Relative Effectiveness of Cell-Cultured versus Egg-Based Seasonal Influenza Vaccines in Preventing Influenza-Related Outcomes in Subjects 18 Years Old or Older: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction
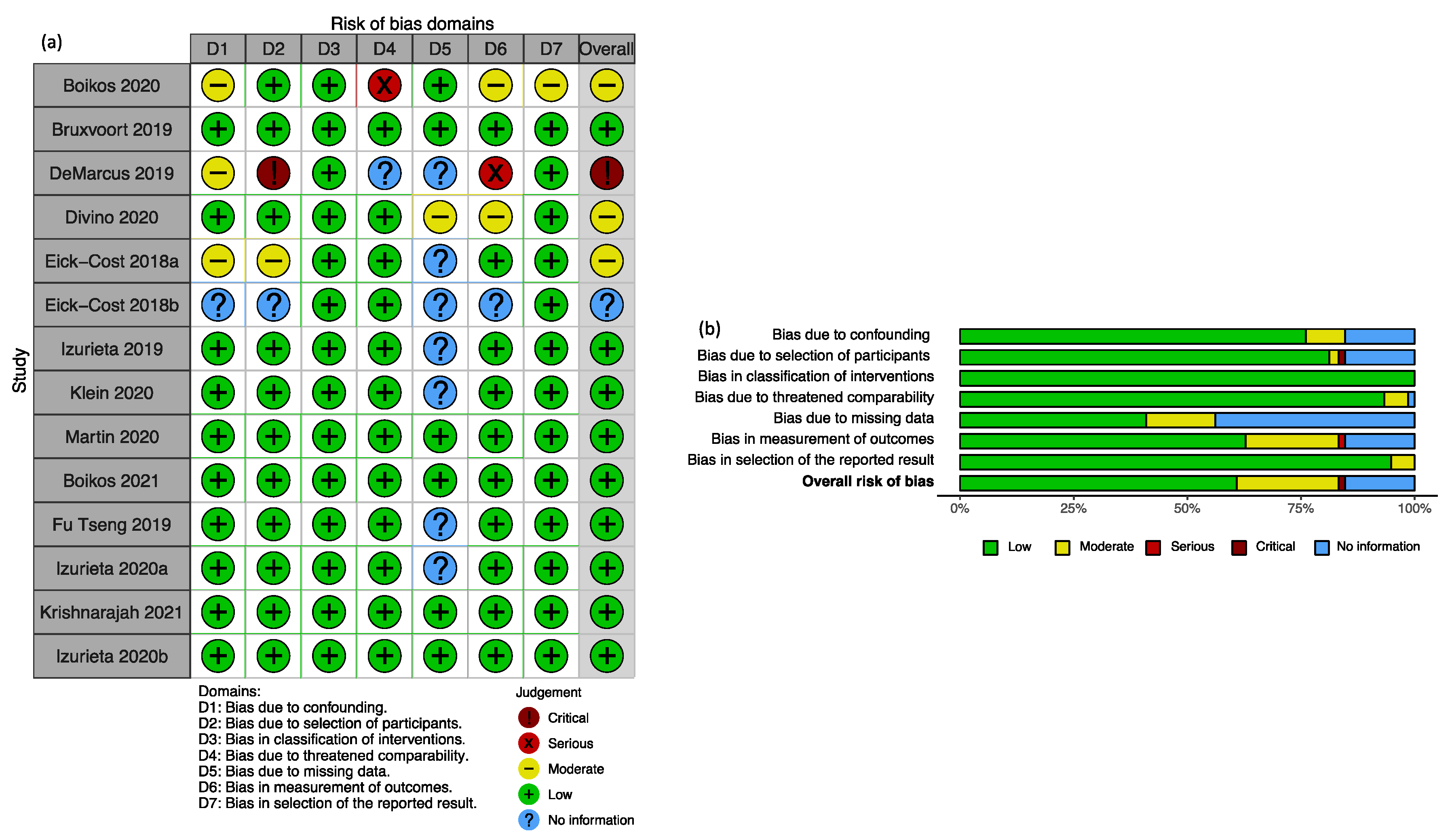
2.3. Study Quality Assessment
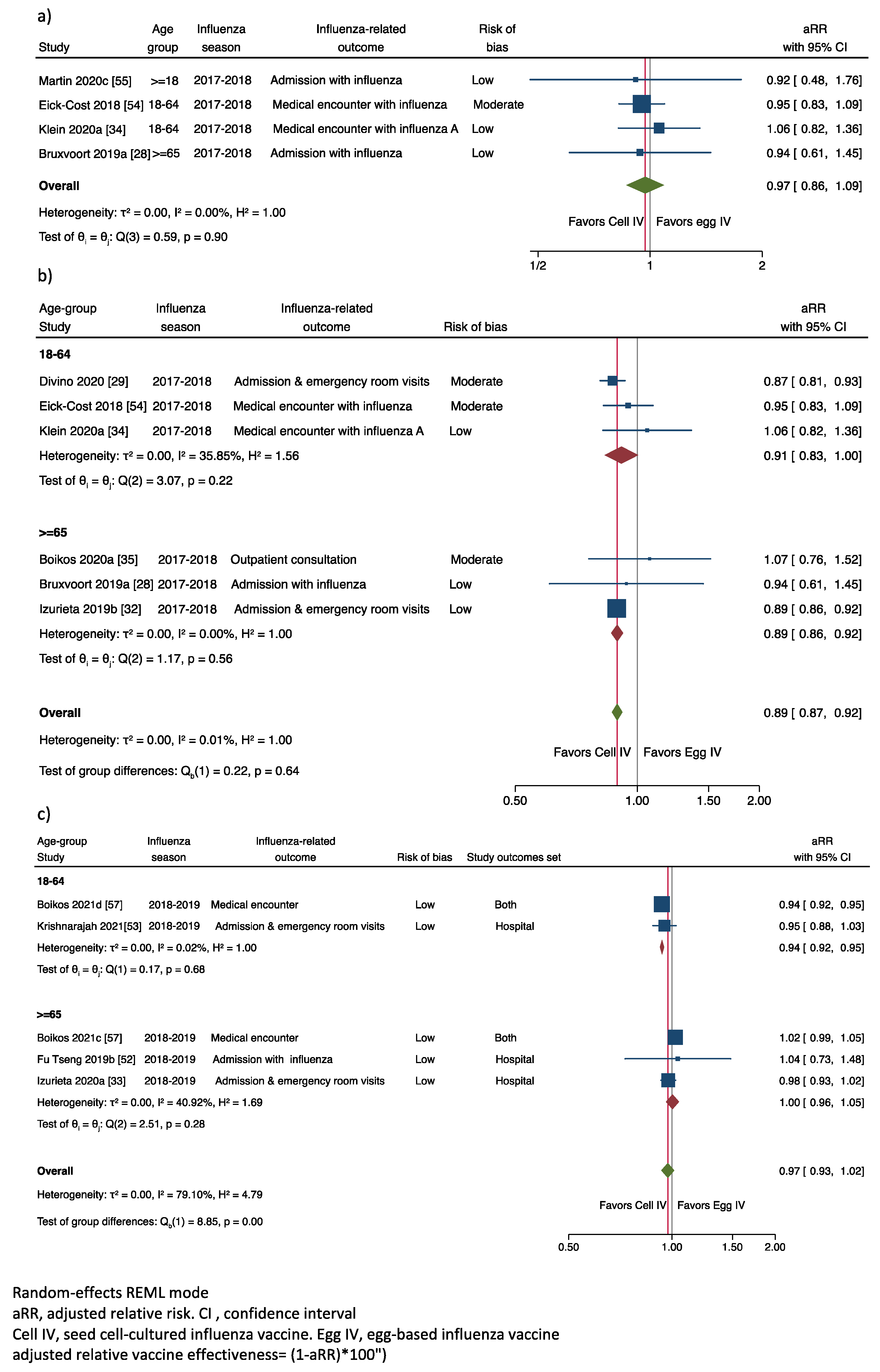
2.4. Data Analysis
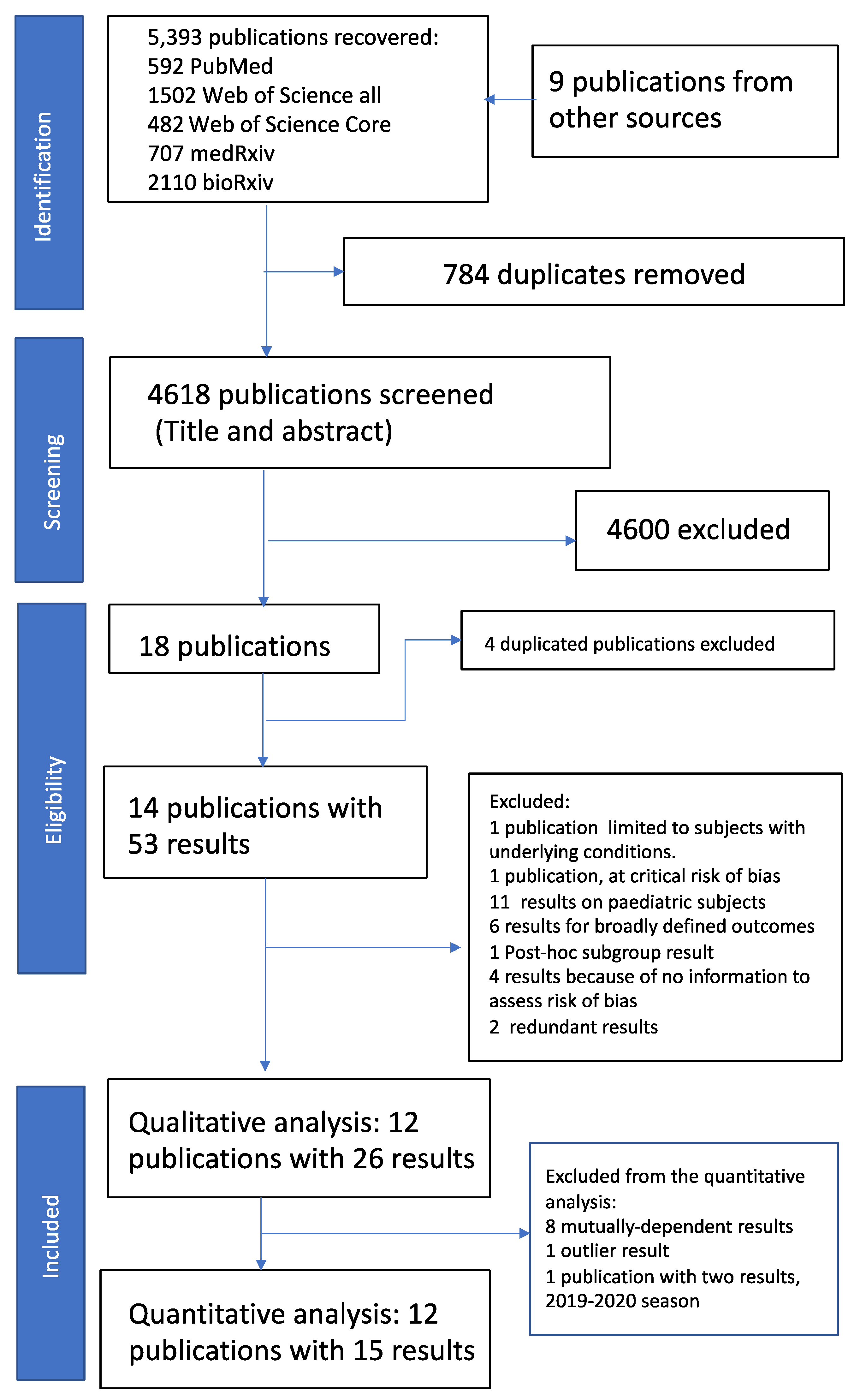
3. Results
3.1. Qualitative Review of Included Publications
3.2. Heterogeneity
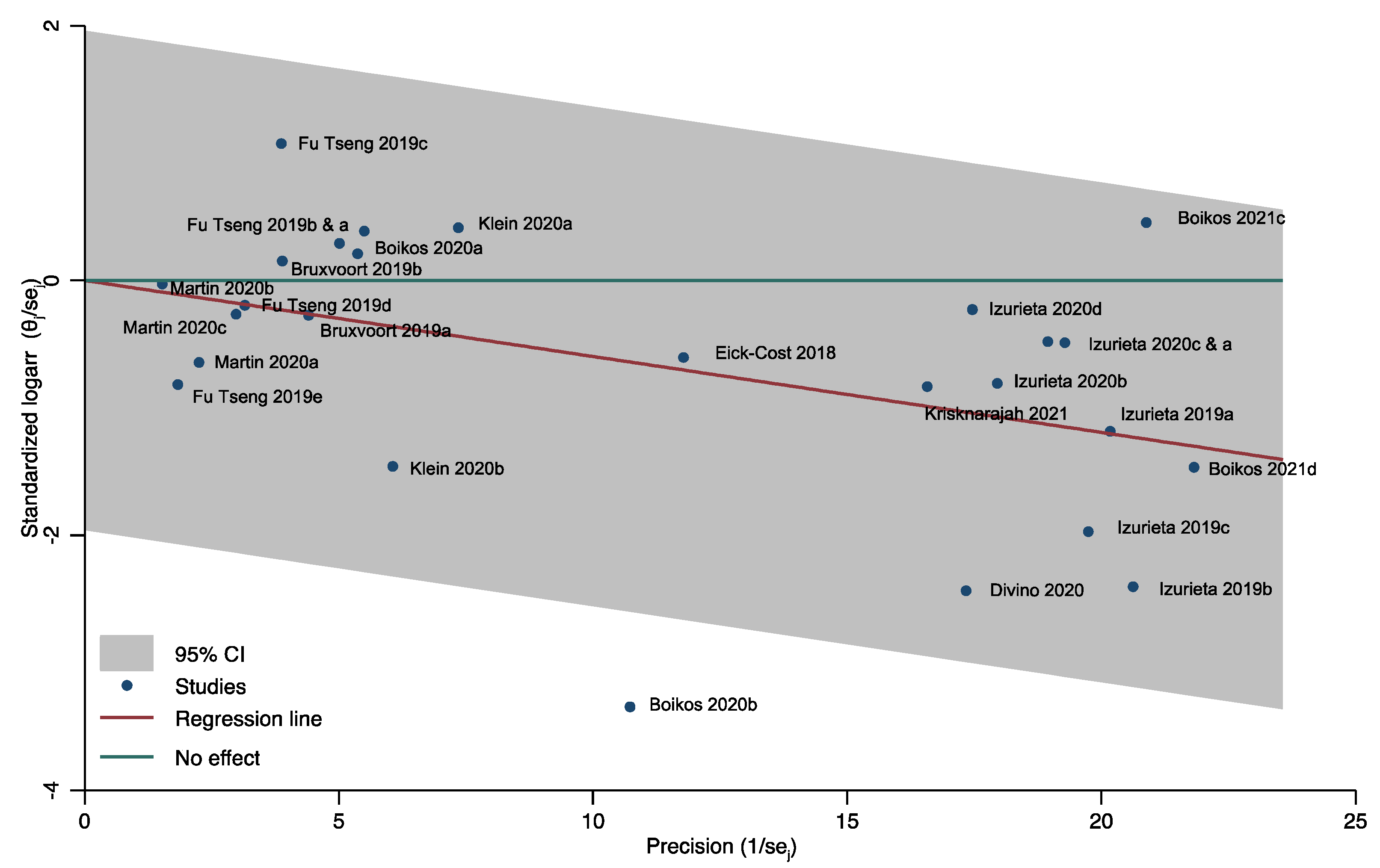
3.3. Publication Bias
3.4. Meta-Analysis of the Published Relative Vaccine Effectiveness Results
3.4.1. Adjusted Relative Vaccine Effectiveness in Preventing Laboratory-Confirmed IRO
3.4.2. Adjusted Relative Vaccine Effectiveness in Preventing Any IRO
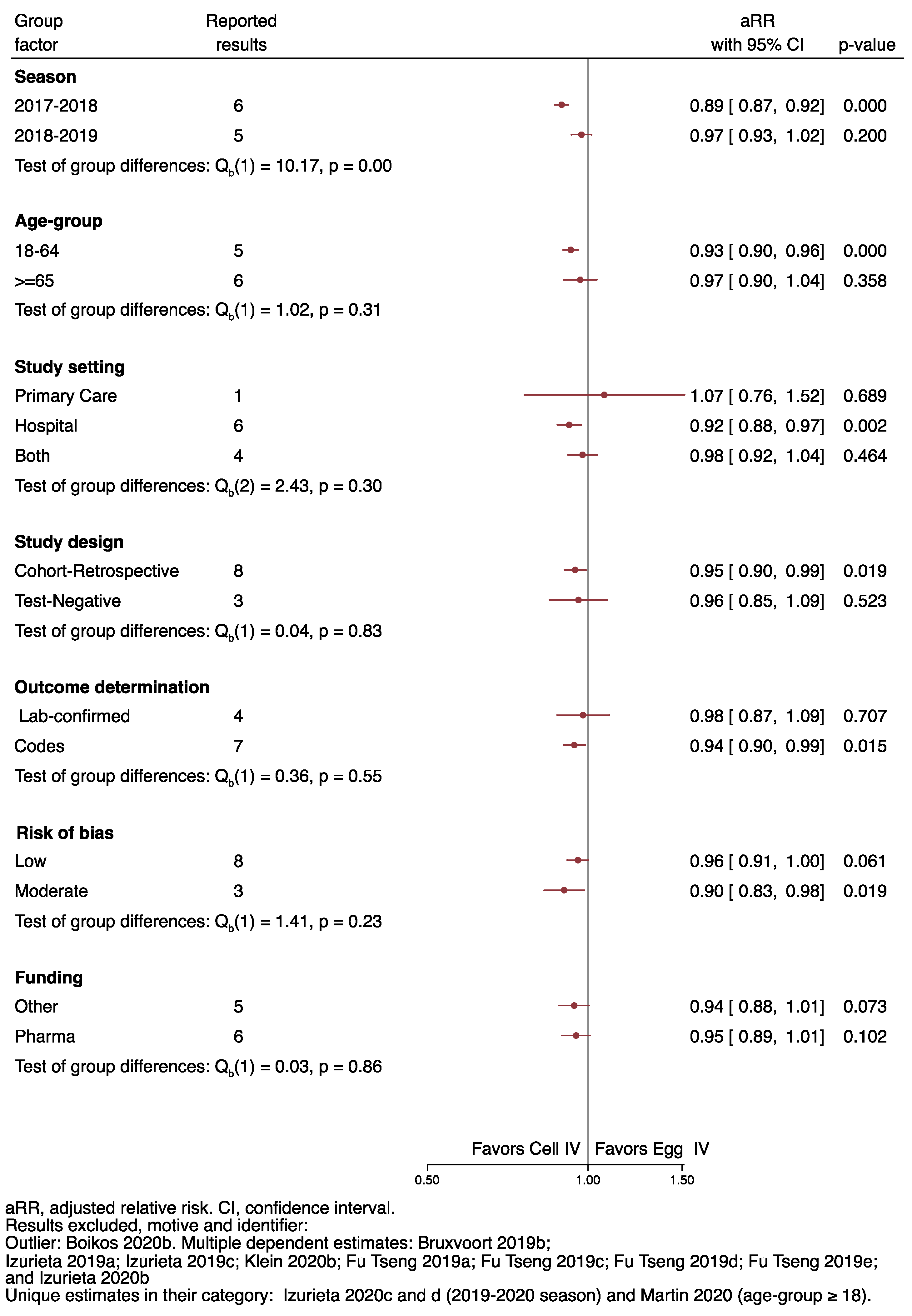
3.5. Sensitivity Analysis
4. Discussion
4.1. Interpretation and Validity
4.2. Limitations
4.3. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Troeger, C.E.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.M.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef] [Green Version]
- A Walker, T.; Waite, B.; Thompson, M.G.; McArthur, C.; Wong, C.; Baker, M.G.; Wood, T.; Haubrock, J.; Roberts, S.; Gross, D.K.; et al. Risk of Severe Influenza Among Adults With Chronic Medical Conditions. J. Infect. Dis. 2019, 221, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, P.; McLean, H.Q.; A Belongia, E.; Cobey, S. Earliest infections predict the age distribution of seasonal influenza A cases. eLife 2020, 9, e50060. [Google Scholar] [CrossRef]
