Application of Rice Husk Biochar and Earthworm on Concentration and Speciation of Heavy Metals in Industrial Sludge Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Substrate and Earthworm
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
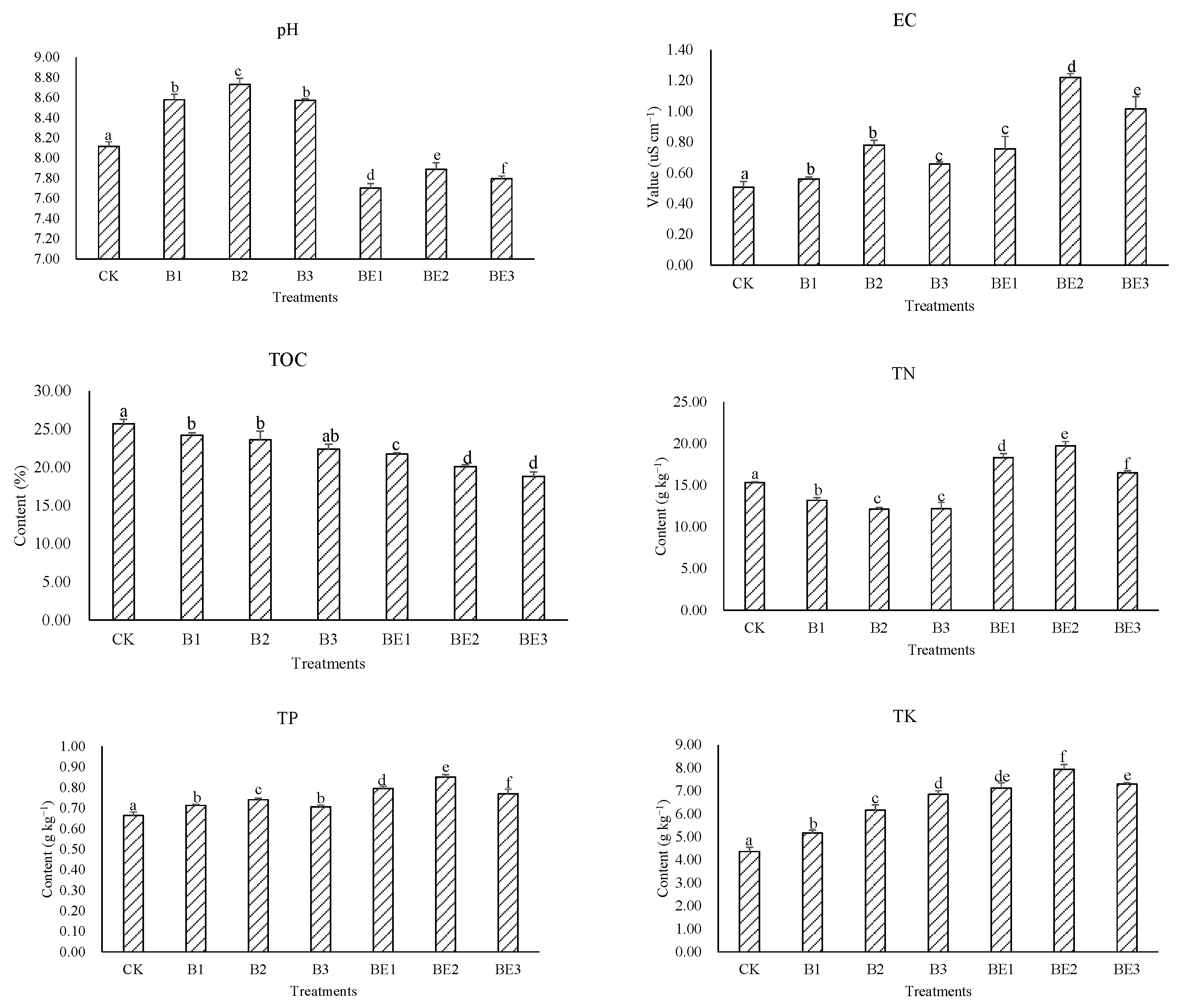
3.1. Physicochemical Properties of Industrial Sludge
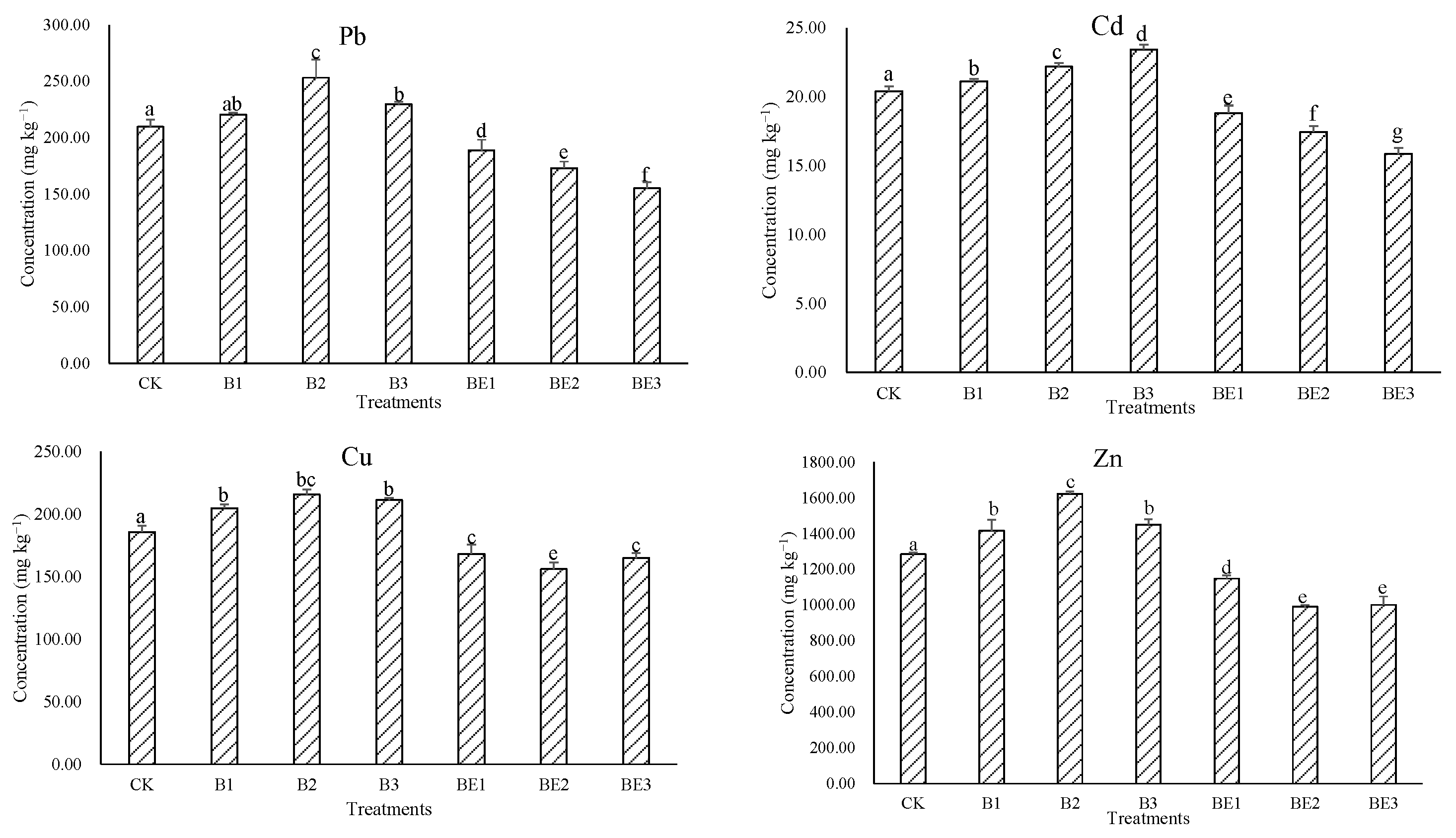
3.2. Heavy Metal Contents in Industrial Sludge
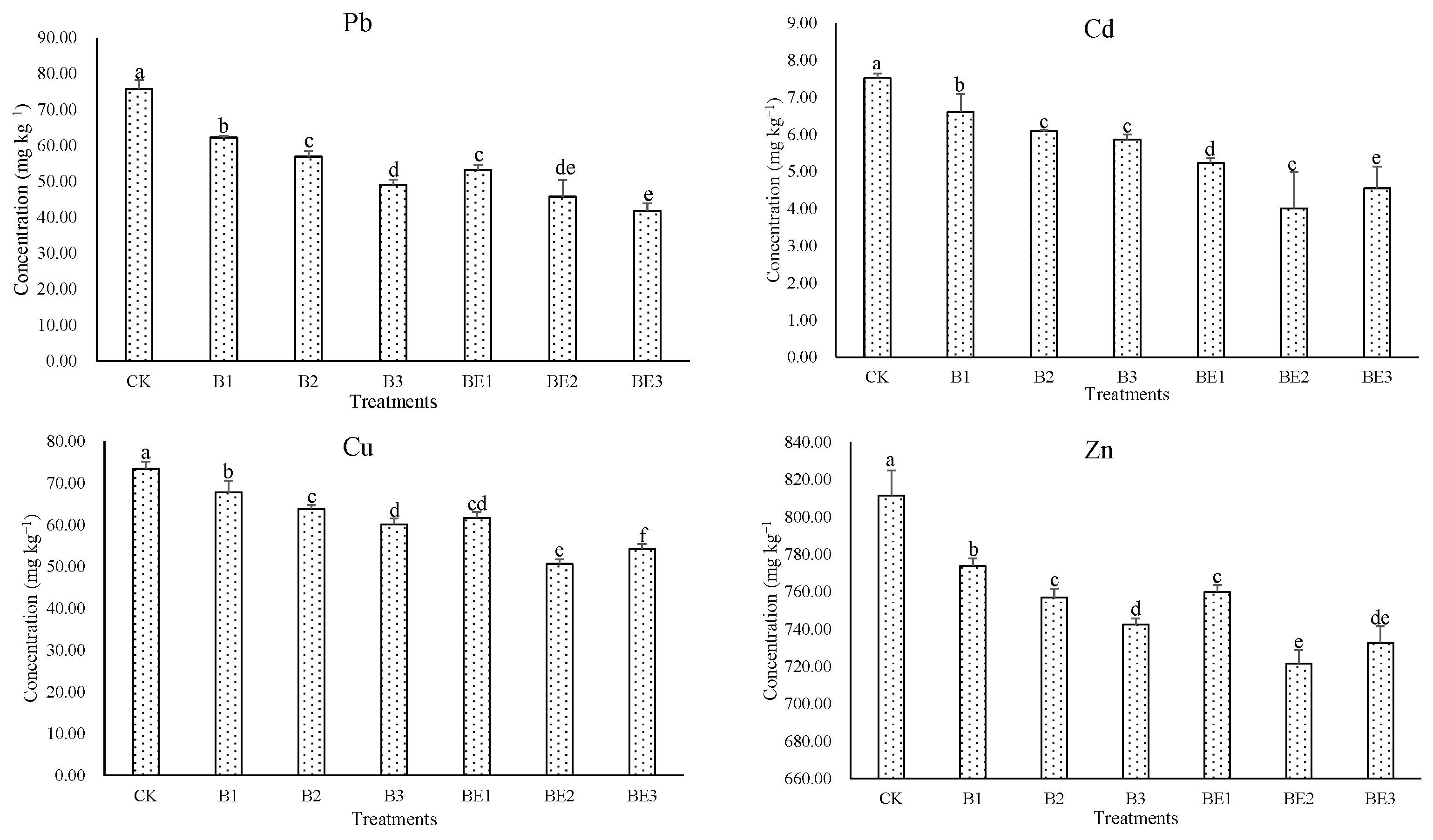
3.3. Bioavailability of Heavy Metals in Industrial Sludge
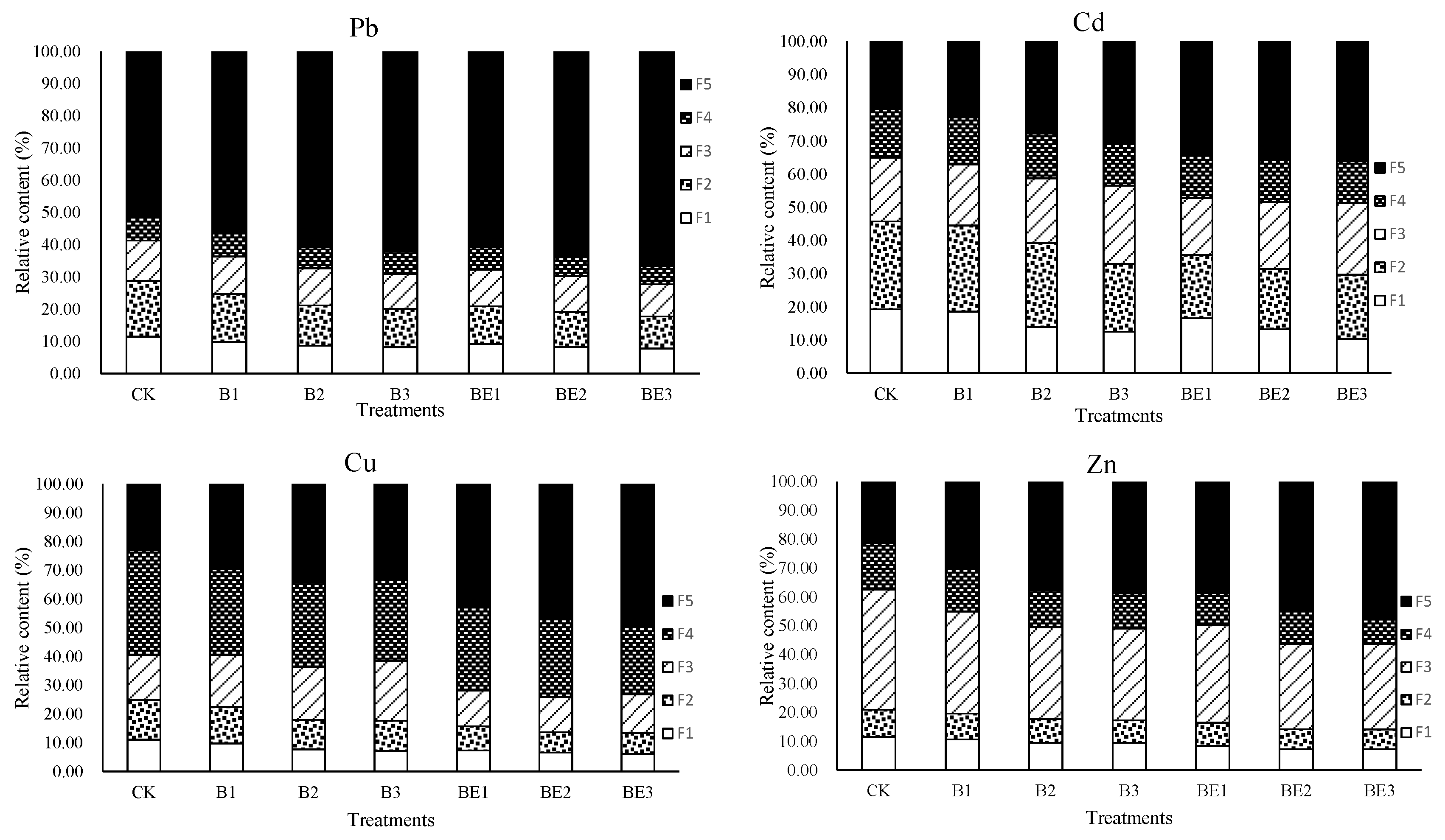
3.4. Heavy Metal Speciation in Industrial Sludge
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agyarko-Mintah, E.; Cowie, A.; Zwieten, L.V.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.V.; Golder, A.K.; Ghosh, P.K. Production of composite clay bricks: A value-added solution to hazardous sludge through effective heavy metal fixation. Constr. Build. Mater. 2019, 201, 391–400. [Google Scholar] [CrossRef]
- Lee, L.H.; Wu, T.Y.; Shak, K.P.Y.; Lim, S.L.; Ng, K.Y.; Nguyen, M.N.; Teoh, W.H. Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting: A mini-review: Sustainable approach to biotransform industrial sludge into organic fertilizer via vermicomposting. J. Chem. Technol. Biotechnol. 2018, 93, 925–935. [Google Scholar] [CrossRef]
