Do Patients with Prostate Cancer Benefit from Exercise Interventions? A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection
2.5. Data Collection Process
2.6. Data Items
2.7. Risk of Bias in Individual Studies
2.8. Summary of Measurements
2.9. Synthesis of Results
2.10. Risk of Bias across Studies
2.11. Additional Analyses
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
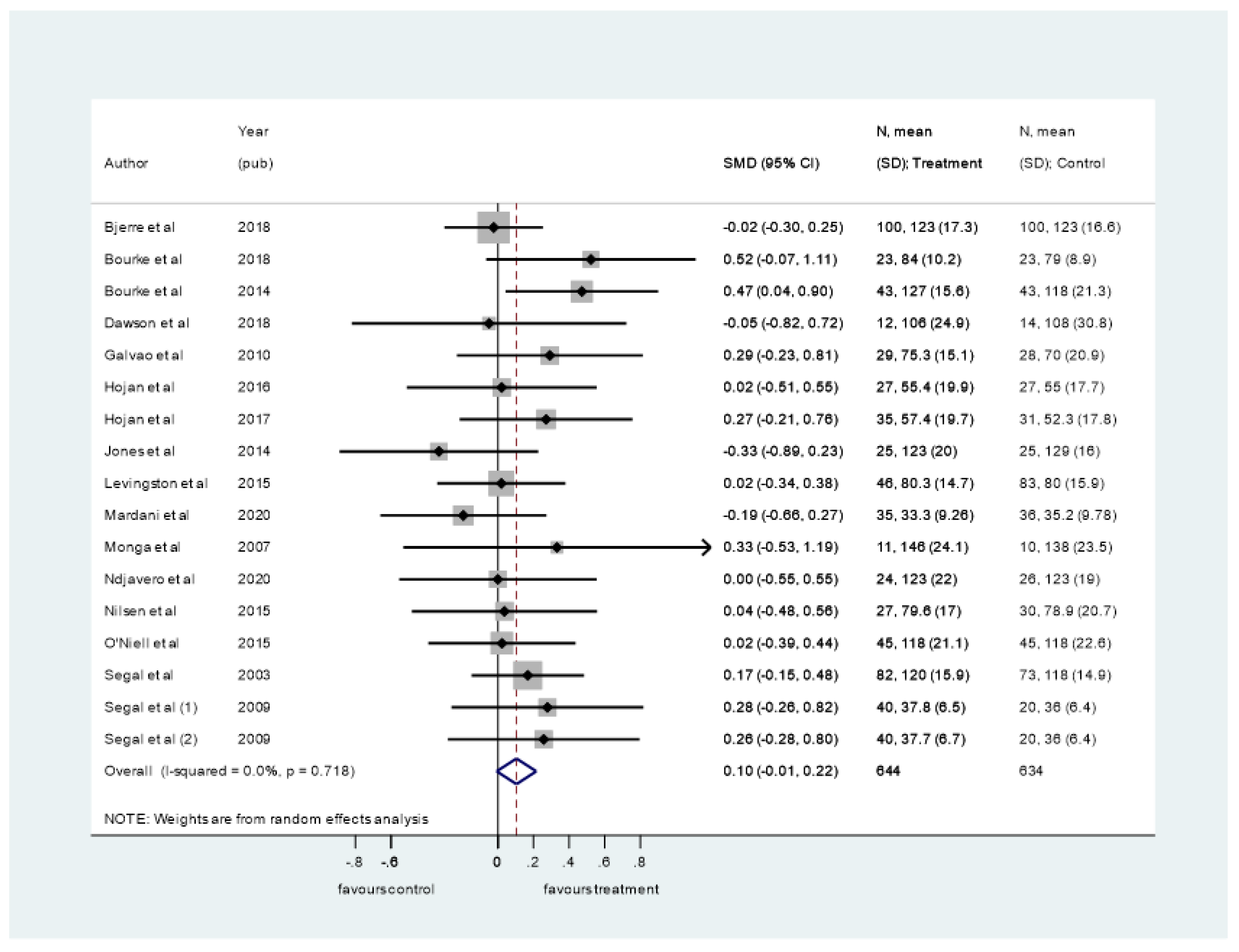
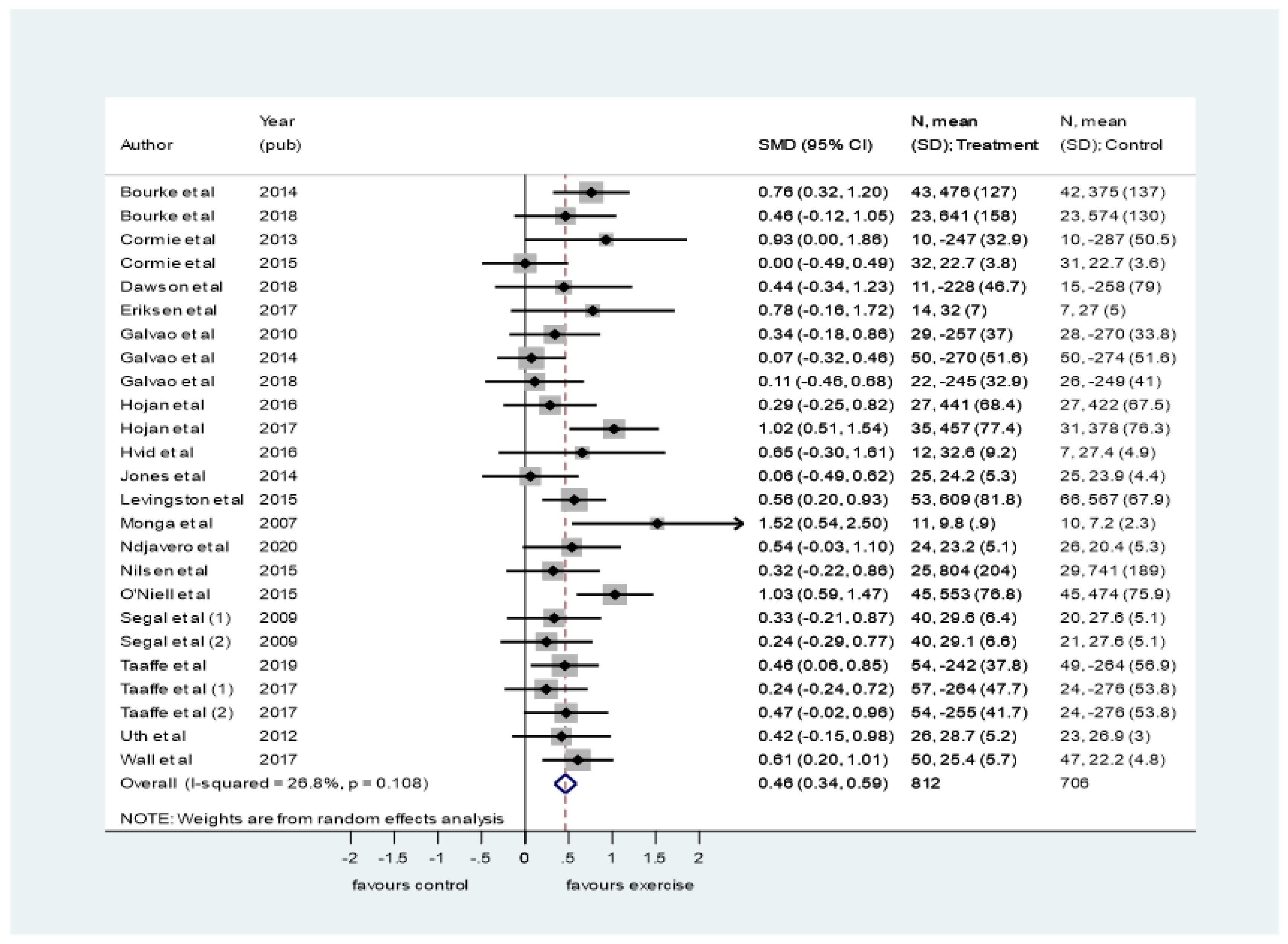
3.4. Synthesis of Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Correction in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.L.; Alibhai, S.M.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, K.; Tuffaha, H.; Galvão, D.A.; Scuffham, P.; Newton, R.U. Incidence of the adverse effects of androgen deprivation therapy for prostate cancer: A systematic literature review. Support. Care Cancer 2020, 28, 2079–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, S.M.; Kuo, Y.F.; Shahinian, V.B. Prevalent and incident use of androgen deprivation therapy among men with prostate cancer in the United States. Urol. Oncol. 2011, 29, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, M.; Hamilton, E.J.; Gilfillan, C.; Bolton, D.; Joon, D.L.; Zajac, J.D. Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med. J. Aust. 2011, 194, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Abdollah, F.; Sammon, J.D.; Reznor, G.; Sood, A.; Schmid, M.; Klett, D.E.; Sun, M.; Aizer, A.A.; Choueiri, T.K.; Hu, J.C. Medical androgen deprivation therapy and increased non-cancer mortality in non-metastatic prostate cancer patients aged ≥66 years. Eur. J. Surg. Oncol. 2015, 41, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Smith, D.; Steed, L.; Hooper, R.; Carter, A.; Catto, J.; Albertsen, P.C.; Tombal, B.; Payne, H.A.; Rosario, D.J. Exercise for Men with Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016, 69, 693–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, J.R.; Livingston, P.M.; Fraser, S.F. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: A systematic review. J. Clin. Oncol. 2014, 32, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, V.; Singh, B.; Kaur, S.; Prokop, L.J.; Kaushik, D. The Effects of Exercise on Fatigue, Quality of Life, and Psychological Function for Men with Prostate Cancer: Systematic Review and Meta-analyses. Eur. Urol. Focus 2016, 2, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Taaffe, D.R.; Newton, R.U.; Buffart, L.M.; Galvão, D.A. What is the minimal dose for resistance exercise effectiveness in prostate cancer patients? Systematic review and meta-analysis on patient-reported outcomes. Prostate Cancer Prostatic Dis. 2021, 24, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Bigaran, A.; Zopf, E.; Gardner, J.; Gerche, A.L.; Murphy, D.G.; Howden, E.J.; Baker, M.K.; Cormie, P. The effect of exercise training on cardiometabolic health in men with prostate cancer receiving androgen deprivation therapy: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, A.; Hasenöhrl, T.; Palma, S.; Crevenna, R. Effects of resistance exercise in prostate cancer patients: A systematic review update as of March 2020. Wien. Klin. Wochenschr. 2020, 132, 452–463. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Wang, Y.; Xu, D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuttgen, H.; Kraemer, W.J. Terminology and measurement in exercise performance. Sport Sci. Res. 1987, 1, 1–10. [Google Scholar]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2021. [Google Scholar]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V. Chapter 9: Summarizing study characteristics and preparing for synthesis. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: Chichester, UK, 2021. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalton, J.E.; Bolen, S.D.; Mascha, E.J. Publication Bias: The Elephant in the Review. Anesth. Analg. 2016, 123, 812–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjerre, E.D.; Brasso, K.; Jørgensen, A.B.; Petersen, T.H.; Eriksen, A.R.; Tolver, A.; Christensen, J.F.; Poulsen, M.H.; Madsen, S.S.; Østergren, P.B. Football Compared with Usual Care in Men with Prostate Cancer (FC Prostate Community Trial): A Pragmatic Multicentre Randomized Controlled Trial. Sports Med. 2019, 49, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourke, L.; Gilbert, S.; Hooper, R.; Steed, L.A.; Joshi, M.; Catto, J.W.F.; Saxton, J.M.; Rosorio, D.J. Lifestyle changes for improving disease-specific quality of life in sedentary men on long-term androgen-deprivation therapy for advanced prostate cancer: A randomised controlled trial. Eur. Urol. 2014, 65, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Stevenson, R.; Turner, R.; Hooper, R.; Sasieni, P.; Greasley, R.; Morrissey, D.; Loosemore, M.; Fisher, A.; Payne, H.; et al. Exercise training as a novel primary treatment for localised prostate cancer: A multi-site randomised controlled phase II study. Sci. Rep. 2018, 8, 8374. