Relationship between Eye Frailty and Physical, Social, and Psychological/Cognitive Weaknesses among Community-Dwelling Older Adults in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Survey Items
2.3. Analyses
3. Results
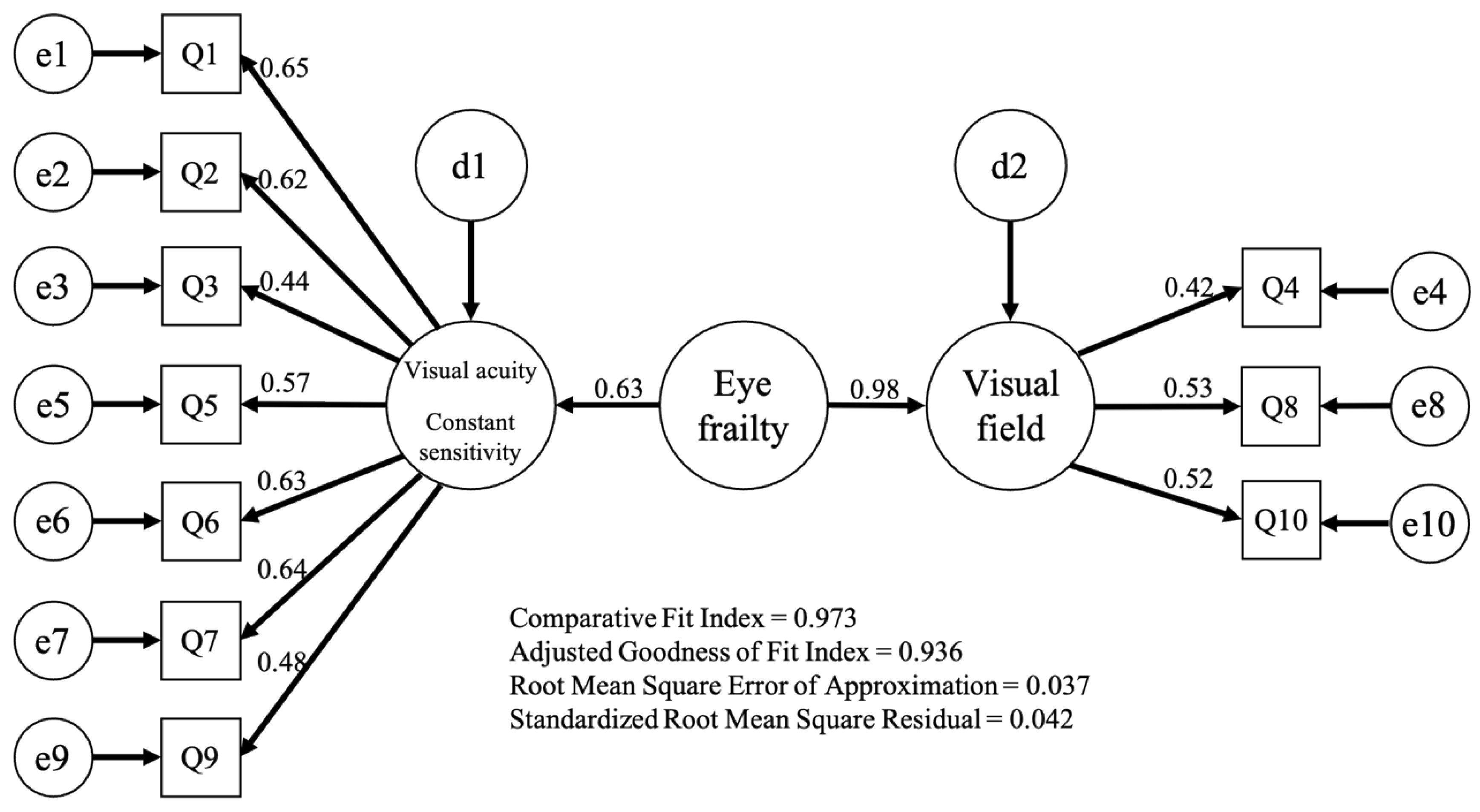
3.1. Exploratory and Confirmatory Factor Analysis of Eye Frailty Self-Check Test
3.2. Comparison of Prevalence of Eye Frailty and Physical Function
3.3. Investigation of Factors Related to Eye Frailty
3.4. Investigation of the Relationship between Eye Frailty and Subordinate Items of the Kihon Checklist
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research, G., Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Stadnyk, K.; MacKnight, C.; McDowell, I.; Hebert, R.; Hogan, D.B. A brief clinical instrument to classify frailty in elderly people. Lancet 1999, 353, 205–206. [Google Scholar] [CrossRef]
- Makizako, H.; Tsutsumimoto, K.; Shimada, H.; Arai, H. Social Frailty Among Community-Dwelling Older Adults: Recommended Assessments and Implications. Ann. Geriatr. Med. Res. 2018, 22, 3–8. [Google Scholar] [CrossRef]
- Gobbens, R.J.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M.G.A. Towards an integral conceptual model of frailty. J. Nutr. Health Aging 2010, 14, 175–181. [Google Scholar] [CrossRef]
- Tan, B.K.J.; Man, R.E.K.; Gan, A.T.L.; Fenwick, E.K.; Varadaraj, V.; Swenor, B.K.; Gupta, P.; Wong, T.Y.; Trevisan, C.; Lorenzo-Lopez, L.; et al. Is Sensory Loss an Understudied Risk Factor for Frailty? A Systematic Review and Meta-analysis. J. Gerontol. -Ser. A Biol. Sci. Med. Sci. 2020, 75, 2461–2470. [Google Scholar] [CrossRef]
- Swenor, B.K.; Lee, M.J.; Tian, J.; Varadaraj, V.; Bandeen-Roche, K. Visual impairment and frailty: Examining an understudied relationship. J. Gerontol. -Ser. A Biol. Sci. Med. Sci. 2020, 75, 596–602. [Google Scholar] [CrossRef]
- Ng, T.P.; Feng, L.; Nyunt, M.S.; Larbi, A.; Yap, K.B. Frailty in older persons: Multisystem risk factors and the Frailty Risk Index (FRI). J. Am. Med. Dir. Assoc. 2014, 15, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Samper-Ternent, R.; Al Snih, S.; Raji, M.A.; Markides, K.S.; Ottenbacher, K.J. Relationship between frailty and cognitive decline in older Mexican Americans. J. Am. Geriatr. Soc. 2008, 56, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, H.; Gu, X.; Sun, X.; Pan, Z.; Zhu, S.; Young, D. Frailty and Associated Risk Factors in Elderly People with Health Examination in Rural Areas of China. Iran J. Public Health 2019, 48, 1663–1670. [Google Scholar] [CrossRef]
