Inactivation of Spores and Vegetative Forms of Clostridioides difficile by Chemical Biocides: Mechanisms of Biocidal Activity, Methods of Evaluation, and Environmental Aspects
Abstract
1. Introduction
2. Characterization of Biocides Against Clostridioides difficile Used in Healthcare Settings
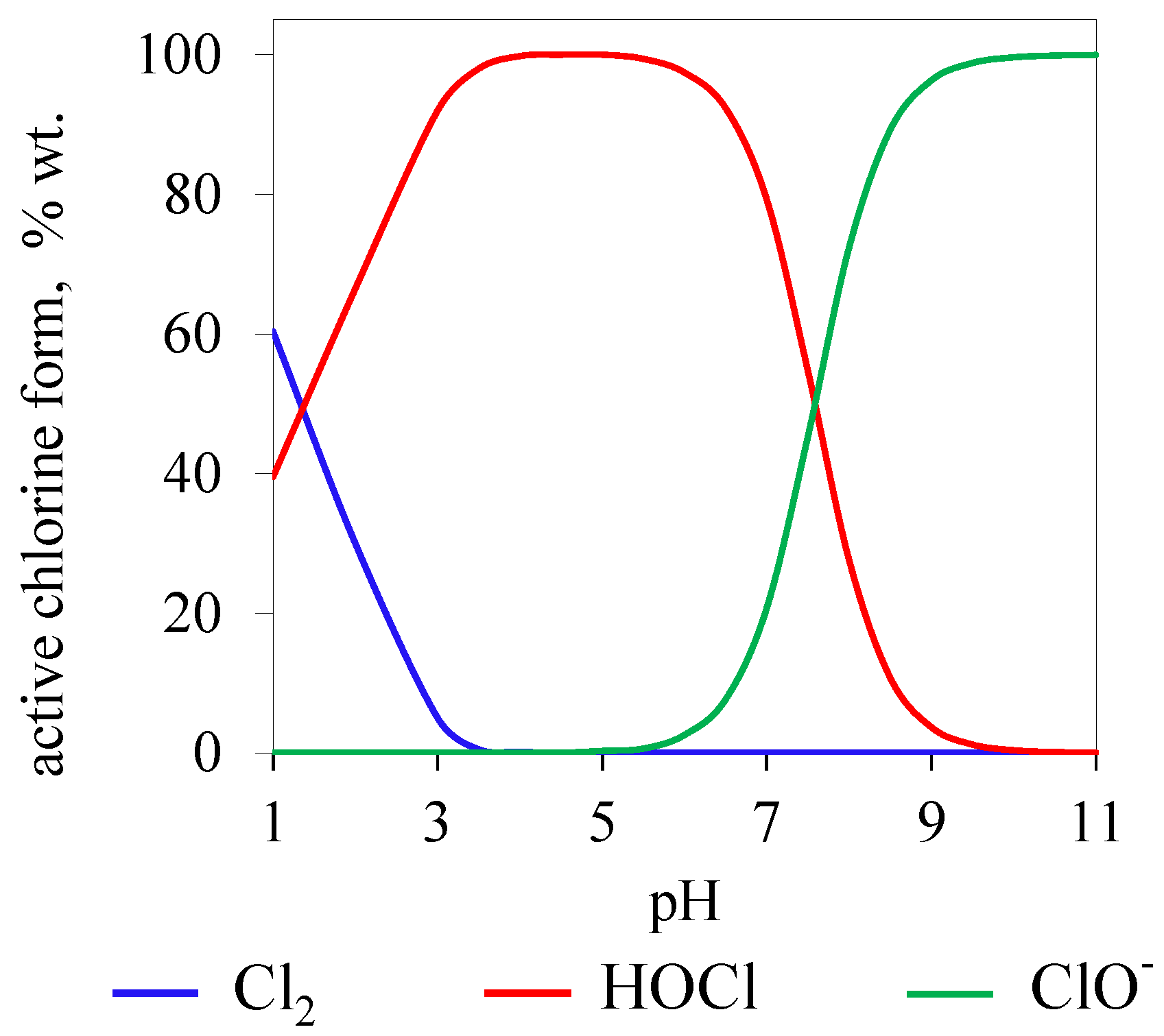
2.1. Active Chlorine

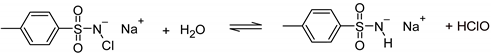
2.2. Glutaraldehyde

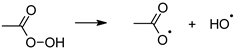
2.3. Hydrogen Peroxide
2.4. Peracetic Acid
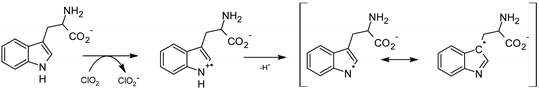
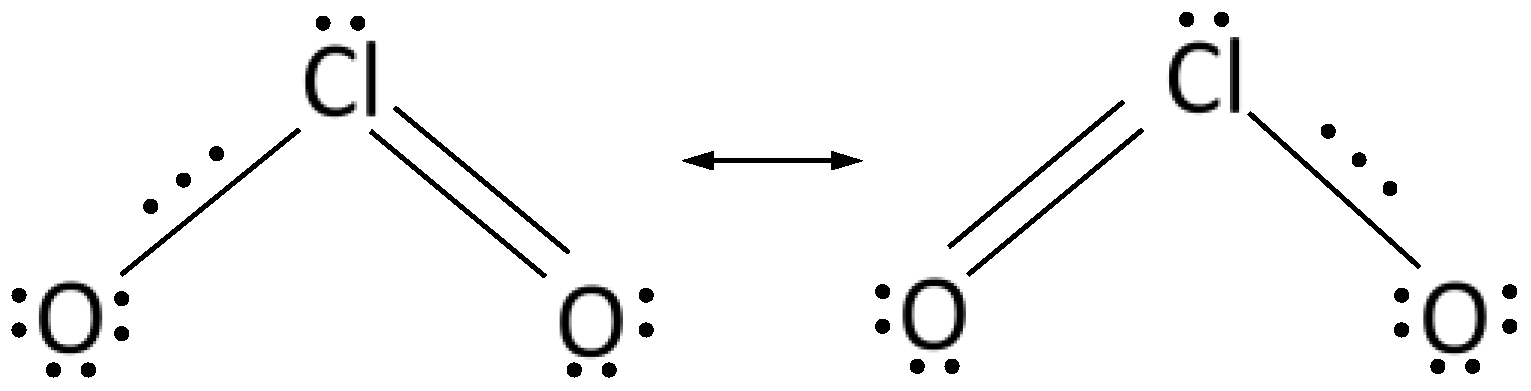
2.5. Chlorine Dioxide
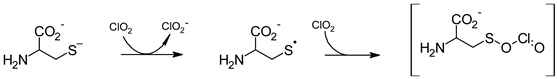
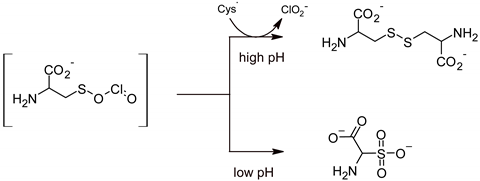
3. Standardized Methods for Assessing the Sporicidal Activity of Disinfectants
3.1. Suspension Methods
3.2. Carrier Methods
3.3. Carrier Methods for Assessing the Effectiveness of Automatic Air Disinfection
4. Conclusions
- oxidation of thiol groups with the formation of disulfide bridges or derivatization of amine compounds by radical oxidation (oxidants);
- formation of imine bonds (Schiff compounds) by condensation of amino groups of amino acids with carbonyl groups (aldehydes).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, B.R.; Leighton, F.A. A History of One Health. Rev. Sci. Tech. 2014, 33, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Stephen, C.; Karesh, W.B. Is One Health Delivering Results? Introduction. Rev. Sci. Tech. 2014, 33, 375–392. [Google Scholar] [PubMed]
- Truszczyński, M.; Pejsak, Z. “One Health”—The Concept Combining Scientific and Practically Applied Activity of Human and Animal Health Protection. Życie Weter. 2015, 90, 280–283. [Google Scholar]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global Trends in Emerging Infectious Diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.; Haydon, D.T.; Antia, R. Emerging Pathogens: The Epidemiology and Evolution of Species Jumps. Trends Ecol. Evol. 2005, 20, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial Pharmaceuticals in the Aquatic Environment—Occurrence and Environmental Implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef]
- Wang, L.F.; Crameri, G. Emerging Zoonotic Viral Diseases. Rev. Sci. Tech. 2014, 33, 569–581. [Google Scholar] [CrossRef]
- Stephenson, B.; Lanzas, C.; Lenhart, S.; Ponce, E.; Bintz, J.; Erik, R. Dubberke, E.R.; Day, J. Comparing Intervention Strategies for Reducing Clostridioides difficile Transmission in Acute Healthcare Settings: An Agent-Based Modeling Study. BMC Infect. Dis. 2020, 20, 799. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Chorepsima, S.; Triarides, N.A.; Falagas, M.E. Tigecycline for the Treatment of Patients with Clostridium Difficile Infection: An Update of the Clinical Evidence. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1053–1058. [Google Scholar] [CrossRef]
- Liu, J.Y.; Dickter, J.K. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest. Endosc. Clin. 2020, 30, 637–652. [Google Scholar] [CrossRef]