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Newton, R.U.; Spry, N.; Joseph, D.; Taaffe, D.R.; Galvão, D.A. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases [published correction appears in Prostate Cancer Prostatic Dis. 2015 Jun;18(2):196]. Prostate Cancer Prostatic Dis. 2013, 16, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormie, P.; Galvão, D.A.; Spry, N.; Joseph, D.; Chee, R.; Taaffe, D.R.; Chambers, S.K.; Newton, R.U. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2015, 115, 256–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, J.K.; Dorff, T.B.; Schroeder, E.T.; Lane, C.J.; Gross, M.E.; Dieli-Conwright, C.M. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer 2018, 18, 368. [Google Scholar] [CrossRef] [PubMed]
- Dieperink, K.B.; Johansen, C.; Hansen, S.; Wagner, L.; Andersen, K.K.; Minet, L.R.; Hansen, O. The effects of multidisciplinary rehabilitation: RePCa-a randomised study among primary prostate cancer patients. Br. J. Cancer 2013, 109, 3005–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, A.K.; Hansen, R.D.; Borre, M.; Larsen, R.G.; Jensen, J.M.; Overgaard, K.; Borre, M.; Kyrø, C.; Landberg, R.; Olsen, A.; et al. A lifestyle intervention among elderly men on active surveillance for non-aggressive prostate cancer: A randomised feasibility study with whole-grain rye and exercise. Trials 2017, 18, 20. [Google Scholar] [CrossRef] [Green Version]
- Focht, B.C.; Lucas, A.R.; Grainger, E.; Simpson, C.; Fairman, C.M.; Thomas-Ahner, J.M.; Buell, J.; Monk, J.P.; Mortazavi, A.; Clinton, S.K. Effects of a Group-Mediated Exercise and Dietary Intervention in the Treatment of Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: Results From the IDEA-P Trial. Ann. Behav. Med. 2018, 52, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial. J. Clin. Oncol. 2010, 28, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Galvão, D.A.; Spry, N.; Denham, J.; Taaffe, D.R.; Cormie, P.; Joseph, D.; Lamb, D.S.; Chambers, S.K.; Newton, R.U. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur. Urol. 2014, 65, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Cormie, P.; Joseph, D.; Chambers, S.K.; Chee, R.; Peddle-Mcintyre, C.J.; Hart, N.H.; Baumann, F.T.; et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med. Sci. Sports Exerc. 2018, 50, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Hébert, J.R.; Hurley, T.G.; Harmon, B.E.; Heiney, S.; Hebert, C.J.; Steck, S.E. A diet, physical activity, and stress reduction intervention in men with rising prostate-specific antigen after treatment for prostate cancer. Cancer Epidemiol. 2012, 36, e128–e136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojan, K.; Kwiatkowska-Borowczyk, E.; Leporowska, E.; Gόrecki, M.; Ozga-Majchrzak, O.; Milecki, T.; Milecki, P. Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high-risk prostate cancer patients during radiotherapy: A prospective, randomized clinical study. Eur. J. Phys. Rehabil. Med. 2016, 52, 489–501. [Google Scholar] [PubMed]
- Hojan, K.; Kwiatkowska-Borowczyk, E.; Leporowska, E.; Milecki, P. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer. A randomized controlled trial of a 12-month exercise program. Pol. Arch. Intern. Med. 2017, 127, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hvid, T.; Winding, K.; Rinnov, A.; Dejgaard, T.; Thomsen, C.; Iversen, P.; Brasso, K.; Mikines, K.J.; van Hall, G.; Lindegaard, B.; et al. Endurance training improves insulin sensitivity and body composition in prostate cancer patients treated with androgen deprivation therapy. Endocr. Relat. Cancer 2013, 20, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Hornsby, W.E.; Freedland, S.J.; Lane, A.; West, M.J.; Moul, J.W.; Ferrandino, M.N.; Allen, J.D.; Kenjale, A.A.; Thomas, S.M.; et al. Effects of nonlinear aerobic training on erectile dysfunction and cardiovascular function following radical prostatectomy for clinically localized prostate cancer. Eur. Urol. 2014, 65, 852–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, P.M.; Craike, M.J.; Salmon, J.; Courneya, K.S.; Gaskin, C.J.; Fraser, S.F.; Mohebbi, M.; Broadbent, S.; Botti, M.; Kent, B.; et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: A multicenter cluster randomized controlled trial (ENGAGE). Cancer 2015, 121, 2646–2654. [Google Scholar] [CrossRef] [Green Version]