- Varadaraj, V.; Lee, M.J.; Tian, J.; Ramulu, P.Y.; Bandeen-Roche, K.; Swenor, B.K. Near Vision Impairment and Frailty: Evidence of an Association. Am. J. Ophthalmol. 2019, 208, 234–241. [Google Scholar] [CrossRef]
- Moon, J.H.; Oh, Y.H.; Kong, M.H.; Kim, H.J. Relationship between visual acuity and muscle mass in the Korean older population: A cross-sectional study using Korean National Health and Nutrition Examination Survey. BMJ Open 2019, 9, e033846. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Varadaraj, V.; Tian, J.; Bandeen-Roche, K.; Swenor, B.K. The Association between Frailty and Uncorrected Refractive Error in Older Adults. Ophthalmic Epidemiol. 2020, 27, 219–225. [Google Scholar] [CrossRef] [PubMed]
- White, U.E.; Black, A.A.; Wood, J.M.; Delbaere, K. Fear of falling in vision impairment. Optom. Vis. Sci. 2015, 92, 730–735. [Google Scholar] [CrossRef]
- Delbaere, K.; Crombez, G.; Vanderstraeten, G.; Willems, T.; Cambier, D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing 2004, 33, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Brunes, A.; Hanson, M.B.; Heir, T. Loneliness among adults with visual impairment: Prevalence, associated factors, and relationship to life satisfaction. Health Qual Life Outcomes 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Liljas, A.E.; Wannamethee, S.G.; Whincup, P.H.; Papacosta, O.; Walters, K.; Iliffe, S.; Lennon, L.T.; Carvalho, L.A.; Ramsay, S.E. Socio-demographic characteristics, lifestyle factors and burden of morbidity associated with self-reported hearing and vision impairments in older British community-dwelling men: A cross-sectional study. J. Public Health 2016, 38, e21–e28. [Google Scholar] [CrossRef] [PubMed]
- Amir, N.N.; Kamaruzzaman, S.B.; Effendi-Tenang, I.; Jamaluddin, M.; Tan, M.P.; Ramli, N.; Khaliddin, N.; Zahari, M. Contrast sensitivity is associated with frailty. Eur. Geriatr. Med. 2021, 12, 313–319. [Google Scholar] [CrossRef]
- Japan Ophthalmology Awareness Council. Japan Ophthalmology Awareness Council Eye Frailty Awareness Official Website. Available online: https://www.eye-frail.jp (accessed on 17 August 2022).
