacientes Graves com COVID-19. Available online: https://docs.bvsalud.org/biblioref/2021/05/1179870/orientacoes-sobre-otimizacao-do-uso-de-oxigenio-e-suporte-vent_DRIvHhs.pdf (accessed on 15 March 2022).
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes/Metabolism Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef]
- Benski, C.; Di Filippo, D.; Taraschi, G.; Reich, M.R. Guidelines for pregnancy management during the COVID-19 pandemic: A public health conundrum. Int. J. Environ. Res. Public Health 2020, 17, 8277. [Google Scholar] [CrossRef]
- Souza, A.S.R.; Amorim, M.M.R. Maternal mortality by COVID-19 in Brazil. Rev. Bras. Saúde Matern. Infant. 2021, 21, 253–256. [Google Scholar] [CrossRef]
- Salem, D.; Katranji, F.; Bakdash, T. COVID-19 infection in pregnant women: Review of maternal and fetal outcomes. Int. J. Gynecol. Obstet. 2021, 152, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Yeni, C.M.; Utami, N.A.; Masand, R.; Asrani, R.K.; Patel, S.K.; Kumar, A.; Yatoo, M.I.; Tiwari, R.; Natesan, S.; et al. SARS-CoV-2 infection during pregnancy and pregnancy-related conditions: Concerns, challenges, management and mitigation strategies–a narrative review. J. Infect. Public Health 2021, 14, 863–875. [Google Scholar] [CrossRef]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, e041868. [Google Scholar] [CrossRef]
- Takemoto, M.L.; Menezes, M.d.O.; Andreucci, C.B.; Nakamura-Pereira, M.; Amorim, M.M.; Katz, L.; Knobel, R. The tragedy of COVID-19 in Brazil: 124 maternal deaths and counting. Int. J. Gynecol. Obstet. 2020, 151, 154–156. [Google Scholar] [CrossRef]
- Kotlar, B.; Gerson, E.; Petrillo, S.; Langer, A.; Tiemeier, H. The impact of the COVID-19 pandemic on maternal and perinatal health: A scoping review. Reprod. Health 2021, 18, 1–39. [Google Scholar] [CrossRef]
- Brasil. Ministério da Saúde. Guia de Vigilância Epidemiológica Emergência de Saúde Pública de Importância Nacional pela Doença pelo Coronavírus 2019. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/guias-e-manuais/2021/guia-de-vigilancia-epidemiologica-covid-19-3.pdf/view (accessed on 15 March 2022).
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID multinational cohort study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Godoi, A.P.N.; Bernardes, G.C.S.; Almeida, N.A.d.; Melo, S.N.d.; Belo, V.S.; Nogueira, L.S.; Pinheiro, M.d.B. Severe Acute Respiratory Syndrome by COVID-19 in pregnant and postpartum women. Rev. Bras. Saúde Matern. Infant. 2021, 21, 461–469. [Google Scholar] [CrossRef]
