Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ultrasonographic Examination
2.3. Statistical Analysis
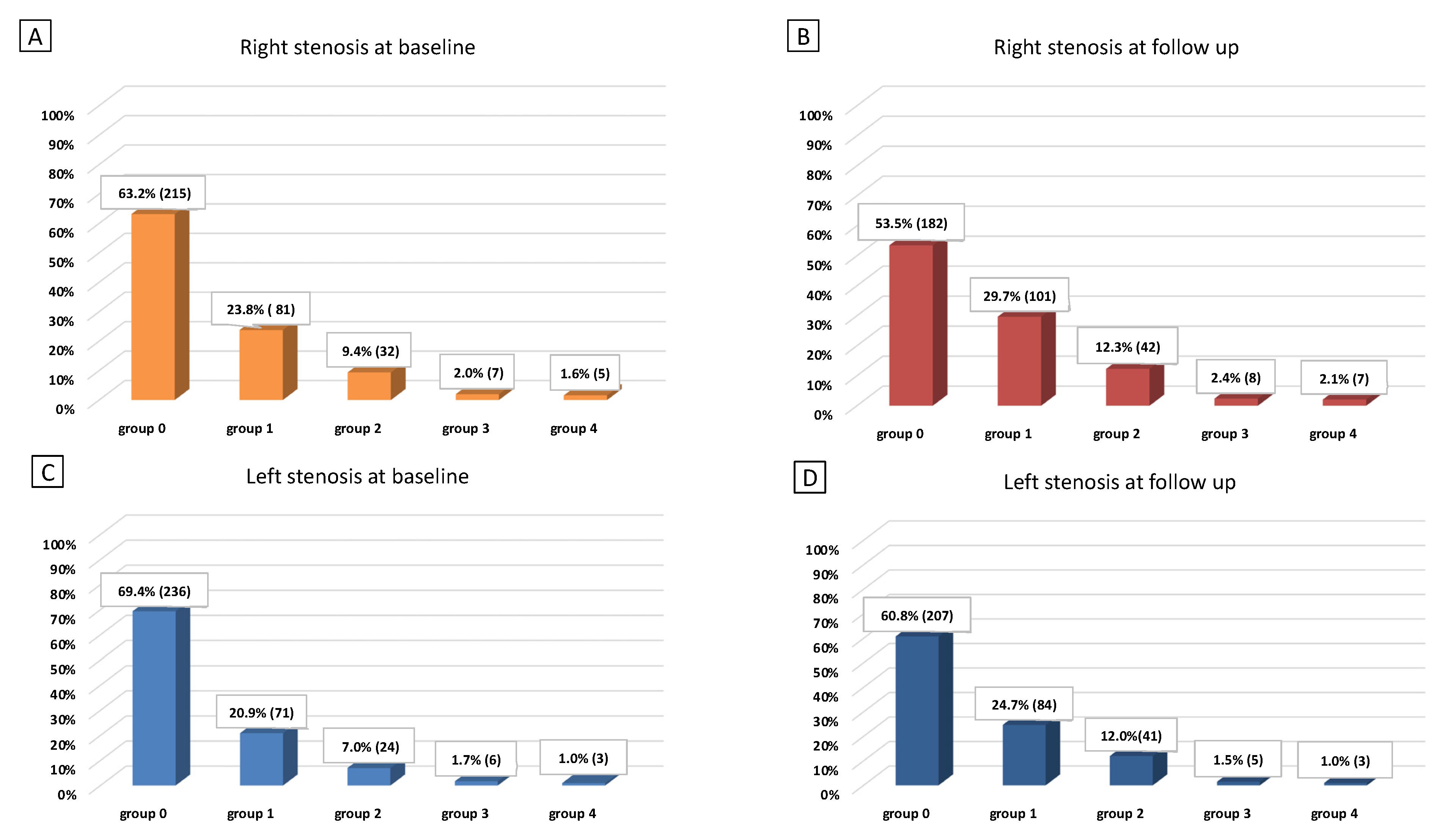
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, L.C.; Lin, R.T.; Chen, C.F.; Chen, C.H.; Hank Juo, S.H.; Lin, H.F. Predictors of carotid intima-media thickness and plaque pro-gression in a chinese population. J. Atheroscler. Thromb. 2016, 23, 940–949. [Google Scholar] [CrossRef]
- Greenland, P.; Abrams, J.; Aurigemma, G.P.; Bond, M.G.; Clark, L.T.; Criqui, M.H.; CrouseIII, J.R.; Friedman, L.; Fuster, V.; Herrington, D.M.; et al. Prevention Conference V: Beyond secondary prevention: Identifying the high-risk patient for primary prevention: Noninvasive tests of atherosclerotic burden: Writing Group III. Circulation 2000, 101, e16–e22. [Google Scholar] [CrossRef]
- Balestrini, S.; Lupidi, F.; Balucani, C.; Altamura, C.; Vernieri, F.; Provinciali, L.; Silvestrini, M. One-Year Progression of Moderate Asymptomatic Carotid Stenosis Predicts the Risk of Vascular Events. Stroke 2013, 44, 792–794. [Google Scholar] [CrossRef]
- Mackinnon, A.D.; Jerrard-Dunne, P.; Sitzer, M.; Buehler, A.; Von Kegler, S.; Markus, H.S. Rates and determinants of site-specific progression of carotid artery intima-media thickness: The carotid atherosclerosis progression study. Stroke 2004, 35, 2150–2154. [Google Scholar] [CrossRef]
- Sturlaugsdottir, R.; Aspelund, T.; Bjornsdottir, G.; Sigurdsson, S.; Thorsson, B.; Eiriksdottir, G.; Gudnason, V. Predictors of carotid plaque progression over a 4-year follow-up in the Reykjavik REFINE-study. Atherosclerosis 2018, 269, 57–62. [Google Scholar] [CrossRef]
- Hollander, M.; Hak, A.E.; Koudstaal, P.J.; Bots, M.L.; Grobbee, D.E.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Comparison between measures of atherosclerosis and risk of stroke: The Rotterdam study. Stroke 2003, 34, 2367–2372. [Google Scholar] [CrossRef]
- Rosvall, M.; Janzon, L.; Berglund, G.; Engström, G.; Hedblad, B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis 2005, 179, 325–331. [Google Scholar] [CrossRef]