- Dai, Q.X.; Ma, L.P.; Ren, N.Q.; Ning, P.; Guo, Z.Y.; Xie, L.G. Research on the variations of organics and heavy metals in municipal sludge with additive acetic acid and modified phosphogypsum. Water Res. 2019, 155, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bhattacharyya, P.; Ghosh, B.C.; Banik, P. Effect of vermicomposting on calcium, sulphur and some heavy metal content of different biodegradable organic wastes under liming and microbial inoculation. J. Environ. Sci. Health B 2012, 47, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhou, Q.; Ma, L.Q. Availability and assessment of fixing additives for the in situ remediation of heavy metal contaminated soils: A review. Environ. Monit. Assess. 2006, 116, 513–528. [Google Scholar] [CrossRef]
- He, X.; Zhang, Y.X.; Shen, M.C.; Zeng, G.M.; Zhou, M.C.; Li, M.R. Effect of vermicomposting on concentration and speciation of heavy metals in sewage sludge with additive materials. Bioresour. Technol. 2016, 218, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Cai, L.; Li, S.; Chang, S.X.; Sun, X.; An, Z. Bamboo biochar amendment improves the growth and reproduction of Eisenia fetida and the quality of green waste vermicompost. Ecotoxicol. Environ. Saf. 2018, 156, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Lu, H.H.; Da, D.; Hui, P.; Strong, P.J.; Wang, H.L.; Wu, W.H. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef]