- Monga, U.; Garber, S.L.; Thornby, J.; Vallbona, C.; Kerrigan, A.J.; Monga, T.N.; Zimmermann, K.P. Exercise prevents fatigue and improves quality of life in prostate cancer patients undergoing radiotherapy. Arch. Phys. Med. Rehabil. 2007, 88, 1416–1422. [Google Scholar] [CrossRef]
- Ndjavera, W.; Orange, S.T.; O’Doherty, A.F.; Leicht, A.S.; Rochester, M.; Mills, R.; Saxton, J. Exercise-induced attenuation of treatment side-effects in patients with newly diagnosed prostate cancer beginning androgen-deprivation therapy: A randomised controlled trial. BJU Int. 2020, 125, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, T.S.; Raastad, T.; Skovlund, E.; Courneya, K.S.; Langberg, C.W.; Lilleby, W.; Fosså, S.D.; Thorsen, L. Effects of strength training on body composition, physical functioning, and quality of life in prostate cancer patients during androgen deprivation therapy. Acta Oncol. 2015, 54, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.F.; Haseen, F.; Murray, L.J.; O’Sullivan, J.M.; Cantwell, M.M. A randomised controlled trial to evaluate the efficacy of a 6-month dietary and physical activity intervention for patients receiving androgen deprivation therapy for prostate cancer. J. Cancer Surviv. 2015, 9, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, T.N.; Nam, J.K.; Ha, H.K.; Shin, D.G.; Lee, W.; Kim, M.-S.; Chung, M.K. Recovery of overall exercise ability, quality of life, and continence after 12-week combined exercise intervention in elderly patients who underwent radical prostatectomy: A randomized controlled study. Urology 2012, 80, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Malone, S.C.; Parliament, M.B.; Scott, C.G.; Venner, P.M.; Quinney, H.A.; Jones, L.W.; Lovinec, M.E.; et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2003, 21, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.J.; Reid, R.D.; Courneya, K.S.; Sigal, R.J.; Kenny, G.P.; Prud’ Homme, D.G.; Malone, S.C.; Wells, G.A.; Scott, C.G.; D’Angelo, M.E.S. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J. Clin. Oncol. 2009, 27, 344–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taaffe, D.R.; Newton, R.U.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Wall, B.A.; Cormie, P.; Bolam, K.A.; Galvāo, D.A. Effects of Different Exercise Modalities on Fatigue in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Year-long Randomised Controlled Trial. Eur. Urol. 2017, 72, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taaffe, D.R.; Galvão, D.A.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Hayne, D.; Cormie, P.; Shum, D.H.K.; Newton, R.U. Immediate versus delayed exercise in men initiating androgen deprivation: Effects on bone density and soft tissue composition. BJU Int. 2019, 123, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Uth, J.; Hornstrup, T.; Schmidt, J.F.; Christensen, J.F.; Frandsen, C.; Christensen, K.B.; Helge, E.W.; Brasso, K.; Rørth, M.; Midtgaard, J.; et al. Football training improves lean body mass in men with prostate cancer undergoing androgen deprivation therapy. Scand. J. Med. Sci. Sports 2014, 24 (Suppl. 1), 105–112. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.M.; Nicol, K.F.; Potter, J. A randomized, controlled trial of aerobic exercise for treatment-related fatigue in men receiving radical external beam radiotherapy for localized prostate carcinoma. Cancer 2004, 101, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.A.; Galvão, D.A.; Fatehee, N.; Taaffe, D.R.; Spry, N.; Joseph, D.; Hebert, J.J.; Newton, R.U. Exercise Improves V O2max and Body Composition in Androgen Deprivation Therapy-treated Prostate Cancer Patients. Med. Sci. Sports Exerc. 2017, 49, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Dobek, J.C.; Bennett, J.A.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Skeletal response to resistance and impact training in prostate cancer survivors. Med. Sci. Sports Exerc. 2014, 46, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winters-Stone, K.M.; Lyons, K.S.; Dobek, J.; Dieckmann, N.F.; Bennett, J.A.; Nail, L.; Beer, T.M. Benefits of partnered strength training for prostate cancer survivors and spouses: Results from a randomized controlled trial of the Exercising Together project. J. Cancer Surviv. 2016, 10, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Mardani, A.; Pedram Razi, S.; Mazaheri, R.; Haghani, S.; Vaismoradi, M. Effect of the exercise programme on the quality of life of prostate cancer survivors: A randomized controlled trial. Int. J. Nurs. Pract. 2021, 27, e12883. [Google Scholar] [CrossRef] [PubMed]
