Development and Validation of the Chinese Frailty Screening Scale: A Study among Community-Dwelling Older Adults in Shanghai
Abstract
1. Introduction
2. Methods
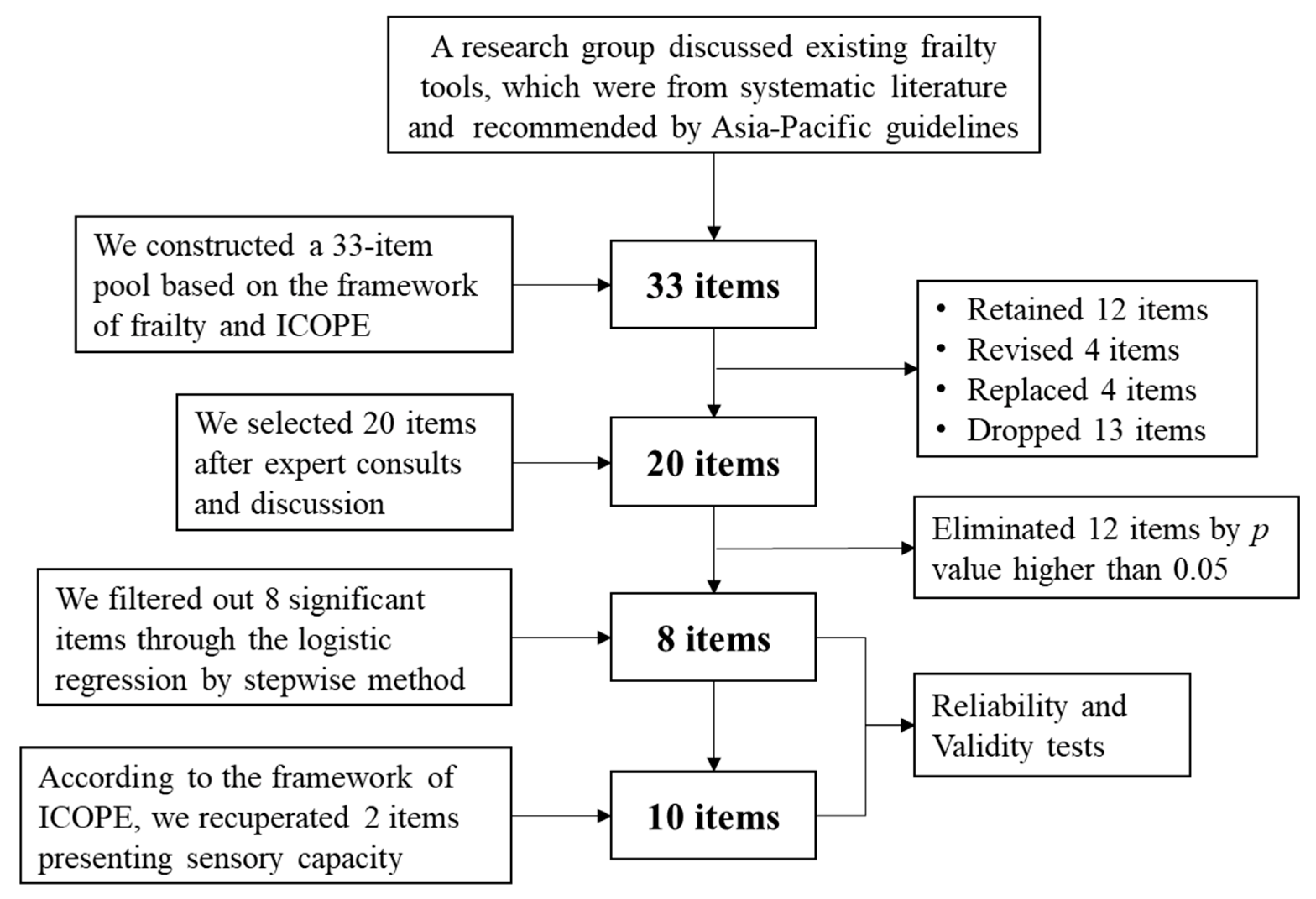
2.1. Part One: Drafting the Chinese Frailty Screening Scale (CFSS)
2.2. Part Two: Revising and Validating the CFSS
3. Measurements
- (1)
- The fatigue, resistance, ambulation, illness, and loss of weight (FRAIL) scale was derived from a consensus of a European, Canadian, and American geriatric advisory panel [31]. It is a quick and valid tool applied globally [32] and had been used and validated among Chinese older populations [21]. It consists of 5 simple questions and ranges from 0 to 5 with 1 point for each question (0 = best; 5 = worst): The scores were frail (3–5), prefrail (1–2), and robust (0).