- Malińska, K.; Golańska, M.; Caceres, R.; Rorat, A.; Weisser, P.; Ślęzak, E. Biochar amendment for integrated composting and vermicomposting of sewage sludge—The effect of biochar on the activity of Eisenia fetida and the obtained vermicompost. Bioresour. Technol. 2017, 225, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.Y.; Xing, M.Y.; Yang, J. Speciation and transformation of heavy metals during vermicomposting of animal manure. Bioresour. Technol. 2016, 209, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Goswami, L.; Sarkar, S.; Mukherjee, S.; Das, S.; Barman, S.; Raul, P.; Bhattacharyya, P.; Mandal, N.C.; Bhattacharya, S.; Bhattacharya, S.S. Vermicomposting of Tea Factory Coal Ash: Metal accumulation and metallothionein response in Eisenia fetida (Savigny) and Lampito mauritii (Kinberg). Bioresour. Technol. 2014, 166, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Varma, S.; Kalamdhad, A.S. Effects of various C/N ratios during vermicomposting of sewage sludge using Eisenia fetida. Environ. Sci. Technol. 2013, 6, 63–78. [Google Scholar] [CrossRef][Green Version]
- Ndegwa, P.M.; Thompson, S.A.; Das, K.C. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12. [Google Scholar] [CrossRef]
- Malińska, K.; Zabochnicka-Świątek, M.; Cáceres, R.; Marfà, O. The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol. Eng. 2016, 90, 35–41. [Google Scholar] [CrossRef]