- Van Kerkhove, M.D.; Vandemaele, K.A.H.; Shinde, V.; Jaramillo-Gutierrez, G.; Koukounari, A.; Donnelly, C.A.; Carlino, L.O.; Owen, R.; Paterson, B.; Pelletier, L.; et al. Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis. PLoS Med. 2011, 8, e1001053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.-L.; Dickinson, J.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H.; et al. Age-Related Differences in Influenza B Infection by Lineage in a Community-Based Sentinel System, 2010–2011 to 2015–2016, Canada. J. Infect. Dis. 2017, 216, 697–702. [Google Scholar] [CrossRef]
- Song, J.Y.; Noh, J.Y.; Lee, J.S.; Wie, S.-H.; Kim, Y.K.; Lee, J.; Jeong, H.W.; Kim, S.W.; Lee, S.H.; Park, K.-H.; et al. Effectiveness of repeated influenza vaccination among the elderly population with high annual vaccine uptake rates during the three consecutive A/H3N2 epidemics. Vaccine 2019, 38, 318–322. [Google Scholar] [CrossRef]
- Lafond, K.E.; Porter, R.M.; Whaley, M.J.; Suizan, Z.; Ran, Z.; Aleem, M.A.; Thapa, B.; Sar, B.; Proschle, V.S.; Peng, Z.; et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: A systematic review and meta-analysis. PLoS Med. 2021, 18, e1003550. [Google