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Doi, T.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Lee, S. Social Frailty Leads to the Development of Physical Frailty among Physically Non-Frail Adults: A Four-Year Follow-Up Longitudinal Cohort Study. Int. J. Environ. Res. Public Health 2018, 15, 490. [Google Scholar] [CrossRef]
- Hu, L.-t.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Schermelleh-Engel, K.; Moosbrugger, H.; Müller, H. Evaluating the Fit of Structural Equation Models: Tests of Significance and Descriptive Goodness-of-Fit Measures. Methods Psychol. Res. Online 2003, 8, 23–74. [Google Scholar]
- Satake, S.; Senda, K.; Hong, Y.-J.; Miura, H.; Endo, H.; Sakurai, T.; Kondo, I.; Toba, K. Validity of the Kihon Checklist for assessing frailty status. Geriatr. Gerontol. Int. 2016, 16, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef]
- Khadka, J.; Ratcliffe, J.; Caughey, G.E.; Wesselingh, S.L.; Inacio, M.C. Prevalence of Eye Conditions, Utilization of Eye Health Care Services, and Ophthalmic Medications After Entering Residential Aged Care in Australia. Transl. Vis. Sci. Technol. 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.K.; Klein, R.; Knudtson, M.D.; Lee, K.E. Relationship of measures of frailty to visual function: The beaver dam eye study. Trans. Am. Ophthalmol. Soc. 2003, 101, 191–200. [Google Scholar]
- Fujiwara, Y.; Nishi, M.; Fukaya, T.; Hasebe, M.; Nonaka, K.; Koike, T.; Suzuki, H.; Murayama, Y.; Saito, M.; Kobayashi, E. Synergistic or independent impacts of low frequency of going outside the home and social isolation on functional decline: A 4-year prospective study of urban Japanese older adults. Geriatr. Gerontol. Int. 2016, 17, 500–508. [Google Scholar] [CrossRef]
- Yoshida, Y.; Hiratsuka, Y.; Kawachi, I.; Murakami, A.; Kondo, K.; Aida, J. Association between visual status and social participation in older Japanese: The JAGES cross-sectional study. Soc. Sci. Med. 2020, 253, 112959. [Google Scholar] [CrossRef]
- Igarashi, A.; Aida, J.; Yamamoto, T.; Hiratsuka, Y.; Kondo, K.; Osaka, K. Associations between vision, hearing and tooth loss and social interactions: The JAGES cross-sectional study. J. Epidemiol. Community Health 2021, 75, 171–176. [Google Scholar] [CrossRef]
- Soler, V.; Sourdet, S.; Balardy, L.; Abellan Van Kan, G.; Brechemier, D.; Rouge Bugat, M.E.; Tavassoli, N.; Cassagne, M.; Malecaze, F.; Nourhashemi, F.; et al. Visual impairment screening at the Geriatric Frailty Clinic for Assessment of Frailty and Prevention of Disability at the Gérontopôle. J. Nutr. Health Aging 2016, 20, 870–877. [Google Scholar] [CrossRef]
- Nelson, L.A.; Noonan, C.; Goldberg, J.; Buchwald, D.S. Social Engagement and Physical and Cognitive Health Among American Indian Participants in the Health and Retirement Study. J. Cross-Cult. Gerontol. 2013, 28, 453. [Google Scholar] [CrossRef]
- Hikichi, H.; Kondo, N.; Kondo, K.; Aida, J.; Takeda, T.; Kawachi, I. Effect of a community intervention programme promoting social interactions on functional disability prevention for older adults: Propensity score matching and instrumental variable analyses, JAGES Taketoyo study. J. Epidemiol. Community Health 2015, 69, 905–910. [Google Scholar] [CrossRef] [PubMed]