- Balsells, E.; Shi, T.; Leese, C.; Lyell, I.; Burrows, J.; Wiuff, C.; Campbell, H.; Kyaw, M.H.; Nair, H. Global Burden of Clostridium Difficile Infections: A Systematic Review and Meta-Analysis. J. Glob. Health. 2019, 9, 010407. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.H.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium Difficile Infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Palazuelos-Munoz, S.; Balsells, E.M.; Nair, H.; Chit, A.; Kyaw, M.H. Cost of Hospital Management of Clostridium Difficile Infection in United States—A Meta-Analysis and Modelling Study. BMC Infect. Dis. 2016, 16, 447. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch-Institute. Infection Epidemiological Yearbook of Reportable Diseases for 2019; Robert Koch-Institute: Berlin, Germany, 2020. [Google Scholar]
- Gemein, S.; Gebel, J.; Christiansen, B.; Martiny, B.; Vossebein, L.; Brill, F.H.H.; Decius, M.; Eggers, M.; Koburger-Janssen, T.; Meckel, M.; et al. Interlaboratory Reproducibility of a Test Method Following 4-Field Test Methodology to Evaluate the Susceptibility of Clostridium Difficile Spores. J. Hosp. Infect. 2019, 103, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.; O’toole, E. Intestinal Flora in New-Born Infants: With a Description of a New Pathogenic Anaerobe, Bacillus Difficilis. JAMA Pediatr. 1935, 49, 390–402. [Google Scholar] [CrossRef]
- Barbut, F.; Jones, G.; Eckert, C. Epidemiology and Control of Clostridium Difficile Infections in Healthcare Settings: An Update. Curr. Opin. Infect. Dis. 2011, 24, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, A.; Tiwari, M.K.; Mamah Mustapha, S.S.; Navarrete, S.J.; Henriksen, A.B.; Møller, I.M.; Krogfelt, K.A.; Bjerrum, M.J.; Jørgensen, R. Detoxification of Toxin A and Toxin B by Copper Ion-Catalyzed Oxidation in Production of a Toxoid-Based Vaccine against Clostridioides difficile. Free Radic. Biol. Med. 2020, 160, 433–446. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Chagot, B.; Cover, M.; Chazin, W.J.; Spiller, B.; Lacy, D.B. Structure-Function Analysis of Inositol Hexakisphosphate-Induced Autoprocessing in Clostridium Difficile Toxin A. J. Biol. Chem. 2009, 284, 21934–21940. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Pepin, J.; Fang, A.; Killgore, G.; Thompson, A.; Brazier, J.; Frost, E.; McDonald, L.C. Toxin Production by an Emerging Strain of Clostridium Difficile Associated with Outbreaks of Severe Disease in North America and Europe. Lancet 2005, 366, 1079–1084. [Google Scholar] [CrossRef]
- Gonçalves, C.; Decré, D.; Barbut, F.; Burghoffer, B.; Petit, J.C. Prevalence and Characterization of a Binary Toxin (Actin-Specific ADP-Ribosyltransferase) from Clostridium difficile. J. Clin. Microbiol. 2004, 42, 1933–1939. [Google Scholar] [CrossRef]
- Schoyer, E.; Hall, K. Environmental Cleaning and Decontamination to Prevent Clostridioides difficile Infection in Health Care Settings: A Systematic Review. J. Patient. Saf. 2020, 16, S12–S15. [Google Scholar] [CrossRef]
- Surawicz, C.M.; Brandt, L.J.; Binion, D.G.; Ananthakrishnan, A.N.; Curry, S.R.; Gilligan, P.H.; McFarland, L.V.; Mellow, M.; Zuckerbraun, B.S. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium dfficile Infections. Am. J. Gastroenterol. 2013, 108, 478–498, quiz 99. [Google Scholar] [CrossRef] [PubMed]
- Macleod-Glover, N.; Sadowski, C. Efficacy of Cleaning Products for C. Difficile: Environmental Strategies to Reduce the Spread of Clostridium Difficile-Associated Diarrhea in Geriatric Rehabilitation. Can. Fam. Physician. 2010, 56, 417–423. [Google Scholar]