- (2)
- The TFI, a self-reported 15-item questionnaire, addressed three domains. The physical domain consisted of 8 items (e.g., difficulty in walking or hearing) ranging from 0 to 8 points. The psychological domain had 4 items (e.g., depressive symptoms, coping) ranging from 0 to 4 points. The social domain had 3 items (e.g., living alone, social support) ranging from 0 to 3 points. A high score indicates more frailty, and a total score of 5 or more was regarded as frail [33]. The Chinese version of TFI has been validated in China [24].
- (3)
- The FI, which was constructed using a standardized procedure [34] included 49 self-reported items referring to the list of FI constructed by previous studies [35,36]. A summary score of 0–49 with one point per item was used to construct a total score/49. It consisted of the self-reported presence of diseases (20 items: e.g., hypertension, diabetes mellitus, coronary heart disease); geriatric symptoms (12 items: e.g., vision impairment, hearing loss, falls in the previous year); difficulties in performing basic and instrumental activities of daily living (14 items: e.g., dressing, transferring, shopping); cognitive decline (1 item) using a Chinese version of the mini-mental state examination (MMSE) [37] total score < 27; depression (1 item) used a Chinese version of the geriatric depression scale (GDS-15) [38] for a total score ≥ 8; and self-rated poor health (1 item). An FI score more than 0.25 was classified as frail.
- (4)