- Vig, A.P.A.; Singh, J.; Wani, S.H.; Dhaliwal, S.S. Vermicomposting of tannery sludge mixed with cattle dung into valuable manure using earthworm Eisenia fetida (Savigny). Bioresour. Technol. 2011, 102, 7941–7945. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Agronomy, No. 9; Soil Science Society of America: Madison, WI, USA, 1982; p. 1159. [Google Scholar] [CrossRef]
- Fernández-Gómez, M.J.; Quirantes, M.; Vivas, A.; Nogales, R. Vermicomposts and/or Arbuscular Mycorrhizal Fungal Inoculation in Relation to Metal Availability and Biochemical Quality of a Soil Contaminated with Heavy Metals. Water Air Soil Pollut. 2012, 223, 2707–2718. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. J. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Pramanik, P.; Ghosh, G.K.; Ghosal, P.K.; Banik, P. Changes in organic—C, N, P and K and enzyme activities in vermicompost of biodegradable organic wastes under liming and microbial inoculants. Bioresour. Technol. 2007, 98, 2485–2494. [Google Scholar] [CrossRef]
- Wu, S.H.; He, H.J.; Inthapanya, X.; Yang, C.P.; Lu, L.; Zeng, G.M.; Han, Z.F. Role of biochar on composting of organic wastes and remediation of contaminated soils—A review. Environ. Sci. Pollut. Res. 2017, 24, 16560–16577. [Google Scholar] [CrossRef]
- Wang, Q.; Li, R.H.; Cai, H.Z.; Awasthi, M.K.; Zhang, Z.Q.; Wang, J.J.; Ali, A.; Amanullah, M. Improving pig manure composting efficiency employing Ca-bentonite. Ecol. Eng. 2016, 87, 157–161. [Google Scholar] [CrossRef]