- Francisco, R.P.V.; Lacerda, L.; Rodrigues, A.S. Obstetric Observatory BRAZIL-COVID-19: 1031 maternal deaths because of COVID-19 and the unequal access to health care services. Clinics 2021, 76, e3120. [Google Scholar] [CrossRef]
- Diniz, D.; Brito, L.; Rondon, G. Maternal mortality and the lack of women-centered care in Brazil during COVID-19: Preliminary findings of a qualitative study. Lancet Reg. Health —Am. 2022, 10, 100239. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; Volume 112. [Google Scholar]
- Li, Z.; Wang, L.; Huang, L.s.; Zhang, M.; Cai, X.; Xu, F.; Wu, F.; Li, H.; Huang, W.; Zhou, Q.; et al. Efficient management strategy of COVID-19 patients based on cluster analysis and clinical decision tree classification. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- San-Cristobal, R.; Martín-Hernández, R.; Ramos-Lopez, O.; Martinez-Urbistondo, D.; Micó, V.; Colmenarejo, G.; Villares Fernandez, P.; Daimiel, L.; Martínez, J.A. Longwise Cluster Analysis for the Prediction of COVID-19 Severity within 72 h of Admission: COVID-DATA-SAVE-LIFES Cohort. J. Clin. Med. 2022, 11, 3327. [Google Scholar] [CrossRef]
- Cui, W.; Cabrera, M.; Finkelstein, J. Latent COVID-19 clusters in patients with chronic respiratory conditions. Integr. Citiz. Centered Digit. Health Soc. Care 2020, 275, 32–36. [Google Scholar] [CrossRef]
- Raposo, L.M.; Abreu, G.F.D.; de Medeiros Cardoso, F.B.; Alves, A.T.J.; Rosa, P.T.C.R.; Nobre, F.F. Symptom-based clusters of hospitalized patients with severe acute respiratory illness by SARS-CoV-2 in Brazil. J. Infect. Public Health 2022, 15, 621–627. [Google Scholar] [CrossRef]
- Kenny, G.; McCann, K.; O’Brien, C.; Savinelli, S.; Tinago, W.; Yousif, O.; Lambert, J.S.; O’Broin, C.; Feeney, E.R.; De Barra, E.; et al. Identification of distinct long COVID clinical phenotypes through cluster analysis of self-reported symptoms. Open Forum Infect Dis. 2022, 9, ofac060. [Google Scholar] [CrossRef]
- Benham, J.L.; Atabati, O.; Oxoby, R.J.; Mourali, M.; Shaffer, B.; Sheikh, H.; Boucher, J.C.; Constantinescu, C.; Leigh, J.P.; Ivers, N.M.; et al. COVID-19 vaccine–related attitudes and beliefs in Canada: National cross-sectional survey and cluster analysis. JMIR Public Health Surveill. 2021, 7, e30424. [Google Scholar] [CrossRef]
- Husson, F.; Lê, S.; Pagès, J. Exploratory Multivariate Analysis by Example using R; CRC Press: Boca Raton, FL, USA, 2011; Volume 15. [Google Scholar]
- Greenacre, M.J. Theory and Applications of Correspondence Analysis; Academic Press: London, UK, 1984. [Google Scholar]