- Physical function was evaluated by Katz as the activity of daily living (ADL) and by Lawton as the instrumental activity of daily living (IADL). Katz’s ADL consisted of feeding, continence, transferring, toileting, dressing, and bathing [39]. Lawton’s IADL comprises using the telephone, shopping, preparing food, housekeeping, doing laundry, using transportation, handling medications, and handling finances [40]. For each activity, participants were asked if they had no difficulty, had difficulty, or were unable to perform the tasks from 0 to 2 points. The presence of ADL or IADL deficits to any positive individual score (i.e., equivalent to more than ‘‘0”) indicated disability [41]. Body mass index (BMI, the ratio of weight and squared height, kg/m2) was also included.
4. Statistical Analysis
5. Results
5.1. Participant Characteristics
5.2. Simplified CFSS
5.3. Reliability
5.4. Construct Validity
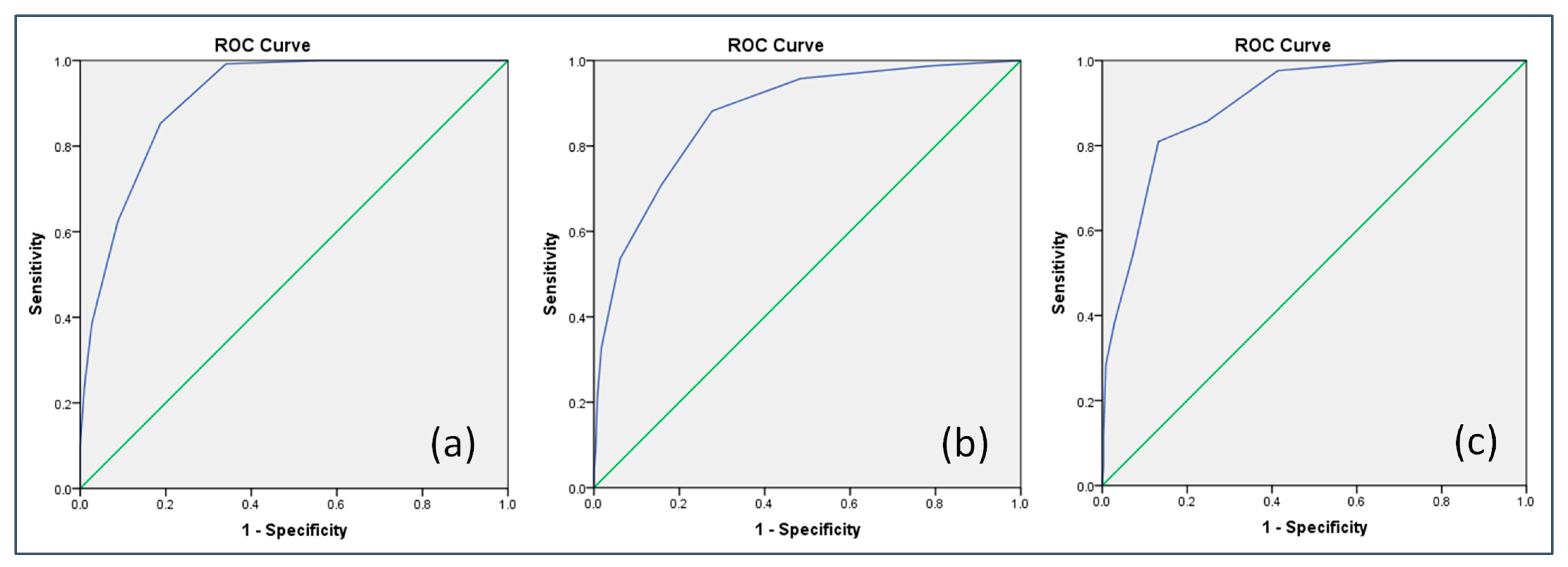
5.5. Criteria Validity
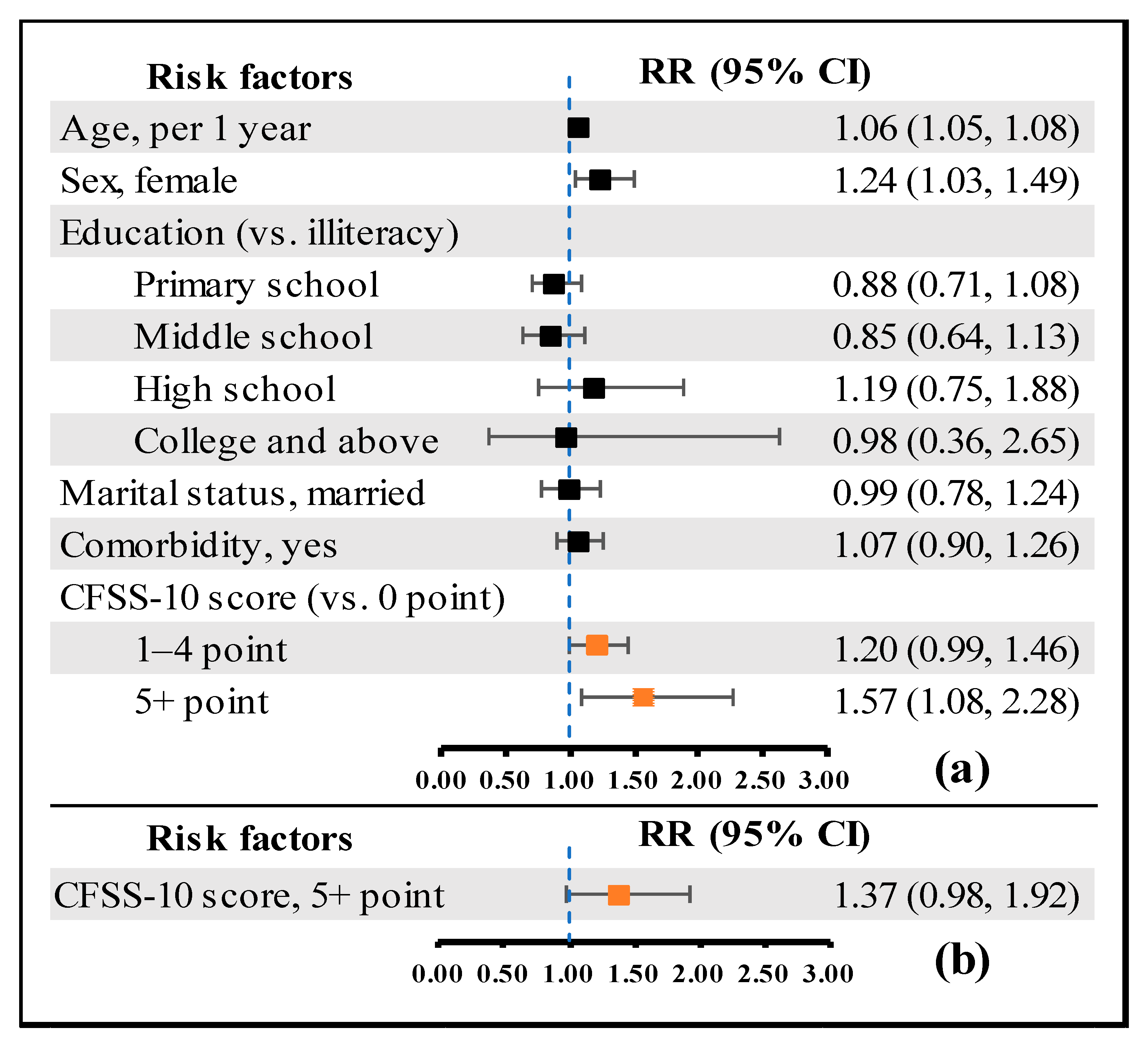
5.6. Predictive Validity
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Aging 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ma, Y.; Wang, C.; Jiang, M.; Geng, C.; Chang, X.; Ma, B.; Han, L. Prevalence and risk factors for frailty among community-dwelling older people in china: A systematic review and meta-analysis. J. Nutr. Health Aging 2019, 23, 442–450. [Google Scholar] [CrossRef]
- Cesari, M.; Prince, M.; Thiyagarajan, J.A.; De Carvalho, I.A.; Bernabei, R.; Chan, P.; Gutierrez-Robledo, L.M.; Michel, J.-P.; Morley, J.E.; Ong, P.; et al. Frailty: An emerging public health priority. J. Am. Med. Dir. Assoc. 2016, 17, 188–192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Ageing and Health. 2015. Available online: https://www.who.int/publications/i/item/9789241565042 (accessed on 25 June 2022).