- Brinza, L.; Schofield, P.F.; Mosselmans, J.F.W.; Donner, E.; Lombi, E.; Paterson, D.; Hodson, M.E. Can earthworm-secreted calcium carbonate immobilise Zn in contaminated soils? Soil Biol. Biochem. 2014, 74, 1–10. [Google Scholar] [CrossRef]
- Paul, S.; Kauser, H.; Jain, M.S.; Khwairakpam, M.; Kalamdhad, A.S. Biogenic stabilization and heavy metal immobilization during vermicomposting of vegetable waste with biochar amendment. J. Hazard. Mater. 2020, 390, 121366. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.B.; Cui, X.; Jilani, G.; Lazzat, U.; Zehra, A.; Hamid, Y.; Hussain, B.; Tang, L.; Yang, X.; He, Z. Eisenia fetida and biochar synergistically alleviate the heavy metals content during valorization of biosolids via enhancing vermicompost quality. Sci. Total Environ. 2019, 684, 597–609. [Google Scholar] [CrossRef]
- Hait, S.; Tare, V. Optimizing vermistabilization of waste activated sludge using vermicompost as bulking material. Waste Manag. 2011, 31, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Bhardwaj, P. Comparative studies on biomass production, life cycles and composting efficiency of Eisenia fetida (Savigny) and Lampito mauritii (Kinberg). Bioresour. Technol. 2004, 92, 275–283. [Google Scholar] [CrossRef]
- Surindra, S. Pilot-scale vermireactors for sewage sludge stabilization and metal remediation process: Comparison with small-scale vermireactors. Ecol. Eng. 2009, 36, 703–712. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sugir, M.E.; Li, Y.Y.; Yuan, L.; Zhou, M.; Lv, P.; Yu, Z.M.; Wang, L.M.; Zhou, D.X. Effects of vermicomposting on the main chemical properties and bioavailability of Cd/Zn in pure sludge. Environ. Sci. Pollut. Res. 2019, 26, 20949–20960. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.S.; Jambhulkar, R.; Kalamdhad, A.S. Biochar amendment for batch composting of nitrogen rich organic waste: Effect on degradation kinetics, composting physics and nutritional properties. Bioresour. Technol. 2018, 253, 204–213. [Google Scholar] [CrossRef]
- Renuka, G.K.G. Stabilization of primary sewage sludge during vermicomposting. J. Hazard. Mater. 2008, 153, 1023–1030. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Pratap, O.; Sik, Y.; Cao, X.D. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Blanka, M.; Jan, K.; Markéta, S.; Jakub, H. Effects of combined composting and vermicomposting of waste sludge on arsenic fate and bioavailability. J. Hazard. Mater. 2014, 280, 544–551. [Google Scholar] [CrossRef]
- Brinton, W.F. Compost Quality Standards and Guidelines; Final Report by Woods End Research Laboratories for the New York State Association of Recyclers; Woods End Research Laboratory, Inc.: New York, NY, USA, 2000. [Google Scholar]
- BSI PAS 100:2011; Specification for Composted Materials. British Standards Institution: London, UK, 2011.
- GB 4284-2018; Agricultural Sludge Pollutant Control Standard. Ecology Environment & Conservation. NSCO: Beijing, China, 2017.