- Backhaus, K.; Erichson, B.; Gensler, S.; Weiber, R.; Weiber, T. Multivariate Analysis: An Application-Oriented Introduction; Springer: New York, NY, USA, 2021. [Google Scholar]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis; Prentice Hall: Hoboken, NJ, USA, 1992; Volume 405. [Google Scholar]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 5–38. [Google Scholar] [CrossRef] [PubMed]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.d.L.B.; Costa Ferreira Júnior, O.d.; João, E.; Fuller, T.; Silva Esteves, J.; Mendes-Silva, W.; Carvalho Mocarzel, C.; Araújo Maia, R.; Theodoro Boullosa, L.; Gonçalves, C.C.A.; et al. Maternal and neonatal outcomes of SARS-CoV-2 infection in a cohort of pregnant women with comorbid disorders. Viruses 2021, 13, 1277. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, S.; Ashtari, S.; Vahedian-Azimi, A. Manifestations of COVID-19 in pregnant women with focus on gastrointestinal symptoms: A systematic review. Gastroenterol. Hepatol. Bed Bench 2020, 13, 305. [Google Scholar] [PubMed]
- Mutiawati, E.; Fahriani, M.; Mamada, S.S.; Fajar, J.K.; Frediansyah, A.; Maliga, H.A.; Ilmawan, M.; Emran, T.B.; Ophinni, Y.; Ichsan, I.; et al. Anosmia and dysgeusia in SARS-CoV-2 infection: Incidence and effects on COVID-19 severity and mortality, and the possible pathobiology mechanisms-a systematic review and meta-analysis. F1000Research 2021, 10, 40. [Google Scholar] [CrossRef]
- Takemoto, M.L.; Menezes, M.d.O.; Andreucci, C.B.; Knobel, R.; Sousa, L.; Katz, L.; Fonseca, E.B.; Nakamura-Pereira, M.; Magalhães, C.G.; Diniz, C.S.; et al. Clinical characteristics and risk factors for mortality in obstetric patients with severe COVID-19 in Brazil: A surveillance database analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 1618–1626. [Google Scholar] [CrossRef]
- Menezes, M.O.; Takemoto, M.L.; Nakamura-Pereira, M.; Katz, L.; Amorim, M.M.; Salgado, H.O.; Melo, A.; Diniz, C.S.; de Sousa, L.A.; Magalhaes, C.G.; et al. Risk factors for adverse outcomes among pregnant and postpartum women with acute respiratory distress syndrome due to COVID-19 in Brazil. Int. J. Gynecol. Obstet. 2020, 151, 415–423. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Ali, A.; Das, J.K.; Salam, R.A.; Padhani, Z.A.; Irfan, O.; Bhutta, Z.A. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: Clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J. Glob. Health 2021, 11, 05018. [Google Scholar] [CrossRef]