- Cesari, M.; De Carvalho, I.A.; Thiyagarajan, J.A.; Cooper, C.; Martin, F.C.; Reginster, J.-Y.; Vellas, B.; Beard, J.R. Evidence for the domains supporting the construct of intrinsic capacity. J. Gerontol. Ser. A 2018, 73, 1653–1660. [Google Scholar] [CrossRef]
- Belloni, G.; Cesari, M. Frailty and intrinsic capacity: Two distinct but related constructs. Front. Med. 2019, 6, 133. [Google Scholar] [CrossRef] [PubMed]
- Integrated Care for Older People (icope): Guidance for Person-Centred Assessment and Pathways in Primary Care. 2019. Available online: https://www.who.int/publications/i/item/WHO-FWC-ALC-19.1 (accessed on 25 June 2022).
- Woo, J. Frailty, Successful Aging, Resilience, and Intrinsic Capacity: A Cross-disciplinary Discourse of the Aging Process. Curr. Geriatr. Rep. 2019, 8, 67–71. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Gobbens, R.J.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M.G.A. Towards an integral conceptual model of frailty. J. Nutr. Health Aging 2010, 14, 175–181. [Google Scholar] [CrossRef]
- Ruiz, J.G.; Dent, E.; Morley, J.E.; Merchant, R.A.; Beilby, J.; Beard, J.; Tripathy, C.; Sorin, M.; Andrieu, S.; Aprahamian, I. Screening for and managing the person with frailty in primary care: Icfsr consensus guidelines. J. Nutr. Health Aging 2020, 24, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, Y.; Chan, P.; Ma, L. Reliability and validity of the self-reported frailty screening questionnaire in older adults. Ther. Adv. Chronic. Dis. 2020, 11, 254077125. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shang, N.; Chhetri, J.K.; Liu, L.; Guo, W.; Li, P.; Guo, S.; Ma, L. A frailty screening questionnaire (fsq) to rapidly predict negative health outcomes of older adults in emergency care settings. J. Nutr. Health Aging 2020, 24, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Yang, Y.; Zhang, C.-M.; Luo, R.-Z.; Liu, Y.-H. Development of a frailty scale for elderly people in china. Chin. Nurs. Res. 2017, 4, 64–70. [Google Scholar] [CrossRef]
- Fan, J.; Yu, C.; Guo, Y.; Bian, Z.; Sun, Z.; Yang, L.; Chen, Y.; Du, H.; Li, Z.; Lei, Y.; et al. Frailty index and all-cause and cause-specific mortality in chinese adults: A prospective cohort study. Lancet Public Health 2020, 5, e650–e660. [Google Scholar] [CrossRef]
- Bollen, K.A. Structural Equations with Latent Variables; John Wiley & Sons, Inc.: New York, NY, USA, 1989; pp. 289–308. [Google Scholar]
- Woo, J.; Yu, R.; Wong, M.; Yeung, F.; Wong, M.; Lum, C. Frailty screening in the community using the frail scale. J. Am. Med. Dir. Assoc. 2015, 16, 412–419. [Google Scholar] [CrossRef]
- Dent, E.; Lien, C.; Lim, W.S.; Wong, W.C.; Wong, C.H.; Ng, T.P.; Woo, J.; Dong, B.; de la Vega, S.; Poi, P.J.H.; et al. The asia-pacific clinical practice guidelines for the management of frailty. J. Am. Med. Dir. Assoc. 2017, 18, 564–575. [Google Scholar] [CrossRef]
- Chen, S.; Chen, T.; Kishimoto, H.; Susaki, Y.; Kumagai, S. Development of a fried frailty phenotype questionnaire for use in screening community-dwelling older adults. J. Am. Med. Dir. Assoc. 2020, 21, 272–276. [Google Scholar] [CrossRef]