- Polak, J.F.; Pencina, M.J.; Meisner, A.; Pencina, K.M.; Brown, L.S.; Wolf, P.A.; D’Agostino Sr, R.B. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: Comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. J. Ultrasound Med. 2010, 29, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; Pasqualetti, P.; Baruffaldi, R.; Catani, S.; Tibuzzi, F.; Altamura, C.; Bartolini, M.; Provinciali, L.; Vernieri, F. Markers of lacunar stroke in patients with moderate internal carotid artery stenosis. J. Neurol. 2005, 253, 321–327. [Google Scholar] [CrossRef]
- Silvestrini, M.; Altamura, C.; Cerqua, R.; Pasqualetti, P.; Viticchi, G.; Provinciali, L.; Paulon, L.; Vernieri, F. Ultrasonographic Markers of Vascular Risk in Patients with Asymptomatic Carotid Stenosis. Br. J. Pharmacol. 2013, 33, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Zureik, M.; Ducimetière, P.; Touboul, P.J.; Courbon, D.; Bonithon-Kopp, C.; Berr, C.; Magne, C. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques longitudinal results from the aging vascular study (EVA) study. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Von Sarnowski, B.; Lüdemann, J.; Völzke, H.; Dörr, M.; Kessler, C.; Schminke, U. Common carotid intima-media thickness and framingham risk score predict incident carotid atherosclerotic plaque formation: Longitudinal results from the study of health in Pomerania. Stroke 2010, 41, 2375–2377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diomedi, M.; Scacciatelli, D.; Misaggi, G.; Balestrini, S.; Balucani, C.; Sallustio, F.; Di Legge, S.; Stanzione, P.; Silvestrini, M. Increased Common Carotid Artery Wall Thickness Is Associated with Rapid Progression of Asymptomatic Carotid Stenosis. J. Neuroimaging 2013, 24, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Herder, M.; Johnsen, S.H.; Arntzen, K.A.; Mathiesen, E.B. Risk factors for progression of carotid intima-media thickness and total plaque area: A 13-year follow-up study: The Tromsø study. Stroke 2012, 43, 1818–1823. [Google Scholar] [CrossRef] [PubMed]
- De Bray, J.; Glatt, B. Quantification of Atheromatous Stenosis in the Extracranial Internal Carotid Artery. Cerebrovasc. Dis. 1995, 5, 414–426. [Google Scholar] [CrossRef]