- Chenjiao, W.; Hongyan, Z.; Qing, G.; Xiaoqi, Z.; Liying, G.; Ying, F. In-Use Evaluation of Peracetic Acid for High-Level Disinfection of Endoscopes. Gastroenterol. Nurs. 2016, 39, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Louh, I.K.; Greendyke, W.G.; Hermann, E.A.; Davidson, K.W.; Falzon, L.; Vawdrey, D.K.; Shaffer, J.A.; Calfee, D.P.; Furuya, E.Y.; Ting, H.H. Clostridium Difficile Infection in Acute Care Hospitals: Systematic Review and Best Practices for Prevention. Infect. Control Hosp. Epidemiol. 2017, 38, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kierat, W.; Augustyn, W.; Koper, P.; Pawlyta, M.; Chruściel, A.; Wyrwol, B. The Use of UVC Irradiation to Sterilize Filtering Facepiece Masks Limiting Airborne Cross-Infection. Int. J. Environ. Res. Public Health 2020, 17, 7396. [Google Scholar] [CrossRef] [PubMed]
- Connick, R.E.; Chia, Y.-T. The Hydrolysis of Chlorine and Its Variation with Temperature. J. Am. Chem. Soc. 1959, 81, 1280–1284. [Google Scholar] [CrossRef]
- Aieta, E.M.; Roberts, P.V. Henry Constant of Molecular Chlorine in Aqueous Solution. J. Chem. Eng. Data. 1986, 31, 51–53. [Google Scholar] [CrossRef]
- Block, S.S. Disinfection, Sterilization, and Preservation; Lea & Febiger: Philadelphia, PA, USA, 1991. [Google Scholar]
- Ascenzi, J.M. Handbook of Disinfectants and Antiseptics; M. Dekker: New York, NY, USA, 1996. [Google Scholar]
- Black & Veatch Corporation. White’s Handbook of Chlorination and Alternative Disinfectants; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Lu Shih, K.; Lederberg, J. Effects of Chloramine on Bacillus Subtilis Deoxyribonucleic Acid. J. Bacteriol. 1976, 125, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Dukan, S.; Touati, D. Hypochlorous Acid Stress in Escherichia Coli: Resistance, DNA Damage, and Comparison with Hydrogen Peroxide Stress. J. Bacteriol. 1996, 178, 6145–6150. [Google Scholar] [CrossRef]
- Barrette, W.C., Jr.; Hannum, D.M.; Wheeler, W.D.; Hurst, J.K. General Mechanism for the Bacterial Toxicity of Hypochlorous Acid: Abolition of ATP Production. Biochemistry 1989, 28, 9172–9178. [Google Scholar] [CrossRef] [PubMed]
- Camper, A.K.; McFeters, G.A. Chlorine Injury and the Enumeration of Waterborne Coliform Bacteria. Appl. Environ. Microbiol. 1979, 37, 633–641. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.M.; Davies, K.J. The Inhibition of Bacterial Growth by Hypochlorous Acid. Possible Role in the Bactericidal Activity of Phagocytes. Biochem. J. 1988, 254, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Fawley, W.N.; Wigglesworth, N.; Parnell, P.; Verity, P.; Freeman, J. Comparison of the Effect of Detergent versus Hypochlorite Cleaning on Environmental Contamination and Incidence of Clostridium Difficile Infection. J. Hosp. Infect. 2003, 54, 109–114. [Google Scholar] [CrossRef]
- Ungurs, M.; Wand, M.; Vassey, M.; O’Brien, S.; Dixon, D.; Walker, J.; Sutton, J. The Effectiveness of Sodium Dichloroisocyanurate Treatments against Clostridium Difficile Spores Contaminating Stainless Steel. Am. J. Infect. Control 2011, 39, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.F.; Arthur, M. Interaction of Bacillus Subtilis Spores with Sodium Hypochlorite, Sodium Dichloroisocyanurate and Chloramine-T. J. Appl. Bacteriol. 1992, 72, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D.; Day, M.J. Antibiotic and Biocide Resistance in Bacteria. Microbios 1996, 85, 45–65. [Google Scholar] [PubMed]