- Dong, L.; Liu, N.; Tian, X.; Qiao, X.; Gobbens, R.J.; Kane, R.L.; Wang, C. Reliability and validity of the tilburg frailty indicator (tfi) among chinese community-dwelling older people. Arch. Gerontol. Geriatr. 2017, 73, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Westbury, L.; Cooper, C. Social isolation and loneliness as risk factors for the progression of frailty: The english longitudinal study of ageing. Age Aging 2018, 47, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Egashira, R.; Sato, T.; Miyake, A.; Takeuchi, M.; Nakano, M.; Saito, H.; Moriguchi, M.; Tonari, S.; Hagihara, K. The Japan Frailty Scale is a promising screening test for frailty and pre-frailty in Japanese elderly people. Gene 2022, 844, 146775. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tang, H.; Zeng, J.; Pan, X.; Luo, X.; Liao, J.; Liang, D.; Zhang, L.; Zhou, S.; Yin, M.; et al. Traditional Chinese Medicine Constitution Is Associated with the Frailty Status of Older Adults: A Cross-Sectional Study in the Community. Evid. Based Complement. Altern. Med. 2022, 2022, 8345563. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Who Clinical Consortium on Healthy Ageing: Topic Focus—Frailty and Intrinsic Capacity. 2017. Available online: https://www.who.int/publications/i/item/WHO-FWC-ALC-17.2 (accessed on 25 June 2022).
- Azzopardi, R.V.; Vermeiren, S.; Gorus, E.; Habbig, A.-K.; Petrovic, M.; Noortgate, N.V.D.; De Vriendt, P.; Bautmans, I.; Beyer, I.; Verté, D.; et al. Linking frailty instruments to the international classification of functioning, disability, and health: A systematic review. J. Am. Med. Dir. Assoc. 2016, 17, 1061–1066. [Google Scholar] [CrossRef]
- Martino, J.P. The delphi method: Techniques and applications. Technol. Forecast. Soc. Chang. 1976, 8, 441–442. [Google Scholar] [CrossRef]
- Van Kan, G.A.; Rolland, Y.; Bergman, H.; Morley, J.E.; Kritchevsky, S.B.; Vellas, B. The I.A.N.A. Task force on frailty assessment of older people in clinical practice. J. Nutr. Health Aging 2008, 12, 29–37. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical frailty: Icfsr international clinical practice guidelines for identification and management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef]
- Gobbens, R.J.J.; van Assen, M.A.L.M.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M.G.A. The tilburg frailty indicator: Psychometric properties. J. Am. Med. Dir. Assoc. 2010, 11, 344–355. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Q.; Zhi, T.; Zhu, Y.; Wang, Y.; Wang, Z.; Shi, J.; Xie, X.; Chu, X.; Wang, X.; et al. Frailty index and its relation to falls and overnight hospitalizations in elderly chinese people: A population-based study. J. Nutr. Health Aging 2016, 20, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R.; Zhang, M.Y.; Ouang, Y.-Q.; Wang, Z.; Liu, W.T.; Yu, E.; Wong, S.-C.; Salmon, D.P.; Grant, I.A. Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988, 41, 971–978. [Google Scholar] [CrossRef]
- Tang, W.K.; Wong, E.; Chiu, H.F.K.; Lum, C.M.; Ungvari, G.S. The Geriatric Depression Scale should be shortened: Results of Rasch analysis. Int. J. Geriatr. Psychiatry 2005, 20, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The index of adl: A standardized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Wu, C.; Geldhof, G.J.; Xue, Q.-L.; Kim, D.H.; Newman, A.B.; Odden, M.C. Development, construct validity, and predictive validity of a continuous frailty scale: Results from 2 large us cohorts. Am. J. Epidemiol. 2018, 187, 1752–1762. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420. [Google Scholar] [CrossRef]