- Overtoom, E.M.; Rosman, A.N.; Zwart, J.J.; Vogelvang, T.E.; Schaap, T.P.; van den Akker, T.; Bloemenkamp, K.W. SARS-CoV-2 infection in pregnancy during the first wave of COVID-19 in the Netherlands: A prospective nationwide population-based cohort study (NethOSS). BJOG Int. J. Obstet. Gynaecol. 2022, 129, 91–100. [Google Scholar] [CrossRef]
- La Verde, M.; Riemma, G.; Torella, M.; Cianci, S.; Savoia, F.; Licciardi, F.; Scida, S.; Morlando, M.; Colacurci, N.; De Franciscis, P. Maternal death related to COVID-19: A systematic review and meta-analysis focused on maternal co-morbidities and clinical characteristics. Int. J. Gynecol. Obstet. 2021, 154, 212–219. [Google Scholar] [CrossRef]
- Brislane, Á.; Larkin, F.; Jones, H.; Davenport, M.H. Access to and quality of healthcare for pregnant and postpartum women during the COVID-19 pandemic. Front. Glob. Women’S Health 2021, 2, 628625. [Google Scholar] [CrossRef] [PubMed]
- Wastnedge, E.A.; Reynolds, R.M.; Van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Scheler, C.A.; Discacciati, M.G.; Vale, D.B.; Lajos, G.J.; Surita, F.; Teixeira, J.C. Mortality in pregnancy and the postpartum period in women with severe acute respiratory distress syndrome related to COVID-19 in Brazil, 2020. Int. J. Gynecol. Obstet. 2021, 155, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
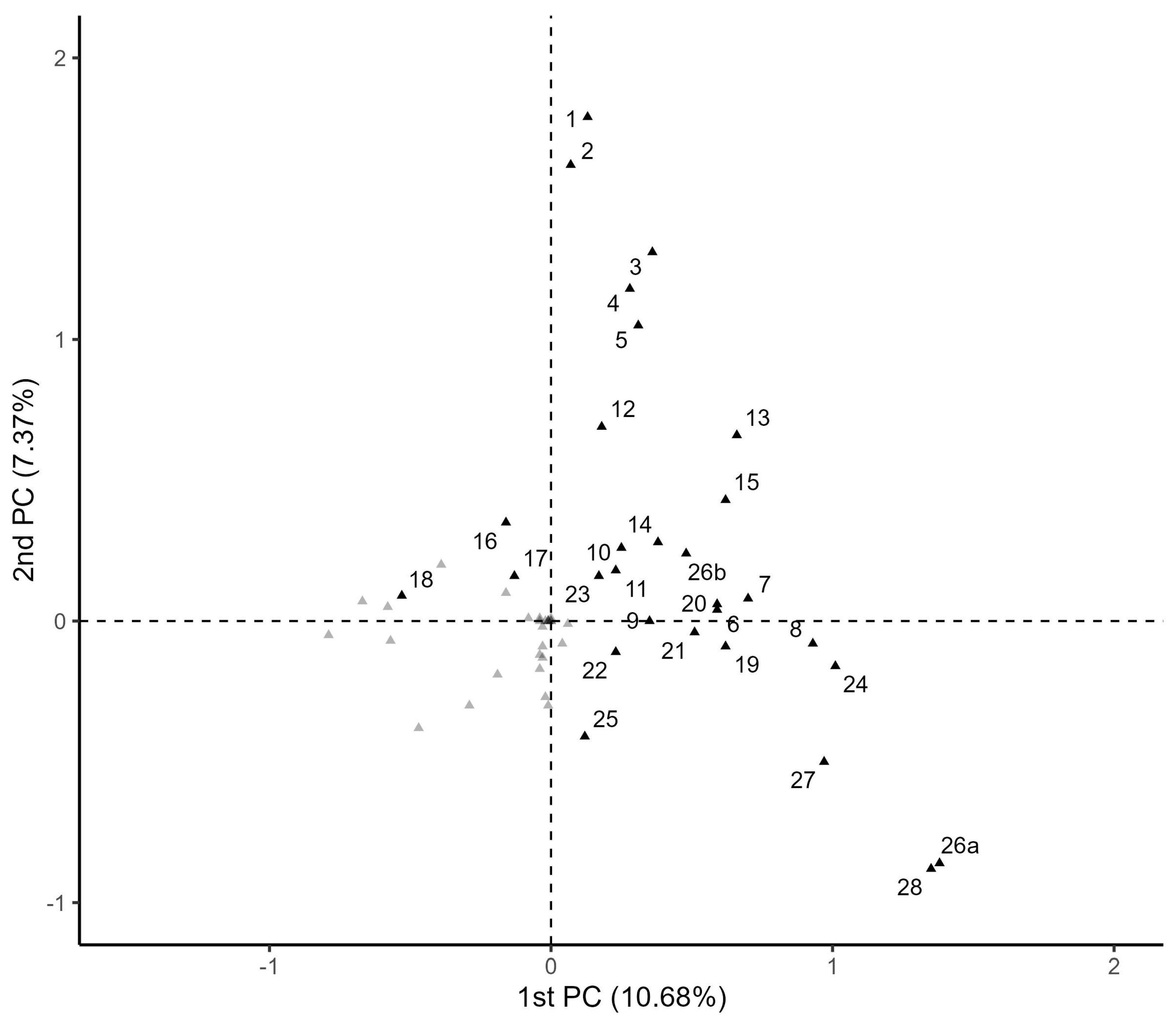
| Principal Components | Eigenvalue | Percentage of Inertia | Cumulative Percentage of Inertia |
|---|---|---|---|
| 1 | 0.110 | 10.681 | 10.681 |
| 2 | 0.076 | 7.368 | 18.049 |
| 3 | 0.054 | 5.259 | 23.309 |
| 4 | 0.048 | 4.675 | 27.984 |
| 5 | 0.044 | 4.326 | 32.310 |
| 6 | 0.044 | 4.265 | 36.575 |
| 7 | 0.040 | 3.950 | 40.526 |
| 8 | 0.037 | 3.604 | 44.130 |
| 9 | 0.036 | 3.545 | 47.675 |
| 10 | 0.036 | 3.499 | 51.175 |
| Variables (Category = Yes) | Cluster 1 (n = 7866) | Cluster 2 (n = 2216) | Cluster 3 (n = 6327) | Total (n = 16,409) |
|---|---|---|---|---|
| Hospital characteristics | ||||
| Death | 1.58 | 5.82 | 23.52 | 10.61 |
| ICU admission | 7.70 | 19.58 | 57.26 | 28.42 |
| Invasive MV | 1.27 | 7.99 | 31.25 | 13.74 |
| Non-invasive MV | 15.34 | 39.76 | 54.39 | 33.69 |
| Lower respiratory symptoms | ||||
| Oxygen saturation less than | 6.79 | 38.63 | 77.64 | 38.41 |
| Respiratory distress | 19.48 | 50.81 | 74.35 | 44.87 |
| Dyspnea | 29.21 | 63.09 | 89.98 | 57.22 |
| Fatigue | 9.71 | 43.23 | 30.47 | 22.24 |
| Gastrointestinal symptoms | ||||
| Abdominal pain | 4.73 | 16.43 | 5.83 | 6.73 |
| Vomiting | 8.56 | 19.04 | 9.80 | 10.45 |
| Diarrhea | 7.56 | 20.49 | 8.60 | 9.71 |