- Russell, A.D. The Destruction of Bacterial Spores; Academic Press: London, UK, 1982. [Google Scholar]
- Dye, M.; Mead, G.C. The Effect of Chlorine on the Viability of Clostridial Spores. Int. J. Food Sci. Technol. 1972, 7, 173–181. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol. Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Joshi, L.T.; Welsch, A.; Hawkins, J.; Baillie, L. The Effect of Hospital Biocide Sodium Dichloroisocyanurate on the Viability and Properties of Clostridium Difficile Spores. Lett. Appl. Microbiol. 2017, 65, 199–205. [Google Scholar] [CrossRef][Green Version]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; Demarini, D.M. Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection By-Products in Drinking Water: A Review and Roadmap for Research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Aslani, H.; Hosseini, M.; Mohammadi, S.; Naghavi-Behzad, M. Drinking Water Disinfection By-products and Their Carcinogenicity, A Review of Unseen Crisis. Int. J. Cancer Manag. 2019, 12, e88930. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Absolute Rate Constants for the Reaction of Hypochlorous Acid with Protein Side Chains and Peptide Bonds. Chem. Res. Toxicol. 2001, 14, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Graham, N.; Templeton, M.R.; Zhang, Y.; Collins, C.; Nieuwenhuijsen, M. A Comparison of the Role of Two Blue–Green Algae in THM and HAA Formation. Water Res. 2009, 43, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Trehy, M.L.; Yost, R.A.; Miles, C.J. Chlorination Byproducts of Amino Acids in Natural Waters. Environ. Sci. Technol. 1986, 20, 1117–1122. [Google Scholar] [CrossRef]
- Ramos, D.R.; García, M.V.; Canle, L.M.; Santaballa, J.A.; Furtmüller, P.G.; Obinger, C. Myeloperoxidase-Catalyzed Chlorination: The Quest for the Active Species. J. Inorg. Biochem. 2008, 102, 1300–1311. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Gorman, S.P.; Scott, E.M.; Russell, A.D. Antimicrobial Activity, Uses and Mechanism of Action of Glutaraldehyde. J. Appl. Bacteriol. 1980, 48, 161–190. [Google Scholar] [CrossRef]
- Beauchamp, R.O., Jr.; Clair, M.B.; Fennell, T.R.; Clarke, D.O.; Morgan, K.T.; Kari, F.W. A Critical Review of the Toxicology of Glutaraldehyde. Crit. Rev. Toxicol. 1992, 22, 143–174. [Google Scholar] [CrossRef]
- European Standard EN 16615:2015; Chemical Disinfectants and Antiseptics—Quantitative Test Method for the Evaluation of Bactericidal and Yeasticidal Activity on Non-Porous Surfaces with Mechanical Action Employing Wipes in the Medical Area (4-Field Test)—Test Method and Requirements (Phase 2, Step 2). European Committee for Standardization: Brussels, Belgium, 2015.
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Mode of Action of Hydrogen Peroxide and Other Oxidizing Agents: Differences between Liquid and Gas Forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef]