- Alberga, A.S.; Segal, R.J.; Reid, R.D.; Scott, C.G.; Sigal, R.G.; Khandwala, F.; Jeffey, J.; Wells, G.A.; Kenny, G.P. Age and androgen-deprivation therapy on exercise outcomes in men with prostate cancer. Support. Care Cancer 2012, 20, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; Doll, H.; Crank, H.; Daley, A.; Rosario, D.; Saxton, J.M. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: A feasibility study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 647–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, G.; Windsor, P.M.; McCowan, C. The effect of aerobic exercise on treatment-related acute toxicity in men receiving radical external beam radiotherapy for localised prostate cancer. Eur. J. Cancer Care Engl. 2010, 19, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.E.; Tew, G.A.; Fairhurst, C.; Bourke, L.; Saxton, J.M.; Winter, E.M.; Rosario, D.J. Effects of a lifestyle intervention on endothelial function in men on long-term androgen deprivation therapy for prostate cancer. Br. J. Cancer 2016, 114, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Newton, R.U. Acute versus chronic exposure to androgen suppression for prostate cancer: Impact on the exercise response. J. Urol. 2011, 186, 1291–1297. [Google Scholar] [CrossRef]
- Cormie, P.; Newton, R.U.; Taaffe, D.R.; Spry, N.; Joseph, D.; Hamid, M.A.; Galvão, D.A. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: A randomized controlled trial. Prostate Cancer Prostatic Dis. 2013, 16, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvāo, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Taaffe, D.R.; Buffart, L.M.; Newton, R.U.; Spry, N.; Denham, J.; Joseph, D.; Lamb, D.; Chambers, S.K.; Galvao, D.A. Time on androgen deprivation therapy and adaptations to exercise: Secondary analysis from a 12-month randomized controlled trial in men with prostate cancer. BJU Int. 2018, 121, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Courneya, K.S.; Segal, R.J.; Reid, R.D.; Jones, L.W.; Malone, S.C.; Venner, P.M.; Parliament, M.B.; Scott, C.G.; Quinney, H.A.; Wells, G.A. Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J. Clin. Epidemiol. 2004, 57, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, C.J.; Fraser, S.F.; Owen, P.J.; Craike, M.; Orellana, L.; Livingston, P.M. Fitness outcomes from a randomised controlled trial of exercise training for men with prostate cancer: The ENGAGE study. J. Cancer Surviv. 2016, 10, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, C.J.; Craike, M.; Mohebbi, M.; Courneya, K.S.; Livingston, P.M. A Clinician Referral and 12-Week Exercise Training Program for Men with Prostate Cancer: Outcomes to 12 Months of the ENGAGE Cluster Randomized Controlled Trial. J. Phys. Act. Health 2017, 14, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.U.; Galvão, D.A.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Wall, B.A.; Bolam, K.A.; Taaffe, D.R. Exercise Mode Specificity for Preserving Spine and Hip Bone Mineral Density in Prostate Cancer Patients. Med. Sci. Sports Exerc. 2019, 51, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Newton, R.U.; Chinapaw, M.J.; Taaffe, D.R.; Spry, N.A.; Denham, J.W.; Joseph, D.J.; Lamb, D.S.; Brug, J.; Galvão, D.A. The effect, moderators, and mediators of resistance and aerobic exercise on health-related quality of life in older long-term survivors of prostate cancer. Cancer 2015, 121, 3367–3368. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, T.S.; Thorsen, L.; Kirkegaard, C.; Ugelstad, I.; Fosså, S.D.; Raastad, T. The effect of strength training on muscle cellular stress in prostate cancer patients on ADT. Endocr. Connect. 2016, 5, 74–82. [Google Scholar] [CrossRef]
- Uth, J.; Hornstrup, T.; Christensen, J.F.; Christensen, K.B.; Jørgensen, N.R.; Brasso, K.; Jakobsen, M.D.; Sundstrup, E.; Anderson, L.L.; Rørth, M.; et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos. Int. 2016, 27, 1507–1518. [Google Scholar] [CrossRef]