Scholar] [CrossRef]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Hardelid, P.; Pebody, R.; Andrews, N. Mortality caused by influenza and respiratory syncytial virus by age group in England and Wales 1999–2010. Influenza Other Respir. Viruses 2012, 7, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.; Simpson, M.D.; King, J.; E Sundaram, M.; Kelley, N.S.; Osterholm, M.T.; McLean, H.Q. Variable influenza vaccine effectiveness by subtype: A systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016, 16, 942–951. [Google Scholar] [CrossRef]
- Belongia, E.; McLean, H.Q. Influenza Vaccine Effectiveness: Defining the H3N2 Problem. Clin. Infect. Dis. 2019, 69, 1817–1823. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.; Fonseca, K.; Winter, A.-L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012–13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS ONE 2014, 9, e92153. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.C.; Zost, S.J.; Thompson, A.J.; Oyen, D.; Nycholat, C.M.; McBride, R.; Paulson, J.C.; Hensley, S.E.; Wilson, I.A. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLoS Pathog. 2017, 13, e1006682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdil, C. The annual production cycle for influenza vaccine. Vaccine 2003, 21, 1776–1779. [Google Scholar] [CrossRef]
- Laurent, P.E.; Bonnet, S.; Alchas, P.; Regolini, P.; Mikszta, J.A.; Pettis, R.; Harvey, N.G. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine 2007, 25, 8833–8842. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Treanor, J.J. New approaches to influenza vaccine—High doses, neuraminidase vaccines, alternative substrates, and new ad-juvants. Infect. Med. 1998, 15, 487–492. [Google Scholar]
- DiazGranados, C.A.; Dunning, A.J.; Kimmel, M.; Kirby, D.; Treanor, J.; Collins, A.; Pollak, R.; Christoff, J.; Earl, J.; Landolfi, V.; et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. N. Engl. J. Med. 2014, 371, 635–645. [Google Scholar] [CrossRef]
- Cox, M.M.; Hashimoto, Y. A fast track influenza virus vaccine produced in insect cells. J. Invertebr. Pathol. 2011, 107, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Milián, E.; Kamen, A.A. Current and Emerging Cell Culture Manufacturing Technologies for Influenza Vaccines. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Galli, C.; Orsi, A.; Pariani, E.; Lai, P.L.; Guarona, G.; Pellegrinelli, L.; Ebranati, E.; Icardi, G.; Panatto, D. In-depth phylogenetic analysis of the hemagglutinin gene of influenza A(H3N2) viruses circulating during the 2016–2017 season revealed egg-adaptive mutations of vaccine strains. Expert Rev. Vaccines 2020, 19, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases (NCIRD). How Influenza (Flu) Vaccines Are Made n.d. Available online: https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm (accessed on 13 March 2020).