- Touboul, P.-J.; Hennerici, M.G.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Hernandez, R.H.; et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011). Cerebrovasc. Dis. 2012, 34, 290–296. [Google Scholar] [CrossRef]
- O’Leary, D.H.; Polak, J.F.; Kronmal, R.A.; Manolio, T.A.; Burke, G.L.; Wolfson, S.K. Carotid-Artery Intima and Media Thickness as a Risk Factor for Myocardial Infarction and Stroke in Older Adults. N. Engl. J. Med. 1999, 340, 14–22. [Google Scholar] [CrossRef]
- Silvestrini, M.; Cagnetti, C.; Pasqualetti, P.; Albanesi, C.; Altamura, C.; Lanciotti, C.; Bartolini, M.; Mattei, F.; Provinciali, L.; Vernieri, F. Carotid wall thickness and stroke risk in patients with asymptomatic internal carotid stenosis. Atherosclerosis 2010, 210, 452–457. [Google Scholar] [CrossRef]
- Touboul, P.-J.; Hennerici, M.; Meairs, S.; Adams, H.; Amarenco, P.; Bornstein, N.; Csiba, L.; Desvarieux, M.; Ebrahim, S.; Fatar, M.; et al. Mannheim Carotid Intima-Media Thickness Consensus (2004–2006). Cerebrovasc. Dis. 2006, 23, 75–80. [Google Scholar] [CrossRef]
- Tschiderer, L.; Klingenschmid, G.; Seekircher, L.; Willeit, P. Carotid intima-media thickness predicts carotid plaque development: Meta-analysis of seven studies involving 9341 participants. Eur. J. Clin. Investig. 2020, 50, e13217. [Google Scholar] [CrossRef]
- Benedetto, F.A.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Rate of Atherosclerotic Plaque Formation Predicts Cardiovascular Events in ESRD. J. Am. Soc. Nephrol. 2008, 19, 757–763. [Google Scholar] [CrossRef]
- Izzo, R.; Stabile, E.; Esposito, G.; Trimarco, V.; Laurino, F.I.; Rao, M.A.E.; De Marco, M.; Losi, M.A.; De Luca, N.T.; Trimarco, B.; et al. Development of new atherosclerotic plaque in hyper-tensive patients: An observational registry study fromthe Campania-Salute network. J. Hypertens. 2015, 33, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Heliopoulos, I.; Papaoiakim, M.; Tsivgoulis, G.; Chatzintounas, T.; Vadikolias, K.; Papanas, N.; Piperidou, C. Common carotid intima media thickness as a marker of clinical severity in patients with symptomatic extracranial carotid artery stenosis. Clin. Neurol. Neurosurg. 