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Q1. Eyes become tired more easily | — | 0.458 ** | 0.347 ** | 0.109 | 0.363 ** | 0.395 ** | 0.412 ** | 0.139 | 0.325 ** | 0.139 |
| Q2. It can sometimes be challenging to see in the evening | — | 0.177 * | 0.132 | 0.376 ** | 0.435 ** | 0.379 ** | 0.173 * | 0.262 ** | 0.173 * | |
| Q3. Opportunities to read newspapers or books for extended periods are now less often | — | 0.176 * | 0.318 ** | 0.242 ** | 0.309 ** | 0.108 | 0.161 * | 0.177 * | ||
| Q4. Sometimes, the table gets dirty when eating | — | 0.168 * | 0.127 | 0.206 ** | 0.221 ** | 0.205 ** | 0.221 ** | |||
| Q5. Often, I feel I cannot see well even with glasses | — | 0.277 ** | 0.328 ** | 0.327 ** | 0.318 ** | 0.216 ** | ||||
| Q6. Often, I feel it is too bright | — | 0.435 ** | 0.215 ** | 0.369 ** | 0.215 ** | |||||
| Q7. I cannot see clearly sometimes without blink | — | 0.234 ** | 0.264 ** | 0.270 ** | ||||||
| Q8. Straight lines appear wavy at time | — | 0.143 * | 0.163 * | |||||||
| Q9. I have sometimes felt stairs were dangerous | — | 0.108 | ||||||||
| Q10. I have overlooked traffic lights or road signs | — |
| Factor Loading | ||
|---|---|---|
| Factor 1 | Factor 2 | |
| Visual Acuity, Contrast Sensitivity | Visual Field | |
| Q1. Eyes become tired more easily | 0.813 | −0.185 |
| Q2. It can sometimes be challenging to see in the evening | 0.678 | −0.066 |
| Q6. Often, I feel it is too bright | 0.606 | 0.034 |
| Q7. I cannot see clearly sometimes without blink | 0.504 | 0.189 |
| Q9. I have sometimes felt stairs were dangerous | 0.413 | 0.097 |
| Q5. Often, I feel I cannot see well even with glasses | 0.380 | 0.278 |
| Q3. Opportunities to read newspapers or books for extended periods are now less often | 0.341 | 0.143 |
| Q4. Sometimes, the table gets dirty when eating | −0.075 | 0.507 |
| Q8. Straight lines appear wavy at time | 0.004 | 0.481 |
| Q10. I have overlooked traffic lights or road signs | 0.048 | 0.399 |
| Robust | Eye Frailty | p Value | ||
|---|---|---|---|---|
| Total, n (%) | 49 (25.5) | 143 (74.5) | ||
| Age | 79.0 ± 5.9 | 79.8 ± 6.7 | 0.44 | |
| Height (cm) | 147.7 ± 5.5 | 148.6 ± 6.1 | 0.33 | |
| BMI (kg/m2) | 23.8 ± 3.3 | 23.7 ± 3.1 | 0.82 | |
| SMI (kg/m2) | 6.41 ± 0.64 | 6.37 ± 0.67 | 0.76 | |
| Calf circumference (cm) | 33.7 ± 3.0 | 33.3 ± 2.9 | 0.48 | |
| Handgrip strength (kg) | 23.1 ± 4.1 | 21.9 ± 4.2 | 0.08 | |
| Usual walking speed (m/s) | 1.30 ± 0.22 | 1.20 ± 0.34 | 0.02 * | |
| Robust | 30 (61.2) | 44 (30.8) | ||
| Physical frailty, N (%) | Pre-frailty | 18 (36.7) | 79 (55.2) | |
| Frailty | 1 (2.0) | 20 (14.0) | 0.0001 † | |
| Robust | 22 (44.9) | 38 (26.6) | ||
| Social frailty, N (%) | Pre-frailty | 23 (46.9) | 83 (58.0) | |
| Frailty | 4 (8.2) | 22 (15.4) | 0.0001 † | |
| fall, N (%) | No fall | 38 (77.6) | 109 (76.2) | |
| Fall | 11 (22.4) | 34 (23.8) | 0.85 | |
| B | SE | Wald | p Value | Odds Ratio | 95%CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | −0.062 | 0.038 | 2.642 | 0.104 | 0.940 | 0.872 | 1.013 |
| Height | 0.088 | 0.048 | 3.424 | 0.064 | 1.092 | 0.995 | 1.199 |