- Barr, I.G.; Donis, R.O.; Katz, J.M.; McCauley, J.W.; Odagiri, T.; Trusheim, H.; Tsai, T.F.; Wentworth, D.E. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: A step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018, 3, 44. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–2019 Influenza Season. MMWR Recomm. Rep. 2018, 67, 1–20. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Flucelvax Tetra n.d. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/flucelvax-tetra (accessed on 12 March 2021).
- European Centre for Disease Prevention and Control. Systematic Review of the Efficacy, Effectiveness and Safety of Newer and Enhanced Seasonal Influenza Vaccines for the Prevention of Laboratory-Confirmed Influenza in Individuals Aged 18 Years and Over; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2020. [CrossRef]
- Bruxvoort, K.J.; Luo, Y.; Ackerson, B.; Tanenbaum, H.; Sy, L.S.; Gandhi, A.; Tseng, H.F. Comparison of vaccine effectiveness against influenza hospitalization of cell-based and egg-based influenza vaccines, 2017–2018. Vaccine 2019, 37, 5807–5811. [Google Scholar] [CrossRef]
- Divino, V.; Krishnarajah, G.; Pelton, S.I.; Mould-Quevedo, J.; Anupindi, V.R.; DeKoven, M.; Postma, M.J. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017–18 influenza season. Vaccine 2020, 38, 6334–6343. [Google Scholar] [CrossRef] [PubMed]
- Pelton, S.; Postma, M.; Divino, V.; Mould-Quevedo, J.; DeKoven, M.; Krishnarajah, G. PIN3 RELATIVE VACCINE EFFECTIVENESS OF QUADRIVALENT CELL-BASED VERSUS EGG-BASED INFLUENZA VACCINES AMONG ADULTS 50-64 YEARS OLD: A U.S. OBSERVATIONAL COHORT STUDY. Value Health 2020, 23, S168. [Google Scholar] [CrossRef]
- DeMarcus, L.; Shoubaki, L.; Federinko, S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017–2018 influenza season. Vaccine 2019, 37, 4015–4021. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017–2018. J. Infect. Dis. 2018, 220, 1255–1264. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Wernecke, M.; MaCurdy, T.; et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019. J. Infect. Dis. 2020, 222, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, N.P.; Fireman, B.; Goddard, K.; Zerbo, O.; Asher, J.; Zhou, J.; King, J.; Lewis, N. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017-18 influenza season. PLoS ONE 2020, 15, e0229279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative Effectiveness of the Cell-Cultured Quadrivalent Influenza Vaccine Compared to Standard, Egg-derived Quadrivalent Influenza Vaccines in Preventing Influenza-like Illness in 2017–2018. Clin. Infect. Dis. 2020, 71, e665–e671. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.D.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097-6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Hernán, M.A.; Robins, J.M. Causal Inference: What If; Champman & Hall/CRC: Boca Raton, FL, USA, 2019; Available online: https://cdn1.sph.harvard.edu/wp-content/uploads/sites/1268/2019/10/ci_hernanrobins_1oct19.pdf (accessed on 13 March 2020).
- Halloran, M.E.; Longini, I.M.; Struchiner, C.J. Design and Analysis of Vaccine Studies; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Berman, N.G.; Parker, R.A. Meta-analysis: Neither quick nor easy. BMC Med. Res. Methodol. 2002, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Raudenbush, S.W. Analyzing effect sizes: Random-effects models. In The Handbook of Research Synthesis and Meta-Analysis, 2nd ed.; Russell Sage Foundation: New York, NY, USA, 2009; pp. 295–316. [Google Scholar]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Gregg, C.; Sylvester, J.A.M. Relative Effectiveness of Cell–Derived Quadrivalent Inactivated Influenza Vaccine (ccIIV4) Versus Egg-Derived IIV4 in Preventing Influenza-Related Medical En-counters during the 2018–2019 Influenza Season in the United States. In Proceedings of the ESWI2020 Virtual Edition, Valencia, Spain, 6–9 December 2020. Abstract 246. [Google Scholar]
- Postma, M.; Pelton, S.I.; Divino, V.; Mould-Quevedo, J.F.; Anupindi, V.R.; Mitchell DeKoven, G.K. Relative vaccine effectiveness against influenza-related hospitalizations and any respiratory event during the 2018/19 high influenza activity period. Real-World Analysis to compare quadrivalent cell-based and egg-based influenza vaccines. In Proceedings of the ESWI2020 Virtual Edition, Valencia, Spain, 6–9 December 2020. Abstract 214. [Google Scholar]
- Boikos, C.; Imran, M.; Nguyen, V.H.; Ducret, T.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of Cell-Derived versus Egg-derived Quadrivalent Influenza Vaccines in Individuals with Underlying Medical Conditions in the U.S. 2018–2019 Influenza Season. In Proceedings of the ESWI2020 Virtual Edition, Valencia, Spain, 6–9 December 2020. Abstract 468. [Google Scholar]