- Pottage, T.; Richardson, C.; Parks, S.; Walker, J.T.; Bennett, A.M. Evaluation of Hydrogen Peroxide Gaseous Disinfection Systems to Decontaminate Viruses. J. Hosp. Infect. 2010, 74, 55–61. [Google Scholar] [CrossRef]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups. Peroxides; Online 2009–2014 John Wiley & Sons, Ltd.: Somerset, UK, 2014; pp. 1–34. [Google Scholar]
- Chojecka, A. Fight against Clostridioides difficile in the Medical Area. Compounds and Preparations Active in the Fight against spores. Zakażenia XXI Wieku 2020, 3, 1–10. [Google Scholar]
- Wallace, R.L.; Ouellette, M.; Jean, J. Effect of UV-C Light or Hydrogen Peroxide Wipes on the Inactivation of Methicillin-Resistant Staphylococcus Aureus, Clostridium Difficile Spores and Norovirus Surrogate. J. Appl. Microbiol. 2019, 127, 586–597. [Google Scholar] [CrossRef]
- Gordon, D.; Carruthers, B.-A.; Theriault, S. Gaseous Decontamination Methods in High-containment Laboratories. Appl. Biosaf. 2012, 17, 31–39. [Google Scholar] [CrossRef]
- Andersen, B.M.; Syversen, G.; Thoresen, H.; Rasch, M.; Hochlin, K.; Seljordslia, B.; Snevold, I.; Berg, E. Failure of Dry Mist of Hydrogen Peroxide 5% to Kill Mycobacterium tuberculosis. J. Hosp. Infect. 2010, 76, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L.; Otter, J.A.; McDonald, L.C.; Adams, N.M.T.; Cooper, T.; Thompson, A.; Wiggs, L.; Killgore, G.; Tauman, A.; et al. Impact of Hydrogen Peroxide Vapor Room Decontamination on Clostridium difficile Environmental Contamination and Transmission in a Healthcare Setting. Infect. Control Hosp. Epidemiol. 2008, 29, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Barbut, F.; Menuet, D.; Verachten, M.; Girou, E. Comparison of the Efficacy of a Hydrogen Peroxide Dry-Mist Disinfection System and Sodium Hypochlorite Solution for Eradication of Clostridium difficile Spores. Infect. Control Hosp. Epidemiol. 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.V.; Sabourin, C.L.K.; Choi, Y.W.; Richter, W.R.; Rudnicki, D.C.; Riggs, K.B.; Taylor, M.L.; Chang, J.C.S. Decontamination Assessment of Bacillus Anthracis, Bacillus Subtilis, and Geobacillus Stearothermophilus Spores on Indoor Surfaces Using a Hydrogen Peroxide Gas Generator. J. Appl. Microbiol. 2005, 99, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Hey, G.; Ledin, A.; Jansen, J.L.C.; Andersen, H.R. Removal of Pharmaceuticals in Biologically Treated Wastewater by Chlorine Dioxide or Peracetic Acid. Environ. Technol. 2012, 33, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Rokhina, E.V.; Makarova, K.; Golovina, E.A.; Van As, H.; Virkutyte, J. Free Radical Reaction Pathway, Thermochemistry of Peracetic Acid Homolysis, and Its Application for Phenol Degradation: Spectroscopic Study and Quantum Chemistry Calculations. Environ. Sci. Technol. 2010, 44, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Sohn, M. Oxidation of Amino Acids, Peptides, and Proteins by Chlorine Dioxide. Implications for Water Treatment. In Environmental Chemistry for a Sustainable World: Remediation of Air and Water Pollution; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer The Netherlands: Dordrecht, The Netherlands, 2012; Volume 2, pp. 237–254. [Google Scholar]
- Pauling, L. General Chemistry; Dover Publications: Dover, UK, 1988. [Google Scholar]