2009, 111, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, I.M.; Del Sol, A.I.; Hak, A.E.; Bots, M.L.; Hofman, A.; Witteman, J.C.M. Risk factors for progression of atherosclerosis measured at multiple sites in the arterial tree: The Rotterdam study. Stroke 2003, 34, 2374–2379. [Google Scholar] [CrossRef]
- Tattersall, M.C.; Gassett, A.; Korcarz, C.E.; Gepner, A.D.; Kaufman, J.D.; Liu, K.J.; Astor, B.C.; Sheppard, L.; Kronmal, R.A.; Stein, J.H. Predictors of carotid thickness and plaque pro-gression during a decade: The multi-ethnic study of atherosclerosis. Stroke 2014, 45, 3257–3262. [Google Scholar] [CrossRef]
- Bonora, E.; Kiechl, S.; Willeit, J.; Oberhollenzer, F.; Egger, G.; Bonadonna, R.C.; Muggeo, M. Carotid atheroscle-rosis and coronary heart disease in the metabolic syndrome: Prospective data from the Bruneck study. Diabetes Care 2003, 26, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
| Patients (N = 340) | ||
|---|---|---|
| Demographic characteristics | ||
| Follow up, months (median, IQR) | 36.6 | 39.6–34.3 |
| Age, years (mean, SD) | 69.9 | 9.1 |
| Sex (n, % of males) | 176 | 51.8% |
| Risk factors (n, %) | ||
| Hypertension | 275 | 80.9% |
| Hyperlipidemia | 238 | 70.0% |
| Diabetes | 79 | 23.2% |
| Smoking | 45 | 13.2% |
| Ultrasound findings | ||
| IMT (mean, SD) | 0.80 | 0.18 |
| Right ICA PSV (median, IQR) | 87 | 39 |
| Right ICA EDV (median, IQR) | 26 | 14 |
| Left ICA PSV (median, IQR) | 90 | 42 |
| Left ICA EDV (median, IQR) | 27 | 13 |
| HAD2S score (median, IQR) | 2 | 1 |
| Non-Progression N = 254 | Progression N = 86 | p Value | |
|---|---|---|---|
| Follow up, months (median, IQR) | 36.2 (5.2) | 37.2 (6.5) | 0.104 |
| Age, years (mean, SD) | 69.2 (9.2) | 71.9 (8.4) | 0.012 |
| Sex (n, % of males) | 126 (49.6) | 50 (58.1) | 0.212 |
| Risk factors, n (%) | |||
| Hypertension | 201 (79.1) | 74 (86.0) | 0.204 |
| Hyperlipidemia | 179 (70.5) | 59 (68.6) | 0.786 |
| Diabetes | 54 (21.3) | 25 (29.1) | 0.142 |
| Smoking | 32 (12.6) | 13 (15.1) | 0.582 |
| Ultrasound findings * | |||