- Zhang, J.; Lv, B.; Xing, M.Y.; Yang, J. Tracking the composition and transformation of humic and fulvic acids during vermicomposting of sewage sludge by elemental analysis and fluorescence excitation–emission matrix. Waste Manag. 2015, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Wang, Q.; Huang, H.; Li, R.; Shen, F.; Lahori, A.H.; Wang, P.; Guo, D.; Guo, Z.; Jiang, S.; et al. Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J. Clean. Prod. 2016, 135, 829–835. [Google Scholar] [CrossRef]
- Kizilkaya, R. Cu and Zn accumulation in earthworm Lumbricus terrestris L. in sewage sludge amended soil and fractions of Cu and Zn in casts and surrounding soil. Ecol. Eng. 2004, 22, 141–151. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.D.; Zhou, D.M.; Wang, Y.J.; Chen, W.F. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.S. Speciation and phytoavailability of lead and antimony in a small arms range soil amended with mussel shell, cow bone and biochar: EXAFS spectroscopy and chemical extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Wang, Q.; Zhang, Z.Q.; Zhang, G.J.; Li, Z.H.; Wang, L.; Zheng, J.Z. Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environ. Technol. 2015, 36, 815–826. [Google Scholar] [CrossRef]
- Hua, L.; Wu, W.X.; Liu, Y.X.; McBride, M.B.; Chen, Y.X. Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ. Sci. Pollut. Res. 2009, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sizmur, T.; Hodson, M.E. Do earthworms impact metal mobility and availability in soil?—A review. Environ. Pollut. 2009, 157, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Sizmur, T.; Watts, M.J.; Brown, G.D.; Palumbo-Roe, B.; Hodson, M.E. Impact of gut passage and mucus secretion by the earthworm Lumbricus terrestris on mobility and speciation of arsenic in contaminated soil. J. Hazard. Mater. 2011, 197, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Narwal, R.P.; Singh, B.R.; Salbu, B. Association of cadmium, zinc, copper, and nickel with components in naturally heavy metal-rich soils studied by parallel and sequential extractions. Commun. Soil Sci. Plant Anal. 1999, 30, 1209–1230. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, R.K.; Jiang, T.Y.; Li, Z. Immobilization of Cu (II), Pb (II) and Cd (II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater. 2012, 229, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; Rahimi, G.; Kolahchi, Z.; Nezhad, A.K.J. Using industrial sewage sludge-derived biochar to immobilize selected heavy metals in a contaminated calcareous soil. Waste Biomass Valorization 2020, 11, 2825–2836. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439. [Google Scholar] [CrossRef]
- Jin, H.P.; Choppala, G.; Bolan, N.; Chung, J.W.; Chuasavathi, T. Biochar and black carbon reduce the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar]
- Zhang, J.N.; Lü, F.; Shao, L.M.; He, P.J. The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 2014, 168, 252–258. [Google Scholar] [CrossRef]
- Liu, X.L.; Hu, C.X.; Zhang, S.Z. Effects of earthworm activity on fertility and heavy metal bioavailability in sewage sludge. Environ. Int. 2005, 31, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.J.; Morris, B. The accumulation and intracellular compartmentation of cadmium, lead, zinc and calcium in two earthworm species (Dendrobaena rubida and Lumbricus rubellus) living in highly contaminated soil. Histochemistry 1982, 75, 269–285. [Google Scholar] [CrossRef]
- Arnold, R.E.; Hodson, M.E.; Comber, S. Does speciation impact on Cu uptake by, and toxicity to, the earthworm Eisenia fetida? Eur. J. Soil Biol. 2007, 43, 230–232. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Cheng, J.M.; Wong, M.H. Earthworm–mycorrhiza interaction on Cd uptake and growth of ryegrass. Earthworm–mycorrhiza interaction on Cd uptake and growth of ryegrass. Soil Biol. Biochem. 2004, 37, 195–201. [Google Scholar] [CrossRef]