| Inappetence | 0.57 | 1.22 | 1.83 | 1.15 |
| Nasopharyngeal symptoms | 1.88 | 87.68 | 1.15 | 13.19 |
| Anosmia | 4.64 | 92.87 | 2.61 | 15.77 |
| Sore throat | 18.04 | 32.76 | 18.60 | 20.24 |
| Flu-like symptoms | ||||
| Nasal congestion | 12.97 | 10.29 | 4.90 | 9.49 |
| Malaise | 2.03 | 1.99 | 1.50 | 1.82 |
| Headache | 19.62 | 25.36 | 13.58 | 18.06 |
| Chest pain | 1.98 | 2.75 | 2.72 | 2.37 |
| Cough | 57.14 | 76.13 | 77.46 | 67.54 |
| Fever | 45.03 | 63.40 | 60.90 | 53.63 |
| Myalgia | 4.64 | 7.76 | 8.38 | 6.50 |
| Comorbidities | ||||
| Obesity | 2.36 | 8.62 | 12.15 | 6.98 |
| Chronic heart disease | 3.83 | 7.13 | 8.98 | 6.26 |
| Asthma | 2.29 | 3.88 | 4.87 | 3.50 |
| Diabetes mellitus | 5.35 | 8.75 | 10.61 | 7.84 |
| Systemic arterial hypertension | 3.13 | 4.20 | 4.08 | 3.64 |
| Immunodeficiency or immunodepression | 1.30 | 1.49 | 1.58 | 1.43 |
| Eclampsia | 1.33 | 0.81 | 1.61 | 1.37 |
| Demographic and obstetric characteristics | ||||
| Age 10–17 | 5.29 | 2.48 | 2.09 | 3.67 |
| Age 18–34 | 71.26 | 69.86 | 64.36 | 68.41 |
| Age 35–42 | 0.21 | 24.68 | 29.84 | 25.06 |
| Age 43–49 | 0.02 | 2.98 | 3.71 | 2.86 |
| 1st trimester | 7.08 | 7.36 | 5.88 | 6.65 |
| 2nd trimester | 15.68 | 23.19 | 24.17 | 19.96 |
| 3rd trimester | 49.28 | 49.46 | 39.24 | 45.43 |
| Puerperium | 23.56 | 17.24 | 27.88 | 24.37 |
| White/Asian | 31.30 | 39.53 | 38.64 | 35.24 |
| Indigenous/Pardo/Black or NA data | 68.70 | 60.47 | 61.36 | 64.76 |
| Variables (Category = yes) | Cluster 2 RR (CI95%) | Cluster 3 RR (CI95%) |
|---|---|---|
| Hospital characteristics | ||
| Death | 3.69 (2.90; 4.70) | 14.92 (12.46; 17.86) |
| ICU admission | 2.54 (2.27; 2.85) | 7.43 (6.87; 8.05) |
| Invasive/Non-invasive Mechanical Ventilation * | 2.87 (2.69; 3.07) | 5.15 (4.90; 5.42) |
| Lower respiratory symptoms | ||
| Oxygen saturation less than | 5.69 (5.16; 6.27) | 11.44 (10.53; 12.43) |
| Respiratory distress | 2.61 (2.46; 2.77) | 3.82 (3.64; 4.00) |
| Dyspnea | 2.16 (2.06; 2.26) | 3.08 (2.97; 3.19) |
| Fatigue | 4.45 (4.10; 4.83) | 3.14 (2.90; 3.39) |
| Gastrointestinal symptoms | ||
| Abdominal pain | 3.47 (3.03; 3.98) | 1.23 (1.07; 1.42) |
| Vomiting | 2.23 (1.99; 2.49) | 1.15 (1.03; 1.27) |
| Diarrhea | 2.71 (2.42; 3.03) | 1.14 (1.02; 1.27) |
| Inappetence | 2.13 (1.32; 3.42) | 3.20 (2.28; 4.51) |
| Nasopharyngeal symptoms | ||
| Ageusia | 46.6 (39.70; 54.71) | 0.61 (0.46; 0.81) |
| Anosmia | 20.01 (18.09; 22.14) | 0.56 (0.47; 0.67) |
| Sore throat | 1.82 (1.68; 1.96) | 1.03 (0.96; 1.11) |
| Flu-like symptoms | ||