- Pelton, S.I.; Postma, M.; Divino, V.; Mould-Quevedo, J.F.; Anupindi, V.R.; Mitchell DeKoven, G.K. Relative vaccine effectiveness against influenza-related hospitalizations and serious respiratory events during the 2018/19 influenza season in children and adults. Comparison between quadrivalent cell-based and egg-based influenza vaccin. In Proceedings of the ESWI2020 Virtual Edition, Valencia, Spain, 6–9 December 2020. Abstrcat 211. [Google Scholar]
- Tseng, H.F.; Bruxvoort, K.J.; Luo, Y.; Anderson, B.; Tanenbaum, H.C.; Sy, L.S. Vaccine effectiveness against influenza hospitalization in the 2018–2019 season: Comparison between cell-based and egg-based influenza vaccines. In Proceedings of the Options X for the Control of Influenza (ISIRV), Singapore, 28 August–1 September 2019. Abstract 10935. [Google Scholar]
- Krishnarajah, G.; Divino, V.; Postma, M.J.; Pelton, S.I.; Anupindi, V.R.; DeKoven, M.; Mould-Quevedo, J. Clinical and Economic Outcomes Associated with Cell-Based Quadrivalent Influenza Vaccine vs. Standard-Dose Egg-Based Quadrivalent Influenza Vaccines during the 2018–19 Influenza Season in the United States. Vaccines 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Eick-Cost, A.A.; Hu, Z. Relative Effectiveness of Cell-Based Influenza Vaccines Compared with Egg-based Influienza Vaccines, Active Component, U.S. Service Members, 2017–2018 Season. In Proceedings of the International Conference on Emerging Infectious Diseases (ICEID2018), Atlanta, GA, USA, 26–29 August 2018; p. 109. [Google Scholar]
- Martin, E.T.; Cheng, C.; Petrie, J.G.; Alyanak, E.; Gaglani, M.; Middleton, D.B.; Ghamande, S.; Silveira, F.P.; Murthy, K.; Zimmerman, R.K.; et al. Low Influenza Vaccine Effectiveness Against A(H3N2)-Associated Hospitalizations in 2016–2017 and 2017–2018 of the Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J. Infect. Dis. 2021, 223, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Izurieta, H.S.; Lu, M.; Kelman, J.; Lu, Y.; Lindaas, A.; Loc, J.; Pratt, D.; Wei, Y.; Chillarige, Y.; Wernecke, M.; et al. Comparative Effectiveness of Influenza Vaccines Among US Medicare Beneficiaries Ages 65 Years and Older During the 2019–2020 Season. Clin. Infect. Dis. 2021, 73, e4251–e4259. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative Effectiveness of the Cell-derived Inactivated Quadrivalent Influenza Vaccine Versus Egg-derived Inactivated Quadrivalent Influenza Vaccines in Preventing Influenza-related Medical Encounters During the 2018–2019 Influenza Season in the United States. Clin. Infect. Dis. 2021, 73, e692–e698. [Google Scholar] [CrossRef]
- Okoli, G.N.; Racovitan, F.; Righolt, C.H.; Mahmud, S.M. Variations in Seasonal Influenza Vaccine Effectiveness due to Study Characteristics: A Systematic Review and Meta-analysis of Test-Negative Design Studies. Open Forum Infect. Dis. 2020, 7, ofaa177. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Sabaiduc, S.; Leir, S.; Rose, C.; Zou, M.; Murti, M.; Dickinson, J.A.; Olsha, R.; Gubbay, J.B.; Croxen, M.; et al. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: Potential imprint-regulated effect of vaccine (I-REV). Eurosurveillance 2019, 24, 1900585. [Google Scholar] [CrossRef] [Green Version]
- Ferdinands, J.M.; Gaglani, M.; Ghamande, S.; Martin, E.T.; Middleton, D.; Monto, A.S.; Silveira, F.; Talbot, H.K.; Zimmerman, R.; Smith, E.R.; et al. Vaccine Effectiveness Against Influenza-Associated Hospitalizations Among Adults, 2018–2019, US Hospitalized Adult Influenza Vaccine Effectiveness Network. J. Infect. Dis. 2020, 224, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing Seasonal Influenza—The Need for a Universal Influenza Vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef]
- Garten, R.; Blanton, L.; Elal, A.I.A.; Alabi, N.; Barnes, J.; Biggerstaff, M.; Brammer, L.; Budd, A.P.; Burns, E.; Cummings, C.N.; et al. Update: Influenza Activity in the United States During the 2017–18 Season and Composition of the 2018–19 Influenza Vaccine. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 634–642. [Google Scholar] [CrossRef]
- Flannery, B.; Kondor, R.J.G.; Chung, J.R.; Gaglani, M.; Reis, M.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; et al. Spread of Antigenically Drifted Influenza A(H3N2) Viruses and Vaccine Effectiveness in the United States During the 2018–2019 Season. J. Infect. Dis. 2019, 221, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Blanton, L.; Elal, A.I.A.; Alabi, N.; Barnes, J.; Biggerstaff, M.; Brammer, L.; Budd, A.P.; Burns, E.; Cummings, C.N.; et al. Update: Influenza Activity in the United States During the 2018–19 Season and Composition of the 2019–20 Influenza Vaccine. Morb. Mortal. Wkly. Rep. 2019, 68, 544–551. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orenstein, E.; De Serres, G.; Haber, M.J.; Shay, D.; Bridges, C.B.; Gargiullo, P.; Orenstein, W.A. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int. J. Epidemiol. 2007, 36, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M.A.; Calzavara, A.; Emerson, S.D.; Djebli, M.; Sundaram, M.E.; Chan, A.K.; Kustra, R.; Baral, S.D.; Mishra, S.; Kwong, J.C. Validating International Classification of Disease 10th Revision algorithms for identifying influenza and respiratory syncytial virus hospitalizations. PLoS ONE 2021, 16, e0244746. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Sullivan, S.G.; Cowling, B. “Crude Vaccine Effectiveness” Is a Misleading Term in Test-negative Studies of Influenza Vaccine Effectiveness. Epidemiology 2015, 26, e60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author, Year | Season | Geographic Location | Study Design | Age Group | Outcome * | Outcome Determination Method † | Risk of Bias | Cell-Cultured IV (n) | Influenza Related Outcomes (n) | Egg-Based IV (n) | Influenza Related Outcomes (n) | arVE (%) | arVE 95%CI | Funding | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boikos 2020a [35] | 2017–2018 | United States | Cohort-retrospective | ≥65 | Outpatient consultation | Codes | Moderate | 29,618 | 521 | 164,151 | 4808 | −7.3 | −51.6 | 24 | Seqirus |
| Boikos 2020b [35] | 2017–2018 | United States | Cohort-retrospective | 18–64 | Outpatient consultation | Codes | Moderate | 55,104 | 1069 | 693,014 | 10,021 | 26.8 | 14.1 | 37.6 | Seqirus |
| Bruxvoort 2019a [28] | 2017–2018 | United States | Test-negative | ≥65 | Admission with influenza | Lab-confirmed | Low | 157 | 25 | 3498 | 612 | 6 | −46 | 39 | Seqirus |
| Bruxvoort 2019b [28] | 2017–2018 | United States | Test-negative | ≥65 | Admission with influenza, A(H3N2) | Lab-confirmed | Low | 151 | 19 | 3321 | 435 | −4 | −70 | 37 | Seqirus |
| Divino 2020 [29] | 2017–2018 | United States | Cohort-retrospective | 18–64 | Admission & emergency room visits | Codes | Moderate | 499,156 | 976 | 1,730,403 | 4053 | 13.1 | 6.8 | 19 | Seqirus |
| Eick-Cost 2018 [54] | 2017–2018 | Not reported | Test-negative | 18–64 | Medical encounter with influenza | Lab-confirmed | Moderate | 2467 | 506 | 3239 | 757 | 5 | −10 | 17 | Defence Health Agency |
| Izurieta 2019a [32] | 2017–2018 | United States | Cohort-retrospective | ≥65 | Outpatient consultation | Codes | Low | 659,249 | 3299 | 1,863,654 | 9607 | 5.7 | 1.9 | 9.4 | FDA |
| Izurieta 2019b [32] | 2017–2018 | United States | Cohort-retrospective | ≥65 | Admission & emergency room visits | Codes | Low | 659,249 | 4370 | 1,863,654 | 14,417 | 11 | 7.9 | 14 | FDA |
| Izurieta 2019c [32] | 2017–2018 | United States | Cohort-retrospective | ≥65 | Admission | Codes | Low | 659,249 | 2527 | 1,863,654 | 8359 | 9.5 | 5.3 | 13.4 | FDA |
| Klein 2020a [34] | 2017–2018 | United States | Cohort-retrospective | 18–64 | Medical encounter, with influenza, A | Lab-confirmed | Low | 40,685 | . | 712,126 | . | −5.8 | −36.1 | 17.7 | DHHS |
| Klein 2020b [34] | 2017–2018 | United States | Cohort-retrospective | 18–64 | Medical encounter, with influenza, B | Lab-confirmed | Low | 40,685 | . | 712,126 | . | 21.4 | −7.3 | 42.4 | DHHS |
| Martin 2020a [55] | 2017–2018 | United States | Test-negative | ≥18 | Admission with lab confirmed A(H3N2) | Lab-confirmed | Low | 56 | 7 | 1459 | 248 | 24.9 | −78.8 | 68.5 | CDC, NIH |
| Martin 2020b [55] | 2017–2018 | United States | Test-negative | ≥18 | Admission with B/Yamagata-lineage | Lab-confirmed | Low | 43 | 3 | 1135 | 83 | 1.8 | −254 | 72.8 | CDC, NIH |
| Martin 2020c [55] | 2017–2018 | United States | Test-negative | ≥18 | Admission with influenza | Lab-confirmed | Low | 65 | 14 | 1676 | 399 | 8.5 | −75.9 | 52.3 | CDC, NIH |
| Boikos 2021c [57] | 2018–2019 | United States | Cohort-retrospective | ≥65 | Medical encounter | Codes | Low | 517,639 | 6321 | 987,943 | 11,545 | −2.2 | −5.4 | 0.9 | Seqirus |
| Boikos 2021d [57] | 2018–2019 | United States | Cohort-retrospective | 18–64 | Medical encounter | Codes | Low | 1,529,189 | 24,084 | 5,384,922 | 87,113 | 6.5 | 5.2 | 7.9 | Seqirus |
| Fu Tseng 2019a [52] | 2018–2019 | United States | Test-negative | ≥65 | Admission with influenza A | Lab-confirmed | Low | 696 | 39 | 2773 | 146 | −6 | −54.3 | 28 | Kaiser Permanente |
| Fu Tseng 2019b [52] | 2018–2019 | United States | Test-negative | ≥65 | Admission with influenza | Lab-confirmed | Low | 696 | 39 | 2773 | 143 | −4 | −50.3 | 26 | Kaiser Permanente |
| Fu Tseng 2019c [52] | 2018–2019 | United States | Test-negative | ≥65 | Admission with influenza A(H1N1) | Lab-confirmed | Low | 696 | 22 | 2773 | 65 | −32 | −117 | 20 | Kaiser Permanente |
| Fu Tseng 2019d [52] | 2018–2019 | United States | Test-negative | ≥65 | Admission with influenza A(H3N2) | Lab-confirmed | Low | 696 | 13 | 2773 | 52 | 6 | −75 | 49 | Kaiser Permanente |