- Uth, J.; Fristrup, B.; Haahr, R.D.; Haahr, R.D.; Brasso, K.; Helge, J.W.; Rørth, M.; Midtgaard, J.; Helge, E.W.; Krustrup, P.; et al. Football training over 5 years is associated with preserved femoral bone mineral density in men with prostate cancer. Scand. J. Med. Sci. Sports 2018, 28 (Suppl. 1), 61–73. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Dobek, J.C.; Bennett, J.A.; Dieckmann, N.F.; Maddalozzo, G.F.; Ryan, C.W.; Beer, T.M. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: Evidence from a randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, R.U.; Galvão, D.A.; Spry, N.; Joseph, D.; Chambers, S.K.; Gardiner, R.A.; Hayne, D.; Taaffe, D.R. Timing of exercise for muscle strength and physical function in men initiating ADT for prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 457–464. [Google Scholar] [CrossRef]
- Dieperink, K.B.; Hansen, S.; Wagner, L.; Minet, L.R.; Hansen, O. Long-term follow-up 3 years after a randomized rehabilitation study among radiated prostate cancer survivors. J. Cancer Surviv. 2021, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Bjerre, E.D.; Weller, S.; Poulsen, M.H.; Poulsen, M.H.; Madsen, S.S.; Bjerre, R.D.; Østergren, P.B.; Borre, M.; Brasso, K.; Midtgaard, J. Safety and Effects of Football in Skeletal Metastatic Prostate Cancer: A Subgroup Analysis of the FC Prostate Community Randomised Controlled Trial. Sports Med. -Open 2021, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, O.; Galvão, D.A.; Taaffe, D.R.; Spry, N.; Joseph, D.; Tang, C.; Chee, R.; Newton, R.U. Effect of Exercise Adjunct to Radiation and Androgen Deprivation Therapy on Patient-Reported Treatment Toxicity in Men With Prostate Cancer: A Secondary Analysis of 2 Randomized Controlled Trials. Pract. Radiat. Oncol. 2021, 11, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Galvão, D.A.; Newton, R.U.; Chambers, S.K.; Spry, N.; Joseph, D.; Gardiner, R.A.; Fairman, C.M.; Taaffe, D.R. Psychological distress in men with prostate cancer undertaking androgen deprivation therapy: Modifying effects of exercise from a year-long randomized controlled trial. Prostate Cancer Prostatic Dis. 2021, 24, 758–766. [Google Scholar] [CrossRef]
- Newton, R.U.; Mavropalias, G.; Fragala, M.S.; Kraemer, W.J.; Häkkkinen, K.; Taaffe, D.R.; Spry, N.; Joseph, D.; Galvāo, D.A. Radiotherapy before or during androgen-deprivation therapy does not blunt the exercise-induced body composition protective effects in prostate cancer patients: A secondary analysis of two randomized controlled trials. Exp. Gerontol. 2021, 151, 111427. [Google Scholar] [CrossRef] [PubMed]
- Mina, D.S.; Alibhai, S.M.H.; Matthew, A.G.; Guglietti, C.L.; Pirbaglou, M.; Trachtenberg, J.; Ritvo, P. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J. Aging Phys. Act. 2013, 21, 455–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, G.A.; Kelley, K.S. Exercise and cancer-related fatigue in adults: A systematic review of previous systematic reviews with meta-analyses. BMC Cancer 2017, 17, 693. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, A.; Kouta, C. Cancer Related Fatigue and Quality of Life in Patients with Advanced Prostate Cancer Undergoing Chemotherapy. Biomed. Res. Int. 2016, 2016, 3989286. [Google Scholar] [CrossRef] [Green Version]
- McCabe, R.M.; Grutsch, J.F.; Braun, D.P.; Nutakki, S.B. Fatigue as a Driver of Overall Quality of Life in Cancer Patients. PLoS ONE 2015, 10, e0130023. [Google Scholar] [CrossRef] [Green Version]
- Buffart, L.M.; Sweegers, M.G.; May, A.M.; Chinapaw, M.J.; van Vulpen, J.K.; Newton, R.U.; Galvão, D.A.; Aaronson, N.K.; Stuiver, M.M.; Jacobsen, P.B.; et al. Targeting Exercise Interventions to Patients with Cancer in Need: An Individual Patient Data Meta-Analysis. J. Natl. Cancer Inst. 2018, 110, 1190–1200. [Google Scholar] [CrossRef] [Green Version]
- Wedell-Neergaard, A.S.; Krogh-Madsen, R.; Petersen, G.L.; Hansen, Å.M.; Pedersen, B.K.; Lund, R.; Bruunsgaard, H. Cardiorespiratory fitness and the metabolic syndrome: Roles of inflammation and abdominal obesity. PLoS ONE 2018, 13, e0194991. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Vogt, L.; Thiel, C.; Jäger, E.; Banzer, W. Validity of the six-minute walk test in cancer patients. Int. J. Sports Med. 2013, 34, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Newton, R.U.; Spence, R.R.; Galvão, D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport 2019, 22, 1175–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Health and Care Excellence (2019). Prostate Cancer: Diagnosis and Management (NICE Guideline 131). Available online: https://www.nice.org.uk/guidance/ng131/chapter/recommendations#terms-used-in-this-guideline (accessed on 1 August 2021).