- Murphy, J.M.; Berwick, D.M.; Weinstein, M.C.; Borus, J.F.; Budman, S.H.; Klerman, G.L. Performance of screening and diagnostic tests: Application of receiver operating characteristic analysis. Arch. Gen. Psychiatry. 1987, 44, 550–555. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- De, K.; Banerjee, J.; Rajan, S.P.; Chatterjee, P.; Chakrawarty, A.; Khan, M.A.; Singh, V.; Dey, A.B. Development and Psychometric Validation of a New Scale for Assessment and Screening of Frailty Among Older Indians. Clin. Interv. Aging 2021, 16, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Won, C.W.; Lee, Y.; Lee, S.; Kim, M. Development of Korean Frailty Index for Primary Care (KFI-PC) and Its Criterion Validity. Ann. Geriatr. Med. Res. 2020, 24, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.-L.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Aging Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Puts, M.T.E.; Lips, P.; Deeg, D.J.H. Sex differences in the risk of frailty for mortality independent of disability and chronic diseases. J. Am. Geriatr. Soc. 2005, 53, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, W.J.; Shema, S.J.; Balfour, J.L.; Higby, H.R.; Kaplan, G.A. Antecedents of frailty over three decades in an older cohort. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 1998, 53, S9–S16. [Google Scholar] [CrossRef]
- Wu, C.; Smit, E.; Xue, Q.-L.; Odden, M.C. Prevalence and correlates of frailty among community-dwelling chinese older adults: The china health and retirement longitudinal study. J. Gerontol. Ser. A 2018, 73, 102–108. [Google Scholar] [CrossRef]
- Kojima, G. Frailty defined by frail scale as a predictor of mortality: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2018, 19, 480–483. [Google Scholar] [CrossRef]
- Romero-Ortuno, R.; Kenny, R.A. The frailty index in europeans: Association with age and mortality. Age Ageing 2012, 41, 684–689. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Graham, J.E.; Mogilner, A.J.; Rockwood, K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002, 2, 1. [Google Scholar] [CrossRef]
- Gobbens, R.J.J.; van Assen, M.A.L.M.; Schalk, M.J.D. The prediction of disability by self-reported physical frailty components of the tilburg frailty indicator (tfi). Arch. Gerontol. Geriatr. 2014, 59, 280–287. [Google Scholar] [CrossRef]
- Shamliyan, T.; Talley, K.M.; Ramakrishnan, R.; Kane, R.L. Association of frailty with survival: A systematic literature review. Ageing Res. Rev. 2013, 12, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhong, G.-C.; Zhou, X.; Guan, L.; Zhou, L. Frailty and risks of all-cause and cause-specific death in community-dwelling adults: A systematic review and meta-analysis. BMC Geriatr. 2022, 22, 725. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Miller, D.K.; Morley, J.E. A comparison of four frailty models. J. Am. Geriatr. Soc. 2014, 62, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.; Van Rossum, E.; Beurskens, A.; Heuvel, W.V.D.; De Witte, L. The predictive validity of three self-report screening instruments for identifying frail older people in the community. BMC Public Health 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, I.; Suemoto, C.K.; Aliberti, M.J.R.; Filho, S.d.Q.F.; Melo, J.d.A.; Lin, S.M.; Filho, W.J. Frailty and cognitive status evaluation can better predict mortality in older adults? Arch. Gerontol. Geriatr. 2018, 77, 51–56. [Google Scholar] [CrossRef]
- Rockwood, K.; Andrew, M.; Mitnitski, A. A comparison of two approaches to measuring frailty in elderly people. The journals of gerontology. J. Gerontol. Ser. A 2007, 62, 738–743. [Google Scholar] [CrossRef]