- Kaczur, J.J.; Cawlfield, D.W. Chlorous Acid, Chlorites, and Chlorine Dioxide (ClO2, HClO2). In Kirk—Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Choshen, E.; Elits, R.; Rav-Acha, C. The Formation of Cation-Radicals by the Action of Chlorine Dioxide on P-Substituted Styrenes Usd Other Alkenes. Tetrahedron Lett. 1986, 27, 5989–5992. [Google Scholar]
- Tratnyek, P.G.; Hoigné, J. Kinetics of Reactions of Chlorine Dioxide (OCIO) in Water—II. Quantitative Structure-Activity Relationships for Phenolic Compounds. Water Res. 1994, 28, 57–66. [Google Scholar] [CrossRef]
- Rosenblatt, D.H.; Hull, L.A.; De Luca, D.C.; Davis, G.T.; Weglein, R.C.; Williams, H.K.R. Oxidations of Amines. II. Substituent Effects in Chlorine Dioxide Oxidations. J. Am. Chem. Soc. 1967, 89, 1158–1163. [Google Scholar] [CrossRef]
- Yakupov, M.Z.; Shereshovets, V.V.; Imashev, U.B.; Ismagilov, F.R. Liquid-Phase Oxidation of Thiols with Chlorine Dioxide. Russ. Chem. Bull. 2001, 50, 2352–2355. [Google Scholar] [CrossRef]
- Hoigne, J.; Bader, H.L. Kinetics of Reactions of Chlorine Dioxide (OClO) in Water. Rate constants for Inorganic and Organic Compounds. Water Res. 1994, 28, 45–55. [Google Scholar] [CrossRef]
- Tan, H.-K.; Wheeler, W.B.; Wei, C.-I. Reaction of Chlorine Dioxide with Amino Acids and Peptides: Kinetics and Mutagenicity Studies. Mutat. Res./Genet. Toxicol. 1987, 188, 259–266. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Ren, N.; Ma, F. Disinfection Effect of Chlorine Dioxide on Bacteria in Water. Water Res. 1997, 31, 607–613. [Google Scholar] [CrossRef]
- Schöneich, C. Mechanisms of Protein Damage Induced by Cysteine Thiyl Radical Formation. Chem. Res. Toxicol. 2008, 21, 1175–1179. [Google Scholar] [CrossRef]
- Ison, A.; Odeh, I.N.; Margerum, D.W. Kinetics and Mechanisms of Chlorine Dioxide and Chlorite Oxidations of Cysteine and Glutathione. Inorg. Chem. 2006, 45, 8768–8775. [Google Scholar] [CrossRef]
- Navalon, S.; Alvaro, M.; Garcia, H. Chlorine Dioxide Reaction with Selected Amino Acids in Water. J. Hazard. Mater. 2009, 164, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Napolitano, M.J.; Bakhmutova-Albert, E.V.; Margerum, D.W. Kinetics and Mechanisms of Chlorine Dioxide Oxidation of Tryptophan. Inorg. Chem. 2008, 47, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N. Denaturation of Protein by Chlorine Dioxide: Oxidative Modification of Tryptophan and Tyrosine Residues. Biochemistry 2007, 46, 4898–4911. [Google Scholar] [CrossRef]
- Bakhmutova-Albert, E.V.; Margerum, D.W.; Auer, J.G.; Applegate, B.M. Chlorine Dioxide Oxidation of Dihydronicotinamide Adenine Dinucleotide (NADH). Inorg. Chem. 2008, 47, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Voet, D.; Voet, J.G. Biochemistry; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Aktories, K. Bacterial Toxins That Target Rho Proteins. J. Clin. Investig. 1997, 99, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, R.; Lacy, D.B. The Role of Toxins in Clostridium Difficile Infection. FEMS Microbiol. Rev. 2017, 41, 723–750. [Google Scholar] [CrossRef]
- Tucker, K.D.; Wilkins, T.D. Toxin A of Clostridium difficile Binds to the Human Carbohydrate Antigens I, X, and Y. Infect. Immun. 1991, 59, 73–78. [Google Scholar] [CrossRef]