| Sample | Composition | ||
|---|---|---|---|
| Industrial Sewage Sludge (g) | Rice Hull Biochar (g) | Earthworm (num) | |
| CK | 200 | 0 | 0 |
| B1 | 196 | 4 | 0 |
| B2 | 192 | 8 | 0 |
| B3 | 184 | 16 | 0 |
| BE1 | 196 | 4 | 15 |
| BE2 | 192 | 8 | 15 |
| BE3 | 184 | 16 | 15 |
| Parameters | Industrial Sewage Sludge | Rice Hull Biochar |
|---|---|---|
| pH | 7.7 ± 0.01 a | 9.51 ± 0.09 |
| Moisture content (%) | 78.24 ± 0.71 | 7.82 ± 0.28 |
| EC (ms cm−1) | 0.45 ± 0.01 | 0.32 ± 0.01 |
| TOC (%) | 28.76 ± 0.70 | 38.39 ± 0.60 |
| TN (g kg−1) | 19.10 ± 0.49 | 6.17 ± 0.46 |
| TP (g kg−1) | 0.75 ± 0.04 | 0.07 ± 0.01 |
| TK (g kg−1) | 4.85 ± 0.41 | 6.79 ± 0.5 |
| Pbtotal (mg kg−1) | 189.73 ± 1.08 | 0.25 ± 0.01 |
| Cdtotal (mg kg−1) | 19.83 ± 0.47 | 0.02 ± 0.01 |
| Cutotal (mg kg−1) | 164.05 ± 0.77 | 0.17 ± 0.02 |
| Zntotal (mg kg−1) | 1242.56 ± 3.87 | 0.15 ± 0.02 |
| Item | Bioavailable Metal | pH | EC | TOC | TN | TP | TK |
|---|---|---|---|---|---|---|---|
| Compost | DTPA-extractable Pb | 0.113 | −0.32 | 0.856 ** | 0.615 | 0.254 | −0.961 ** |
| DTPA-extractable Cd | −0.223 | −0.647 | 0.725 * | 0.757 * | −0.09 | −0.972 ** | |
| DTPA-extractable Cu | 0.023 | −0.443 | 0.685 * | 0.644 | 0.097 | −0.871 ** | |
| DTPA-extractable Zn | −0.032 | −0.433 | 0.725 * | 0.724 * | 0.15 | −0.917 ** | |
| Vermicompost | DTPA-extractable Pb | −0.672 * | −0.611 | 0.872 ** | 0.288 | 0.071 | −0.331 |
| DTPA-extractable Cd | −0.704 * | −0.833 ** | 0.692 * | −0.139 | −0.304 | −0.679 * | |
| DTPA-extractable Cu | −0.849 ** | −0.914 ** | 0.694 * | −0.23 | −0.458 | −0.818 ** | |
| DTPA-extractable Zn | −0.876 ** | −0.958 ** | 0.729 * | −0.258 | −0.498 | −0.765 * |
| Treatments | Mobility Factors | |||
|---|---|---|---|---|
| Pb | Cd | Cu | Zn | |
| CK | 28.72 ± 1.43 a† | 45.75 ± 2.28 a†† | 24.80 ± 1.23 a | 20.94 ± 1.04 a |
| B1 | 24.68 ± 1.23 b | 44.52 ± 2.21 a | 22.41 ± 1.11 b | 19.64 ± 0.98 a |
| B2 | 21.16 ± 1.05 c | 39.22 ± 1.96 b | 17.82 ± 0.88 c | 17.75 ± 0.88 b |
| B3 | 20.05 ± 0.99 cd | 32.86 ± 1.30 cd | 17.70 ± 0.88 c | 17.27 ± 0.86 b |
| BE1 | 20.91 ± 1.04 c | 35.62 ± 1.78 c | 15.68 ± 0.78 d | 16.49 ± 0.82 b |
| BE2 | 19.11 ± 0.95 cd | 31.37 ± 1.56 d | 13.65 ± 0.68 e | 14.19 ± 0.71 c |
| BE3 | 17.71 ± 0.88 d | 29.71 ± 1.48 d | 13.30 ± 0.66 e | 14.10 ± 0.71 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chu, Z.; Fan, T.; Liang, S.; Li, G.; Zhang, J.; Zhen, Q. Application of Rice Husk Biochar and Earthworm on Concentration and Speciation of Heavy Metals in Industrial Sludge Treatment. Int. J. Environ. Res. Public Health 2022, 19, 13463. https://doi.org/10.3390/ijerph192013463
Wang X, Chu Z, Fan T, Liang S, Li G, Zhang J, Zhen Q. Application of Rice Husk Biochar and Earthworm on Concentration and Speciation of Heavy Metals in Industrial Sludge Treatment. International Journal of Environmental Research and Public Health. 2022; 19(20):13463. https://doi.org/10.3390/ijerph192013463
Chicago/Turabian StyleWang, Xingming, Zhaoxia Chu, Tingyu Fan, Shuying Liang, Gang Li, Jiamei Zhang, and Quan Zhen. 2022. "Application of Rice Husk Biochar and Earthworm on Concentration and Speciation of Heavy Metals in Industrial Sludge Treatment" International Journal of Environmental Research and Public Health 19, no. 20: 13463. https://doi.org/10.3390/ijerph192013463
APA StyleWang, X., Chu, Z., Fan, T., Liang, S., Li, G., Zhang, J., & Zhen, Q. (2022). Application of Rice Husk Biochar and Earthworm on Concentration and Speciation of Heavy Metals in Industrial Sludge Treatment. International Journal of Environmental Research and Public Health, 19(20), 13463. https://doi.org/10.3390/ijerph192013463