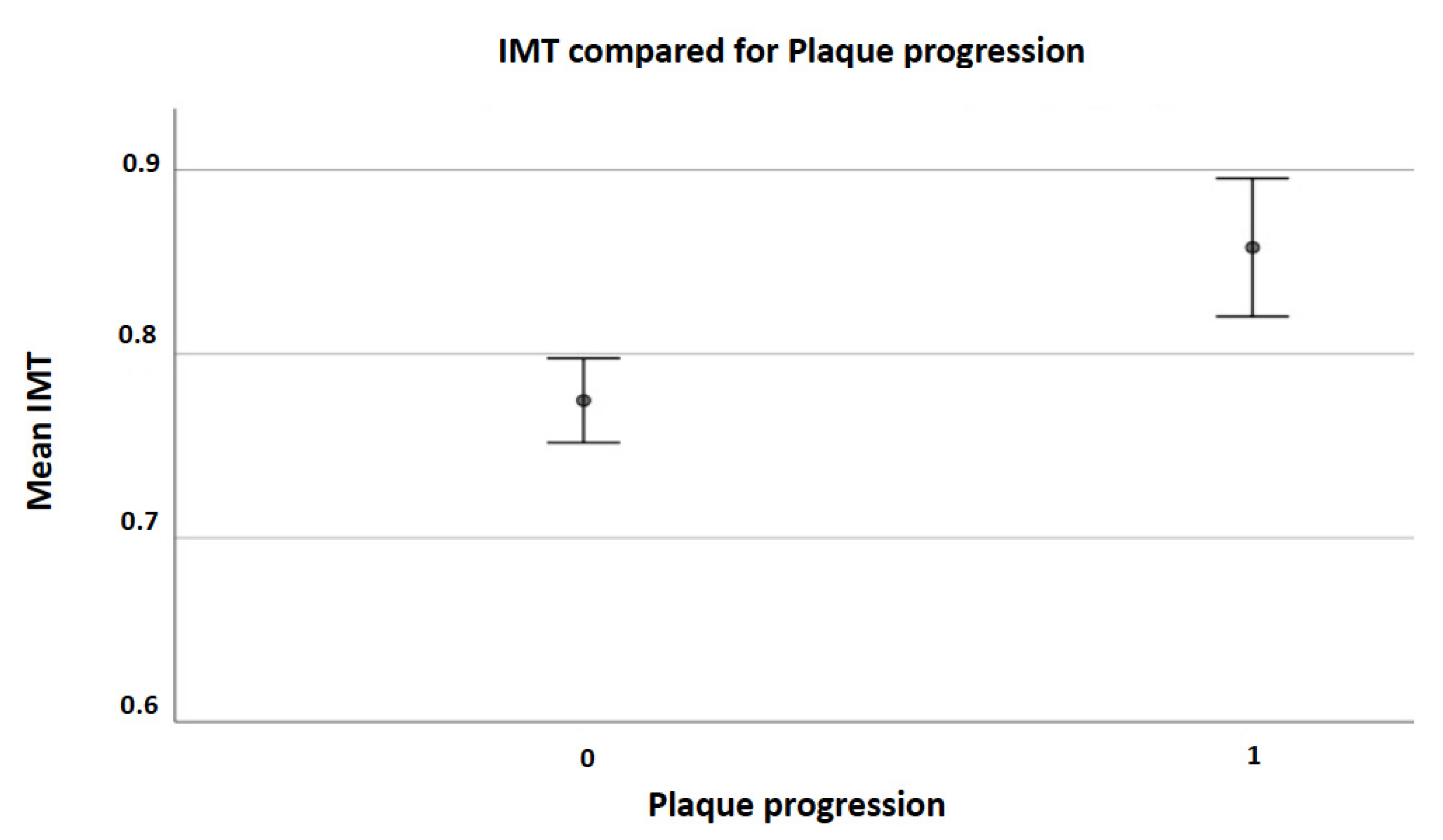
| IMT (mean, SD) | 0.77 (0.18) | 0.86 (0.17) | <0.001 |
| Right ICA PSV (median, IQR) | 85 (38) | 93 (44) | 0.063 |
| Right ICA EDV (median, IQR) | 26 (15) | 26 (11) | 0.808 |
| Left ICA PSV (median, IQR) | 88 (39) | 95.5 (46) | 0.036 |
| Left ICA EDV (median, IQR) | 27 (13) | 27 (15) | 0.822 |
| HAD2S score | 2 (1) | 3 (1) | 0.032 |
| B | S.E. | Wald | Sig. | ODDs Ratio | 95% CI for EXP(B) | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age T0 | 0.012 | 0.017 | 0.500 | 0.479 | 1.012 | 0.980 | 1.045 |
| Left PSV T0 | 0.004 | 0.002 | 2.985 | 0.084 | 1.004 | 0.999 | 1.009 |
| Mean IMT | 2.402 | 0.758 | 10.038 | 0.002 | 11.049 | 2.500 | 48.840 |
| HAD2S score | 0.174 | 0.144 | 1.467 | 0.226 | 1.190 | 0.898 | 1.577 |
| Constant | −4.698 | 1.174 | 16.004 | 0.000 | 0.009 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunelli, N.; Altamura, C.; Costa, C.M.; Altavilla, R.; Palazzo, P.; Maggio, P.; Marcosano, M.; Vernieri, F. Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness. Int. J. Environ. Res. Public Health 2022, 19, 758. https://doi.org/10.3390/ijerph19020758
Brunelli N, Altamura C, Costa CM, Altavilla R, Palazzo P, Maggio P, Marcosano M, Vernieri F. Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness. International Journal of Environmental Research and Public Health. 2022; 19(2):758. https://doi.org/10.3390/ijerph19020758
Chicago/Turabian StyleBrunelli, Nicoletta, Claudia Altamura, Carmelina Maria Costa, Riccardo Altavilla, Paola Palazzo, Paola Maggio, Marilena Marcosano, and Fabrizio Vernieri. 2022. "Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness" International Journal of Environmental Research and Public Health 19, no. 2: 758. https://doi.org/10.3390/ijerph19020758
APA StyleBrunelli, N., Altamura, C., Costa, C. M., Altavilla, R., Palazzo, P., Maggio, P., Marcosano, M., & Vernieri, F. (2022). Carotid Artery Plaque Progression: Proposal of a New Predictive Score and Role of Carotid Intima-Media Thickness. International Journal of Environmental Research and Public Health, 19(2), 758. https://doi.org/10.3390/ijerph19020758