- Hofmann, F.; Busch, C.; Prepens, U.; Just, I.; Aktories, K. Localization of the Glucosyltransferase Activity of Clostridium Difficile Toxin B to the N-Terminal Part of the Holotoxin. J. Biol. Chem. 1997, 272, 11074–11078. [Google Scholar] [CrossRef]
- Barth, H.; Pfeifer, G.; Hofmann, F.; Maier, E.; Benz, R.; Aktories, K. Low pH-Induced Formation of Ion Channels by Clostridium Difficile Toxin B in Target Cells. J. Biol. Chem. 2001, 276, 10670–10676. [Google Scholar] [CrossRef]
- Just, I.; Selzer, J.; Wilm, M.; Eichel-Streiber, C.V.; Mann, M.; Aktories, K. Glucosylation of Rho Proteins by Clostridium Difficile Toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef]
- Just, I.; Wilm, M.; Selzer, J.; Rex, G.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. The Enterotoxin from Clostridium difficile (ToxA) Monoglucosylates the Rho Proteins. J. Biol. Chem. 1995, 270, 13932–13936. [Google Scholar] [CrossRef]
- Chumbler, N.M.; Rutherford, S.A.; Zhang, Z.; Farrow, M.A.; Lisher, J.P.; Farquhar, E.; Giedroc, D.P.; Spiller, B.W.; Melnyk, R.A.; Lacy, D.B. Crystal Structure of Clostridium Difficile Toxin A. Nat. Microbiol. 2016, 1, 15002. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Hofmann, F.; Gerhard, R.; Aktories, K. Involvement of a Conserved Tryptophan Residue in the UDP-Glucose Binding of Large Clostridial Cytotoxin Glycosyltransferases. J. Biol. Chem. 2000, 275, 13228–13234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, C.; Chen, Z.; Yu, G. Fungicidal Mechanism of Chlorine Dioxide on Saccharomyces Cerevisiae. Ann. Microbiol. 2013, 63, 495–502. [Google Scholar] [CrossRef]
- Roller, S.D.; Olivieri, V.P.; Kawata, K. Mode of Bacterial Inactivation by Chlorine Dioxide. Water Res. 1980, 14, 635–641. [Google Scholar] [CrossRef]
- Young, S.B.; Setlow, P. Mechanisms of Killing of Bacillus Subtilis Spores by Hypochlorite and Chlorine Dioxide. J. Appl. Microbiol. 2003, 95, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, D.; Dahlberg, T.; Wiklund, K.; Andersson, P.O.; Henriksson, S.; Andersson, M. Mode of Action of Disinfection Chemicals on the Bacterial Spore Structure and Their Raman Spectra. Anal. Chem. 2021, 93, 3146–3153. [Google Scholar] [CrossRef]
- Foegeding, P.M.; Hemstapat, V.; Giesbrecht, F.G. Chlorine Dioxide Inactivation of Bacillus and Clostridium Spores. J. Food Sci. 1986, 51, 197–201. [Google Scholar] [CrossRef]
- Friedline, A.; Zachariah, M.; Middaugh, A.; Heiser, M.; Khanna, N.; Vaishampayan, P.; Charles, V.R. Sterilization of Hydrogen Peroxide Resistant Bacterial Spores with Stabilized Chlorine Dioxide. AMB Express 2015, 5, 24. [Google Scholar] [CrossRef]
- Perez, J.; Springthorpe, V.S.; Sattar, S.A. Activity of Selected Oxidizing Microbicides against the Spores of Clostridium Difficile: Relevance to Environmental Control. Am. J. Infect. Control 2005, 33, 320–325. [Google Scholar] [CrossRef]
- Hartmann, L. The Sporicidal Effect of Chlorine Dioxide against Clostridium Difficile Spores with and without Presence of Organic Material. Bachelor’s Thesis, School of Medicine, Örebro University, Örebro, Sweden, 2015. [Google Scholar]
- European Standard EN 14347:2005; Chemical Disinfectants and Antiseptics—Basic Sporicidal Activity—Test Method and Requirements (Phase 1, Step 1). European Committee for Standardization: Brussels, Belgium, 2005.