- Aprahamian, I.; Cezar, N.O.D.C.; Izbicki, R.; Lin, S.M.; Paulo, D.L.V.; Fattori, A.; Biella, M.M.; Filho, W.J.; Yassuda, M.S. Screening for frailty with the frail scale: A comparison with the phenotype criteria. J. Am. Med. Dir. Assoc. 2017, 18, 592–596. [Google Scholar] [CrossRef]
| Characteristics | (Mean ± SD) or n (%) | p Value | |
|---|---|---|---|
| Sample 1 (n = 1062) | Sample 2 (n = 2008) | ||
| Area | |||
| Shanghai | 301 (28.3) | 2008 (100.0) | |
| Hangzhou | 96 (9.0) | ||
| Nantong | 319 (30.0) | ||
| Ya’an | 78 (7.3) | ||
| Guangzhou | 100 (9.4) | ||
| Jinan | 122 (11.5) | ||
| Nanchang | 46 (4.3) | ||
| Age, year | 76.7 ± 7.2 | 72.4 ± 6.1 | <0.001 |
| Sex, male | 499 (47.0) | 937 (46.7) | 0.864 |
| Education | <0.001 | ||
| Illiteracy | 309 (29.1) | 890 (44.6) | |
| Primary school | 307 (28.9) | 719 (36.0) | |
| Middle school | 246 (23.2) | 321 (16.1) | |
| High school | 108 (10.2) | 54 (2.7) | |
| College and above | 91 (8.6) | 13 (0.6) | |
| Marital status | <0.001 | ||
| Married | 806 (76.0) | 1678 (84.2) | |
| Other a | 254 (24.0) | 316 (15.8) | |
| BMI, kg/m2 | 24.4 ± 8.7 | ||
| GDS score, point | 3.0 ± 2.7 | ||
| MMSE score, point | 20.6 ± 7.3 | ||
| Comorbidity, ≥2 | 541 (72.8) b | 831 (41.5) | <0.001 |
| FRAIL score | |||
| 1–2 point | 586 (55.2) | ||
| 3–5 point | 252 (23.7) | ||
| TFI score, ≥5 point | 237 (31.9) b | ||
| FI, ≥0.25 | 74 (18.6) c | ||
| ADL, difficulty ≥1 task | 212 (20.0) | 115 (5.7) | <0.001 |
| IADL, difficulty ≥1 task | 493 (46.4) | 435 (21.7) | <0.001 |
| Item | Question | Answer |
|---|---|---|
| Illnesses | Have you been diagnosed with at least 5 illnesses by doctors? (i.e., Hypertension; Dyslipidemia; Diabetes or high blood sugar; Cancer or malignant tumor (excluding minor skin cancers); Chronic lung diseases; Liver disease; Heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems; Stroke; Kidney disease; Stomach or other digestive disease; Alzheimer’s or Parkinson’s disease; Arthritis or rheumatism; Asthma) | □ Yes □ No |
| Exhaustion | Did you often feel tired or fatigue in the last month? | □ Yes □ No |
| Lack of appetite | In the last three months, did you eat less due to loss of appetite, indigestion, teeth problem or dysphagia? | □ Yes □ No |
| Visual impairment | Do you experience problems in your daily life due to poor vision? | □ Yes □ No |
| Hearing loss | Do you experience problems in your daily life due to poor hearing? | □ Yes □ No |
| Resistance | Do you have difficulty with climbing 10 stairs or a flight without resting? | □ Yes □ No |
| Physical inactivity | Did you walk for at least 10 min or 400 m continuously in the last week? | □ Yes □ No |
| Attention | Did you often wander or have difficulty with concentrating in the last month? | □ Yes □ No |
| Orientation | Did you frequently get the date wrong or get lost in the last month? | □ Yes □ No |
| Depressive symptom | Did you feel you were not interested in doing anything in the last month? | □ Yes □ No |
| Items | r a | Component | |||
|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | ||
| Illnesses | 0.39 * | 0.02 | 0.75 | −0.10 | −0.06 |
| Exhaustion | 0.59 * | 0.35 | 0.56 | 0.07 | −0.01 |
| Lack of appetite | 0.44 * | 0.07 | 0.55 | 0.17 | 0.33 |
| Visual impairment | 0.49 * | 0.18 | 0.06 | 0.77 | −0.01 |
| Hearing loss | 0.45 * | 0.02 | 0.12 | 0.82 | 0.01 |