| BMI | 0.028 | 0.111 | 0.065 | 0.798 | 1.029 | 0.828 | 1.278 |
| SMI | 0.094 | 0.367 | 0.066 | 0.797 | 1.099 | 0.536 | 2.256 |
| Calf circumference | −0.090 | 0.130 | 0.479 | 0.489 | 0.914 | 0.709 | 1.179 |
| Handgrip strength | −0.083 | 0.066 | 1.552 | 0.213 | 0.921 | 0.808 | 1.049 |
| Usual walking speed | −0.182 | 0.909 | 0.040 | 0.842 | 0.834 | 0.140 | 4.953 |
| Kihon check list | 0.326 | 0.090 | 13.013 | 0.000 * | 1.385 | 1.160 | 1.654 |
| Physical frailty (J-CHS) | 0.216 | 0.335 | 0.416 | 0.519 | 1.242 | 0.643 | 2.396 |
| Social frailty | 0.225 | 0.237 | 0.903 | 0.342 | 1.253 | 0.787 | 1.994 |
| Fall | −0.269 | 0.450 | 0.356 | 0.551 | 0.764 | 0.316 | 1.847 |
| (Constant) | −5.238 | 7.872 | 0.443 | 0.506 | 0.005 | ||
| B | SE | Wald | p Value | Odds Ratio | 95%CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| IADL | −0.471 | 0.324 | 2.112 | 0.146 | 0.624 | 0.331 | 1.178 |
| Physical functions | 0.265 | 0.186 | 2.038 | 0.153 | 1.304 | 0.906 | 1.878 |
| Nutritional status | −0.045 | 0.679 | 0.004 | 0.947 | 0.956 | 0.253 | 3.617 |
| Oral function | 0.122 | 0.256 | 0.227 | 0.633 | 1.130 | 0.685 | 1.864 |
| Social withdrawal | 0.891 | 0.385 | 5.343 | 0.021 * | 2.437 | 1.145 | 5.188 |
| Cognitive function | 0.716 | 0.340 | 4.440 | 0.035 * | 2.047 | 1.051 | 3.984 |
| Depression mood | 0.599 | 0.228 | 6.877 | 0.009 * | 1.820 | 1.163 | 2.848 |
| (Constant) | 0.039 | 0.456 | 0.007 | 0.932 | 1.039 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itokazu, M.; Ishizaka, M.; Uchikawa, Y.; Takahashi, Y.; Niida, T.; Hirose, T.; Ito, A.; Yakabi, A.; Endo, Y.; Sawaya, Y.; et al. Relationship between Eye Frailty and Physical, Social, and Psychological/Cognitive Weaknesses among Community-Dwelling Older Adults in Japan. Int. J. Environ. Res. Public Health 2022, 19, 13011. https://doi.org/10.3390/ijerph192013011
Itokazu M, Ishizaka M, Uchikawa Y, Takahashi Y, Niida T, Hirose T, Ito A, Yakabi A, Endo Y, Sawaya Y, et al. Relationship between Eye Frailty and Physical, Social, and Psychological/Cognitive Weaknesses among Community-Dwelling Older Adults in Japan. International Journal of Environmental Research and Public Health. 2022; 19(20):13011. https://doi.org/10.3390/ijerph192013011
Chicago/Turabian StyleItokazu, Masafumi, Masahiro Ishizaka, Yoshikazu Uchikawa, Yoshiaki Takahashi, Takahiro Niida, Tamaki Hirose, Akihiro Ito, Akihiro Yakabi, Yoshiaki Endo, Yohei Sawaya, and et al. 2022. "Relationship between Eye Frailty and Physical, Social, and Psychological/Cognitive Weaknesses among Community-Dwelling Older Adults in Japan" International Journal of Environmental Research and Public Health 19, no. 20: 13011. https://doi.org/10.3390/ijerph192013011
APA StyleItokazu, M., Ishizaka, M., Uchikawa, Y., Takahashi, Y., Niida, T., Hirose, T., Ito, A., Yakabi, A., Endo, Y., Sawaya, Y., Igawa, T., Kobayashi, K., Hara, T., Watanabe, M., Kubo, A., & Urano, T. (2022). Relationship between Eye Frailty and Physical, Social, and Psychological/Cognitive Weaknesses among Community-Dwelling Older Adults in Japan. International Journal of Environmental Research and Public Health, 19(20), 13011. https://doi.org/10.3390/ijerph192013011