- Wayne, P.M.; Lee, M.S.; Novakowski, J.; Osypiuk, K.; Ligibel, J.; Carlson, L.E.; Song, R. Tai Chi and Qigong for cancer-related symptoms and quality of life: A systematic review and meta-analysis. J. Cancer Surviv. 2018, 12, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ben-Josef, A.M.; Chen, J.; Wileyto, P.; Doucette, A.; Bakelman, J.; Christodouleas, J.; Deville, C.; Vapiwala, N. Effect of Eischens Yoga During Radiation Therapy on Prostate Cancer Patient Symptoms and Quality of Life: A Randomized Phase II Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Neil-Sztramko, S.E.; Medysky, M.E.; Campbell, K.L.; Bland, K.A.; Winters-Stone, K.M. Attention to the principles of exercise training in exercise studies on prostate cancer survivors: A systematic review. BMC Cancer 2019, 19, 321. [Google Scholar] [CrossRef] [Green Version]

| Author (Year) | Sample (Group Randomised) | Population | Intervention Duration (Follow-Up) | Intervention Frequency | Intervention Description | Control Group | Outcomes of Interest |
|---|---|---|---|---|---|---|---|
| Bjerre et al., (2018) [22] | 121 (109/105) | Tumour stage: Gleason mean 2.0–10.0 Metastatic disease: 19% PCa treatment: No treatment, ADT, castration | 24 weeks | 2 times per week | Supervised AT: 20 min of warm-up, 20 min of dribbling and shooting, 20 min of 5–7-a-side football | Usual care | QoL a Lean body mass(kg) b Body fat mass (kg) b General physical and mental health c |
| Bourke et al., (2014) [23] | 100 (50/50) | Tumour stage: T 3–4 Metastatic disease: 20% PCa treatment: ADT | 12 weeks | First 6 weeks: 2 supervised, 1 home Last 6 weeks: 1 supervised, 2 home | Supervised AE: 30 min 55–75% max heartrate or Borg 11–13 RT: 2–4 sets 8–12 reps, 60% 1RM progressed through intervention Home AE + RT: 30 min of skills taught in supervised sessions | Usual care | QoL a Fatigue d Cardiovascular fitness e Systolic and diastolic BP |
| Bourke et al., (2018) [24] | 50 (25/25) | Tumour stage: T 1–2 Metastatic disease: None PCa treatment: No information | 12 weeks | 2 times per week | Supervised AE: 20–30, 65–85% of age-specific heart rate max Home AE: 150 min moderate cardiovascular fitness per week | Usual care | QoL f Systolic and diastolic BP Cardiovascular fitness e |
| Cormie et al., (2013) [25] | 20 (10/10) | Tumour stage: Gleason mean 8.2 Metastatic disease: All (inclusion criteria) PCa treatment: Previous ADT | 12 weeks | 2 times per week | Supervised RT: 1–4 set (NI reps), 12–6 RM, 8 exercises Home AE: 150 min of moderate cardiovascular fitness per week | Waiting-list protocol | General physical and mental health g Fatigue h Cardiovascular fitness i Lower body strength j Lean body mass (kg) b Body fat mass (kg) b |
| Cormie et al., (2015) [26] | 63 (32/31) | Tumour stage: Gleason mean 7.5 Metastatic disease: None (excluded) PCa treatment: ADT | 12 weeks | 2 times per week | Supervised RT: 1–4 set (NI reps), 12–6 RM, 8 exercises AE: 20–30 min 70–85% of max heart rate Home AE: 150 min of moderate cardiovascular fitness per week | Waiting-list protocol | General physical and mental health g Fatigue k Cardiovascular fitness i Lower body strength k Lean body mass (kg) b Body fat mass (kg) b Systolic and diastolic BP |
| Dawson et al., (2018) [27] | 37 (16/21) | Tumour stage: Gleason mean 7.5 Metastatic disease: 45% PCa treatment: ADT | 12 weeks | 3 times per week | Supervised RT: 3 sets 15–8 reps (decrease over time) 65–83% 1RM (increase over time) 10 exercises with alternatives | Stretching protocol 3 times a week | QoL a Fatigue m Cardiovascular fitness i Lower body strength l Lean body mass (kg) b Body fat mass (kg) b Systolic and diastolic BP |
| Dieperink et al., (2013) [28] | 161 (79/82) | Tumour stage: T 1–3 Metastatic disease: No information PCa treatment: ADT | 20 weeks | 7 times per week | Home RT: Individualised programme, major muscle groups, 10–12 reps, 3 sets, moderate intensity 30 min per day | Usual care | General physical and mental health e |
| Eriksen et al., (2017) [29] | 26 (17/9) | Tumour stage: T 1–2 Metastatic disease: None PCa treatment: Active surveillance | 24 weeks | 3 times per week | Home AE: 45 min of 70% of max heart rate | Usual care | Cardiovascular fitness n Lean body mass (skinfold) Body fat mass (skinfold) |
| Focht et al., (2018) [30] | 32 (16/16) | Tumour stage: Gleason mean 7.7 Metastatic disease: No information PCa treatment: ADT | 12 weeks | 2 times per week | Supervised RT: 3 sets, 8–12 reps (8–12 RM) 3–6 of perceived exertion (1–10), 9 exercises AE: 10–20 min 3–4 on perceived exertion PA guidelines (150 min per week) | Usual care | Cardiovascular fitness i Lower body strength j Lean body mass (kg) b Body fat mass (kg) b |
| Galvao et al., (2010) [31] | 57 (29/28) | Tumour stage: Gleason mean 7.2–7.5 Metastatic disease: 9% PCa treatment: ADT + RT | 12 weeks | 2 times per week | Supervised RT: 2–4 set (NI reps), 12–6 RM 6 exercises AE: 15–20 min 65–80% of max heart rate | Waiting-list protocol | QoL o General physical and mental health g Cardiovascular fitness i Lower body strength l Lean body mass (kg) b Body fat mass (kg) b |