| Resistance | 0.51 * | 0.07 | 0.52 | 0.25 | −0.07 |
| Physical inactivity | 0.28 * | 0.02 | −0.03 | −0.03 | 0.93 |
| Attention | 0.55 * | 0.82 | 0.02 | 0.11 | −0.02 |
| Orientation | 0.53 * | 0.78 | 0.10 | 0.10 | −0.10 |
| Depressive symptom | 0.45 * | 0.62 | 0.21 | 0.03 | 0.22 |
| Criteria | AUC (95% CI) | Cut-Off | Youden Index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Kappa |
|---|---|---|---|---|---|---|---|---|
| FRAIL | 0.91 * (0.89, 0.93) | ≥3 | 65.1 | 99.2 | 65.9 | 47.5 | 99.6 | 0.47 * |
| ≥4 | 66.5 | 85.3 | 81.2 | 58.6 | 94.7 | 0.58 * | ||
| ≥5 | 53.5 | 62.3 | 91.2 | 68.9 | 88.6 | 0.55 * | ||
| TFI | 0.87 * (0.85, 0.90) | ≥3 | 55.1 | 70.9 | 84.2 | 67.7 | 86.1 | 0.54 * |
| ≥4 | 60.5 | 88.2 | 72.3 | 59.9 | 92.9 | 0.54 * | ||
| ≥5 | 47.5 | 53.6 | 93.9 | 80.4 | 81.2 | 0.52 * | ||
| FI | 0.87 * (0.83, 0.92) | ≥3 | 58.0 | 78.4 | 79.6 | 46.8 | 94.1 | 0.46 * |
| ≥4 | 58.0 | 67.6 | 90.4 | 61.7 | 92.4 | 0.56 * | ||
| ≥5 | 44.6 | 48.6 | 96.0 | 73.5 | 89.1 | 0.51 * |
| Number of Positive Items | Disability (n = 1549) | All-Cause Mortality (n = 2008) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | RR (95%CI) | p Value | Adjusted RR (95%CI) a,b | p Value | n (%) | RR (95%CI) | p Value | Adjusted RR (95%CI) a,c | p Value | |
| 0 | 119 (19.0) | reference | reference | 3 (0.42) | reference | reference | ||||
| 1 | 138 (26.2) | 1.38 (1.11, 1.71) | 0.004 | 1.18 (0.95, 1.46) | 0.136 | 9 (1.45) | 3.42 | 0.065 | 2.49 (0.66, 9.46) | 0.179 |
| 2 | 63 (29.6) | 1.55 (1.19, 2.02) | 0.001 | 1.28 (0.99, 1.66) | 0.062 | 12 (3.91) | 9.24 (2.61, 32.74) | <0.001 | 5.49 (1.52, 19.92) | 0.010 |
| 3 | 26 (25.5) | 1.34 (0.93, 1.94) | 0.121 | 1.19 (0.82, 1.72) | 0.357 | 6 (3.59) | 8.49 (2.12, 33.95) | 0.002 | 4.42 (1.06, 18.42) | 0.041 |
| 4 | 12 (26.7) | 1.40 (0.84, 2.33) | 0.196 | 1.13 (0.70, 1.82) | 0.628 | 4 (4.17) | 9.85 (2.20, 44.00) | 0.003 | 3.73 (0.79, 17.64) | 0.100 |
| 5+ | 16 (43.2) | 2.27 (1.52, 3.40) | <0.001 | 1.57 (1.08, 2.27) | 0.017 | 3 (2.78) | 6.56 (1.32, 32.53) | 0.021 | 2.17 (0.40, 11.71) | 0.368 |
| p for trend | <0.001 | 0.035 | <0.001 | 0.244 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, B.; Wang, Y.; Chen, H.; Chen, Y.; Yan, H.; Fu, H.; Bao, Z.; Gao, J. Development and Validation of the Chinese Frailty Screening Scale: A Study among Community-Dwelling Older Adults in Shanghai. Int. J. Environ. Res. Public Health 2022, 19, 11811. https://doi.org/10.3390/ijerph191811811
Ye B, Wang Y, Chen H, Chen Y, Yan H, Fu H, Bao Z, Gao J. Development and Validation of the Chinese Frailty Screening Scale: A Study among Community-Dwelling Older Adults in Shanghai. International Journal of Environmental Research and Public Health. 2022; 19(18):11811. https://doi.org/10.3390/ijerph191811811
Chicago/Turabian StyleYe, Bo, Yi Wang, Hao Chen, Yingwei Chen, Huihui Yan, Hua Fu, Zhijun Bao, and Junling Gao. 2022. "Development and Validation of the Chinese Frailty Screening Scale: A Study among Community-Dwelling Older Adults in Shanghai" International Journal of Environmental Research and Public Health 19, no. 18: 11811. https://doi.org/10.3390/ijerph191811811
APA StyleYe, B., Wang, Y., Chen, H., Chen, Y., Yan, H., Fu, H., Bao, Z., & Gao, J. (2022). Development and Validation of the Chinese Frailty Screening Scale: A Study among Community-Dwelling Older Adults in Shanghai. International Journal of Environmental Research and Public Health, 19(18), 11811. https://doi.org/10.3390/ijerph191811811