| Nasal congestion | 0.79 (0.69; 0.91) | 0.38 (0.33; 0.43) |
| Malaise | 0.98 (0.70; 1.36) | 0.74 (0.57; 0.95) |
| Headache | 1.29 (1.19; 1.41) | 0.69 (0.64; 0.75) |
| Chest pain | 1.39 (1.04; 1.86) | 1.37 (1.11; 1.70) |
| Cough | 1.33 (1.29; 1.37) | 1.36 (1.32; 1.39) |
| Fever | 1.41 (1.35; 1.47) | 1.35 (1.31; 1.40) |
| Myalgia | 1.67 (1.40; 1.99) | 1.81 (1.59; 2.05) |
| Comorbidities | ||
| Obesity | 3.65 (3.00; 4.44) | 5.14 (4.39; 6.01) |
| Chronic heart disease | 1.86 (1.55; 2.25) | 2.35 (2.05; 2.69) |
| Asthma | 1.70 (1.32; 2.18) | 2.13 (1.78; 2.55) |
| Diabetes mellitus | 1.64 (1.39; 1.93) | 1.98 (1.76; 2.23) |
| Systemic arterial hypertension | 1.34 (1.06; 1.70) | 1.30 (1.10; 1.55) |
| Immunodeficiency or immunodepression | 1.15 (0.78; 1.70) | 1.22 (0.93; 1.60) |
| Eclampsia | 0.61 (0.37; 1.00) | 1.21 (0.92; 1.58) |
| Demographic and obstetric characteristics | ||
| Age greater than or equal to 35 * | 1.18 (1.09; 1.28) | 1.43 (1.36; 1.51) |
| Puerperium * | 0.72 (0.65; 0.79) | 1.16 (1.10; 1.23) |
| Indigenous/Pardo/Black or NA data | 0.88 (0.85; 0.91) | 0.89 (0.87; 0.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, I.C.R.; Feronato, S.G.; Silveira, G.F.; Chiavegatto Filho, A.D.P.; Santos, H.G.d. Clusters of Pregnant Women with Severe Acute Respiratory Syndrome Due to COVID-19: An Unsupervised Learning Approach. Int. J. Environ. Res. Public Health 2022, 19, 13522. https://doi.org/10.3390/ijerph192013522
Carneiro ICR, Feronato SG, Silveira GF, Chiavegatto Filho ADP, Santos HGd. Clusters of Pregnant Women with Severe Acute Respiratory Syndrome Due to COVID-19: An Unsupervised Learning Approach. International Journal of Environmental Research and Public Health. 2022; 19(20):13522. https://doi.org/10.3390/ijerph192013522
Chicago/Turabian StyleCarneiro, Isadora Celine Rodrigues, Sofia Galvão Feronato, Guilherme Ferreira Silveira, Alexandre Dias Porto Chiavegatto Filho, and Hellen Geremias dos Santos. 2022. "Clusters of Pregnant Women with Severe Acute Respiratory Syndrome Due to COVID-19: An Unsupervised Learning Approach" International Journal of Environmental Research and Public Health 19, no. 20: 13522. https://doi.org/10.3390/ijerph192013522
APA StyleCarneiro, I. C. R., Feronato, S. G., Silveira, G. F., Chiavegatto Filho, A. D. P., & Santos, H. G. d. (2022). Clusters of Pregnant Women with Severe Acute Respiratory Syndrome Due to COVID-19: An Unsupervised Learning Approach. International Journal of Environmental Research and Public Health, 19(20), 13522. https://doi.org/10.3390/ijerph192013522