| Galvao et al., (2014) [32] | 100 (50/50) | Tumour stage: T 2–4 Metastatic disease: None (excluded) PCa treatment: Previous ADT + RT | 52 weeks | 2 times per week | Supervised RT: 2–4 set (NI reps), 12–6 RM, 6 exercises AE: 15–20 min 65–80% of max heart rate Home AE + RT: After 6 months of replication of supervised programme | Information on physical activity with printed material | General physical and mental health g Cardiovascular fitness i Lower body strength j Lean body mass (kg) b Body fat mass (kg) b Systolic and diastolic BP |
| Galvao et al., (2018) [33] | 57 (28/29) | Tumour stage: No information Metastatic disease: All (bone metastases, inclusion criteria) PCa treatment: ADT | 12 weeks | 3 times per week | Supervised RT: 3 sets, 10–12 RM major trunk, upper and lower body muscles, progressing 5–10% when exceeding RM values AE: 20–30 min at 60–85% of estimated heart rate max | Waiting-list protocol | General physical health c Cardiovascular fitness i Muscle strength j Lean body mass (kg) b Body fat mass (kg) b |
| Hebert et al., (2012) [34] | 54 (29/25) | Tumour stage: Unclear Metastatic disease: No information PCa treatment: Previous surgery, RT or both | 24 weeks | 5 times per week | Home AE: >30 min of moderate intensity exercise | Waiting-list protocol | Body fat mass (%) b |
| Hojan et al., (2016) [35] | 55 (27/28) | Tumour stage: Gleason mean 6.6 Metastatic disease: None (exclusion) PCa treatment: RT + ADT | 8 weeks | 5 times per week | Supervised RT: 2 sets, 8 reps, 5 exercises 70–75% of estimated 1RM AE: 30 min of moderate intensity, 65–70% of age-estimated heart rate max | Usual care | QoL o Fatigue d Cardiovascular fitness p |
| Hojan et al., (2017) [36] | 72 (36/36) | Tumour stage: Gleason mean 8.8 Metastatic disease: None (exclusion) PCa treatment: RT + ADT | 52 weeks | 5 times per week (weeks 1–10), 3 times per week (weeks 11–52) | Supervised RT: 2 sets, 8 reps, 5 exercises 70–75% of estimated 1RM AE: 30 min of moderate intensity, 65–70% of age-estimated heart rate max (weeks 11–52, 70–80% of estimated heart rate max) | Usual care | QoL o Fatigue d Cardiovascular fitness p |
| Hvid et al., (2016) [37] | 25 (12/7) | Tumour stage: T 1–2 Metastatic disease: None (exclusion) PCa treatment: Active surveillance | 104 weeks | 3 times per week | Home AE: 35 min of interval-based exercise varying between 50–100% of VO2max | Usual care | Cardiovascular fitness n Lean body mass (kg) b Body fat mass (kg) b |
| Jones et al., (2014) [38] | 50 (25/25) | Tumour stage: Gleason 62% >7.0 Metastatic disease: No information PCa treatment: Previous prostatectomy | 24 weeks | 5 times per week (supervised at least 3 times) | Supervised/Home AE: 30–45 min of walking with 55–100% of VO2peak | Usual care | QoL a Cardiovascular fitness n Lean body mass (%) b Body fat mass (%) b Systolic and diastolic BP |
| Livingston et al., (2015) [39] | 147 (54/92) | Tumour stage: T 1–3 Metastatic disease: No information PCa treatment: Surgery, RT, ADT or combined | 12 weeks | 3 times per week (2 supervised, 1 home) | Supervised RT: 4–6 exercises, 2 sets, 8–12 reps AE: 20–30 min 40–70% of max heart rate Home RT + AE With bodyweight and resistance band | Usual care | QoL o Cardiovascular fitness p Lower body Strength l Systolic and diastolic BP |
| Mardani et al., (2020) [54] | 80 (40/40) | Tumour stage: Unclear Metastatic disease: Unclear PCa treatment: Radiotherapy, surgery, hormone therapy | 12 weeks | 2 times per week | Supervised RT: 11 exercises for lang muscle groups, low to moderate load (11–13 on BORG), 8–12 reps, 2 sets AT: walk, light to moderate (11–13 on BORG), 60–150 min per week, | Usual Care | QoL o Fatigue t |
| Monga et al., (2007) [40] | (30) 21 (11/10) | Tumour stage: Gleason mean 5.3 Metastatic disease: Unclear PCa treatment: RT | 8 weeks | 3 times per week | Supervised AE: 30 min walking on treadmill, 65% of heart rate reserve | Usual care | QoL a Fatigue r Lower body strength s Cardiovascular fitness e |
| Ndjavero et al., (2020) [41] | 50 (24/26) | Tumour stage: Gleason 96% ≥7.0 Metastatic disease: 45% PCa treatment: ADT | 12 weeks | 2 times per week supervised, 3 times per week home | Supervised AE: 5 × 5 min on 11–15 on 55–85% of heart rate max on ergometer bike RT: Six exercises targeting major muscle groups, 2–4 sets, 10 reps at 11–15 RPE Home AE: 30 min self-directed structured physical activity | Waiting-list protocol | QoL a Fatigue k Cardiovascular fitness n Lean body mass (kg) y Body fat mass (kg) y |
| Nilsen et al., (2015) [42] | 58 (28/30) | Tumour stage: Intermediate high-risk profile Metastatic disease: Unclear PCa treatment: RT + ADT | 18 weeks | 3 times per week | Supervised RT: 9 exercises, Monday: 1–3 sets, 10 RM, Fridays: 2–3 sets, 6RM, Wednesdays: submaximal session, 10 reps, 80–90% of 10 RM, 2–3 sets | Usual care | QoL o Fatigue t Cardiovascular fitness u Lean body mass (kg) b Body fat mass (kg) b Lower body strength l |
| O’Neill et al., (2015) [43] | 94 (47/47) | Tumour stage: Gleason 90% ≥7.0 Metastatic disease: Unclear PCa treatment: ADT | 24 weeks | 5 times per week | Home AE: 30 min of brisk walking (moderate intensity) | Waiting-list protocol | QoL a Fatigue v Lean body mass (kg) b Body fat mass (kg) b Cardiovascular fitness p |