- European Standard EN 13704:2018; Chemical Disinfectants—Quantitative Suspension Test for the Evaluation of Sporicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic and Institutional Areas—Test Method and Requirements (Phase 2, Step 1). European Committee for Standardization: Brussels, Belgium, 2018.
- European Standard EN 17126:2018; Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Sporicidal Activity of Chemical Disinfectants in the Medical Area. Test Method and Requirements (Phase 2, Step 1). European Committee for Standardization: Brussels, Belgium, 2018.
- Tarka, P.; Chojecka, A.; Paduch, O.; Nitsch-Osuch, A.; Kanecki, K. Assessment of the Lethal Activity of Preparations for Chemical and Thermal Disinfection of Hospital Linen in the Light of the New European Standard PN-EN 16616: 2015–10. Chemical and Thermal Disinfection of Textiles. Postępy Mikrobiol. 2017, 56, 113–119. [Google Scholar]
- European Standard EN 13697:2015; Chemical Disinfectants and Antiseptics, Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants Used in Food, Industrial, Domestic and Institutional Areas. Test Method and Requirements without Mechanical Action (Phase 2, Step 2). European Committee for Standardization: Brussels, Belgium, 2015.
- Tarka, P.; Kanecki, K.; Tomasiewicz, K. Evaluation of the Performance of Chemical Disinfectants Intended for Surfaces Using Carrier Methods. Bactericidal, Yeasticidal and Sporicidal Activity. Postępy Mikrobiol. 2016, 55, 99–104. [Google Scholar]
- European Standard EN 17272:2020; Chemical Disinfectants and Antiseptics—Methods of Airborne Room Disinfection by Automated Process—Determination of Bactericidal, Mycobactericidal, Sporicidal, Fungicidal, Yeasticidal, Virucidal and Phagocidal Activities. European Committee for Standardization: Brussels, Belgium, 2020.

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augustyn, W.; Chruściel, A.; Hreczuch, W.; Kalka, J.; Tarka, P.; Kierat, W. Inactivation of Spores and Vegetative Forms of Clostridioides difficile by Chemical Biocides: Mechanisms of Biocidal Activity, Methods of Evaluation, and Environmental Aspects. Int. J. Environ. Res. Public Health 2022, 19, 750. https://doi.org/10.3390/ijerph19020750
Augustyn W, Chruściel A, Hreczuch W, Kalka J, Tarka P, Kierat W. Inactivation of Spores and Vegetative Forms of Clostridioides difficile by Chemical Biocides: Mechanisms of Biocidal Activity, Methods of Evaluation, and Environmental Aspects. International Journal of Environmental Research and Public Health. 2022; 19(2):750. https://doi.org/10.3390/ijerph19020750
Chicago/Turabian StyleAugustyn, Weronika, Arkadiusz Chruściel, Wiesław Hreczuch, Joanna Kalka, Patryk Tarka, and Wojciech Kierat. 2022. "Inactivation of Spores and Vegetative Forms of Clostridioides difficile by Chemical Biocides: Mechanisms of Biocidal Activity, Methods of Evaluation, and Environmental Aspects" International Journal of Environmental Research and Public Health 19, no. 2: 750. https://doi.org/10.3390/ijerph19020750
APA StyleAugustyn, W., Chruściel, A., Hreczuch, W., Kalka, J., Tarka, P., & Kierat, W. (2022). Inactivation of Spores and Vegetative Forms of Clostridioides difficile by Chemical Biocides: Mechanisms of Biocidal Activity, Methods of Evaluation, and Environmental Aspects. International Journal of Environmental Research and Public Health, 19(2), 750. https://doi.org/10.3390/ijerph19020750