| Fu Tseng 2019e [52] | 2018–2019 | United States | Test-negative | ≥65 | Admission with influenza A untyped | Lab-confirmed | Low | 696 | 4 | 2773 | 26 | 36 | −86 | 78 | Kaiser Permanente |
| Izurieta 2020a [33] | 2018–2019 | United States | Cohort-retrospective | ≥65 | Admission & emergency room visits | Codes | Low | 761,037 | 2330 | 1,454,340 | 4582 | 2.5 | −2.4 | 7.3 | FDA |
| Izurieta 2020b [33] | 2018–2019 | United States | Cohort-retrospective | ≥65 | Admission | Codes | Low | 761,037 | 1426 | 1,454,340 | 2790 | 4.4 | −1.9 | 10.3 | FDA |
| Krishnarajah 2021 [53] | 2018–2019 | United States | Cohort-retrospective | 18–64 | Admission & emergency room visits | Codes | Low | 590,705 | 768 | 2,223,435 | 3113 | 4.9 | −2.8 | 12.1 | Seqirus |
| Izurieta 2020c [56] | 2019–2020 | United States | Cohort-retrospective | ≥65 | Admission & emergency room visits | Codes | Low | 824,264 | 2092 | 1,584,451 | 3956 | 2.5 | −2.8 | 7.6 | FDA |
| Izurieta 2020d [56] | 2019–2020 | United States | Cohort-retrospective | ≥65 | Admission | Codes | Low | 824,264 | 1255 | 1,584,451 | 2309 | 1.3 | −5.7 | 7.9 | FDA |
| Confounders, Effect Modifiers | Publications Included | Reported Results | Cell-Cultured IV | IRO | Egg-Based IV | IRO | aRR ¶ | (95% CI) | Heterogeneity | Test of Group Differences | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 12 † | % | n = 26 | % | Mean | Mean | Mean | Mean | I2 (%) § | Q ** | p-Value | ||||
| Season | 16.86 | <0.001 | ||||||||||||
| 2017–2018 | 7 | 58.3 | 14 | 53.8 | 188,995 | 1111 | 686,936 | 4483 | 0.89 | 0.86 | 0.91 | 0.01 | ||
| 2018–2019 | 4 | 33.3 | 10 | 38.5 | 416,309 | 3505 | 1,151,885 | 10,958 | 0.97 | 0.93 | 1.02 | 79.15 | ||
| 2019–2010 | 1 | 8.3 | 2 | 7.7 | 824,264 | 1674 | 1,584,451 | 3133 | 0.98 | 0.92 | 1.02 | 0.00 | ||
| Age group | 2.16 | 0.340 | ||||||||||||
| >=18 | 1 | 11.5 | 3 | 10.7 | 55 | 8 | 1423 | 243 | 0.92 | 0.48 | 1.76 | 0.00 | ||
| 18–64 | 6 | 26.9 | 7 | 25.0 | 393,999 | 5481 | 1,637,038 | 21,011 | 0.91 | 0.85 | 0.97 | 72.11 | ||
| >=65 | 7 | 61.5 | 16 | 57.1 | 356,212 | 1519 | 802,583 | 3991 | 0.97 | 0.92 | 1.02 | 78.23 | ||
| Study design | 0.11 | 0.740 | ||||||||||||
| Cohort-Retrospective | 8 | 66.7 | 15 | 57.7 | 563,409 | 3,926 | 1,618,444 | 12,821 | 0.94 | 0.89 | 0.98 | 89.21 | ||
| Test-Negative | 4 | 33.3 | 11 | 42.3 | 584 | 63 | 2563 | 270 | 0.96 | 0.85 | 1.08 | 0.00 | ||
| Outcome setting | 1.78 | 0.410 | ||||||||||||
| Primary Care | 2 | 16.7 | 3 | 11.5 | 247,990 | 1630 | 906,940 | 8145 | 0.88 | 0.72 | 1.08 | 80.10 | ||
| Hospital | 8 | 66.7 | 18 | 69.2 | 310,162 | 885 | 765,760 | 2544 | 0.93 | 0.89 | 0.98 | 61.20 | ||
| Both | 3 | 25.0 | 5 | 19.2 | 426,133 | 10,304 | 1,560,071 | 33,138 | 0.98 | 0.92 | 1.04 | 85.13 | ||
| Outcome determination | 0.55 | 0.460 | ||||||||||||
| Laboratory confirmed | 5 | 41.7 | 13 | 50.0 | 6753 | 63 | 111,727 | 270 | 0.98 | 0.87 | 1.09 | 0.00 | ||
| Clinical Codes | 7 | 58.3 | 13 | 50.0 | 643,828 | 3,926 | 1,757,878 | 12,821 | 0.93 | 0.89 | 0.98 | 90.60 | ||
| Funding | 0.36 | 0.550 | ||||||||||||
| Other | 7 | 58.3 | 18 | 69.2 | 290,879 | 1122 | 728,565 | 2996 | 0.95 | 0.90 | 1.00 | 57.05 | ||
| Pharma | 5 | 41.7 | 8 | 30.8 | 402,715 | 4223 | 1,398,836 | 15,213 | 0.92 | 0.85 | 1.00 | 92.69 | ||
| Risk of bias | 2.05 | 0.150 | ||||||||||||
| Low | 9 | 75.0 | 22 | 84.6 | 357,782 | 2433 | 987,002 | 7500 | 0.96 | 0.92 | 1.00 | 78.95 | ||
| Moderate | 3 | 25 | 4 | 15.4 | 146,586 | 768 | 647,702 | 4910 | 0.87 | 0.77 | 0.99 | 65.09 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig-Barberà, J.; Tamames-Gómez, S.; Plans-Rubio, P.; Eiros-Bouza, J.M. Relative Effectiveness of Cell-Cultured versus Egg-Based Seasonal Influenza Vaccines in Preventing Influenza-Related Outcomes in Subjects 18 Years Old or Older: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 818. https://doi.org/10.3390/ijerph19020818
Puig-Barberà J, Tamames-Gómez S, Plans-Rubio P, Eiros-Bouza JM. Relative Effectiveness of Cell-Cultured versus Egg-Based Seasonal Influenza Vaccines in Preventing Influenza-Related Outcomes in Subjects 18 Years Old or Older: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(2):818. https://doi.org/10.3390/ijerph19020818
Chicago/Turabian StylePuig-Barberà, Joan, Sonia Tamames-Gómez, Pedro Plans-Rubio, and José María Eiros-Bouza. 2022. "Relative Effectiveness of Cell-Cultured versus Egg-Based Seasonal Influenza Vaccines in Preventing Influenza-Related Outcomes in Subjects 18 Years Old or Older: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 2: 818. https://doi.org/10.3390/ijerph19020818
APA StylePuig-Barberà, J., Tamames-Gómez, S., Plans-Rubio, P., & Eiros-Bouza, J. M. (2022). Relative Effectiveness of Cell-Cultured versus Egg-Based Seasonal Influenza Vaccines in Preventing Influenza-Related Outcomes in Subjects 18 Years Old or Older: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(2), 818. https://doi.org/10.3390/ijerph19020818