| Park et al., (2012) [44] | 66 (33/33) | Tumour stage: T 2–3 Metastatic disease: Unclear PCa treatment: Radical prostatectomy | 12 weeks | 2 times per week | Supervised RT: Elastic band, 50–70% of 1RM, upper extremities (lateral, anterior, posterior) lower extremities (lift and spread), weeks 9–12 | Pelvic floor exercises | General physical and mental health g |
| Segal et al., (2003) [45] | 155 (82/73) | Tumour stage: T 2–4 Metastatic disease: No information PCa treatment: ADT | 12 weeks | 3 times per week | Supervised RT: 2 sets, 8–12 reps, 9 exercises, 60–70% of 1RM, 5 lbs increase when able to complete >12 reps | Waiting-list protocol | QoL a Fatigue d |
| Segal et al., (2009) [46] | 121 (40/40/41) | Tumour stage: T 1–4 Metastatic disease: None (excluded) PCa treatment: RT and ADT | 24 weeks | 3 times per week | Supervised AE: Weeks 1–4: 50–60% of VO2peak, weeks 5–24: 70–75%, duration 15 min progression 5 min every 3 weeks until 45 min RT: 2 sets, 8–12 reps of 10 exercises at 60–70% of 1RM | Waiting-list protocol | QoL a Fatigue d Cardiovascular fitness n Lower body strength w Body fat mass (%) b |
| Taaffe et al., (2017) [47] | 163 (58/54/51) | Tumour stage: Gleason mean 7.8 Metastatic disease: 7% of sample PCa treatment: ADT | 24 weeks | RT + IMP: 2 times per week supervised, 2 times per week home (just IMP) RT + AE: 2 times per week | RT + IMP supervised RT: 2–4 sets (NI on reps), 6–12 RM, 6 exercises IMP: Ground reaction force 3,4–5,2 times body weight Home IMP: As supervised RT + AE supervised RT: 2–4 sets (NI on reps), 6–12 RM, 6 exercises AE: 20–30 min, 60–70% of estimated heart rate max Home AE: 150 min of moderate cardiovascular fitness per week | Waiting-list protocol | Fatigue t Cardiovascular fitness i Lower body strength l Lean body mass (kg) b Body fat mass (kg) b |
| Taaffe et al., (2019) [48] | 104 (54/50) | Tumour stage: Gleason mean 7.6 Metastatic disease: None, exclusion criteria PCa treatment: ADT | 24 weeks | 3 times per week supervised, 2 times per week home | Supervised AE: Treadmill, rower or bike 60–80% heart rate max RT: Major muscle groups 2–4 sets, 6–12 reps IMP: Hopping, skipping, leaping and drop jump (GRF 3.4–5.2 times body weight) Home Walking and modified IMP | Waiting-list protocol | Lean body mass (kg) b Body fat mass (kg) b |
| Uth et al. (2014) [49] | 57 (29/28) | Tumour stage: Gleason mean 7.9 Metastatic disease: Bone metastases 19% of sample PCa treatment: ADT | 12 weeks | 2 times per week (weeks 1–8), 3 times per week (weeks 9–12) | Supervised AE: 2 × 15 min of 5–7 a-side football (weeks 1–4), 3 × 15 min of 5–7 a-side football (weeks 5–12) | Waiting-list protocol | Lean body mass (kg) b Body fat mass (kg) b Cardiovascular fitness n Lower body strength j |
| Windsor et al., (2004) [50] | 66 (33/33) | Tumour stage: T 1–2 (51 out of 66) Metastatic disease: Unclear PCa treatment: RT | 4 (8) weeks | 3 times per week | Home AE: 30 min of walking at moderate intensity (60–70% of heart rate max) | Usual care | Fatigue m |
| Wall et al., (2017) [51] | 97 (50/47) | Tumour stage: Gleason mean 8.0 Metastatic disease: None (bone metastases excluded) PCa treatment: ADT | 24 weeks | 2 times per week | Supervised RT: 6 exercises, weeks 1–4: 2 sets, 12 reps, weeks 5–8: 3 sets, 10 reps, weeks 9–12: 3 sets, 8 reps, weeks 13–16: ? AE: 20–30 min, 70–90% of VO2peak Home 150 min per week 70–90% of VO2peak | Usual care | Cardiovascular fitness n Lean body mass (kg) b Body fat mass (kg) b Systolic and diastolic BP |
| Winters-Stone et al., (2014) [52] | 51 (29/22) | Tumour stage: Unclear Metastatic disease: 27.6% of intervention sample, 13.6% of control sample PCa treatment: ADT | 52 weeks | 2 times per week supervised + 1 time per week home | Supervised RT: Lower body: 2–3 sets progression from 12–14 RM, 12–14 reps to 6–8 RM, 6–8 reps Upper body: 2–3 sets, 8–12 reps, progressing from 0–2% to 9–10% of body weight IMP: Jumps: 10 reps, progressing from 3 to 10 sets Home As supervised | Stretching protocol | Lean body mass (kg) b Body fat mass (kg) b Fatigue x |
| Winters-Stone et al., (2016) [53] | 64 (32/32) | Tumour stage: Unclear Metastatic disease: 9% of sample PCa treatment: Currently on ADT: Intervention (22%), control (13%) | 24 weeks | 2 times per week | Supervised RT exercising with spouse: 8–10 exercises, 8–15 reps, progressing from 15 to 8 RM | Waiting-list protocol | Lean body mass (kg) b Body fat mass (kg) b General physical and mental health g Lower body strength l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, M.F.; Midtgaard, J.; Bjerre, E.D. Do Patients with Prostate Cancer Benefit from Exercise Interventions? A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 972. https://doi.org/10.3390/ijerph19020972
Andersen MF, Midtgaard J, Bjerre ED. Do Patients with Prostate Cancer Benefit from Exercise Interventions? A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(2):972. https://doi.org/10.3390/ijerph19020972
Chicago/Turabian StyleAndersen, Martin Færch, Julie Midtgaard, and Eik Dybboe Bjerre. 2022. "Do Patients with Prostate Cancer Benefit from Exercise Interventions? A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 2: 972. https://doi.org/10.3390/ijerph19020972
APA StyleAndersen, M. F., Midtgaard, J., & Bjerre, E. D. (2022). Do Patients with Prostate Cancer Benefit from Exercise Interventions? A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(2), 972. https://doi.org/10.3390/ijerph19020972






