UltraViolet SANitizing System for Sterilization of Ambulances Fleets and for Real-Time Monitoring of Their Sterilization Level
Abstract
1. Introduction
2. Materials and Methods
2.1. System Description
2.2. Cells
2.3. SARS-CoV-2 Strain
2.4. Inactivation of SARS-CoV-2 by UVC-Irradiation
2.5. SARS-CoV-2 Viral Titration
2.6. UVC Disinfection Test on Ambulance
3. Results
3.1. Evaluation of 254-nm Ultraviolet Light on Disinfecting SARS-CoV-2 Surface Contamination
3.2. On Site Effectiveness of UV-SAN System
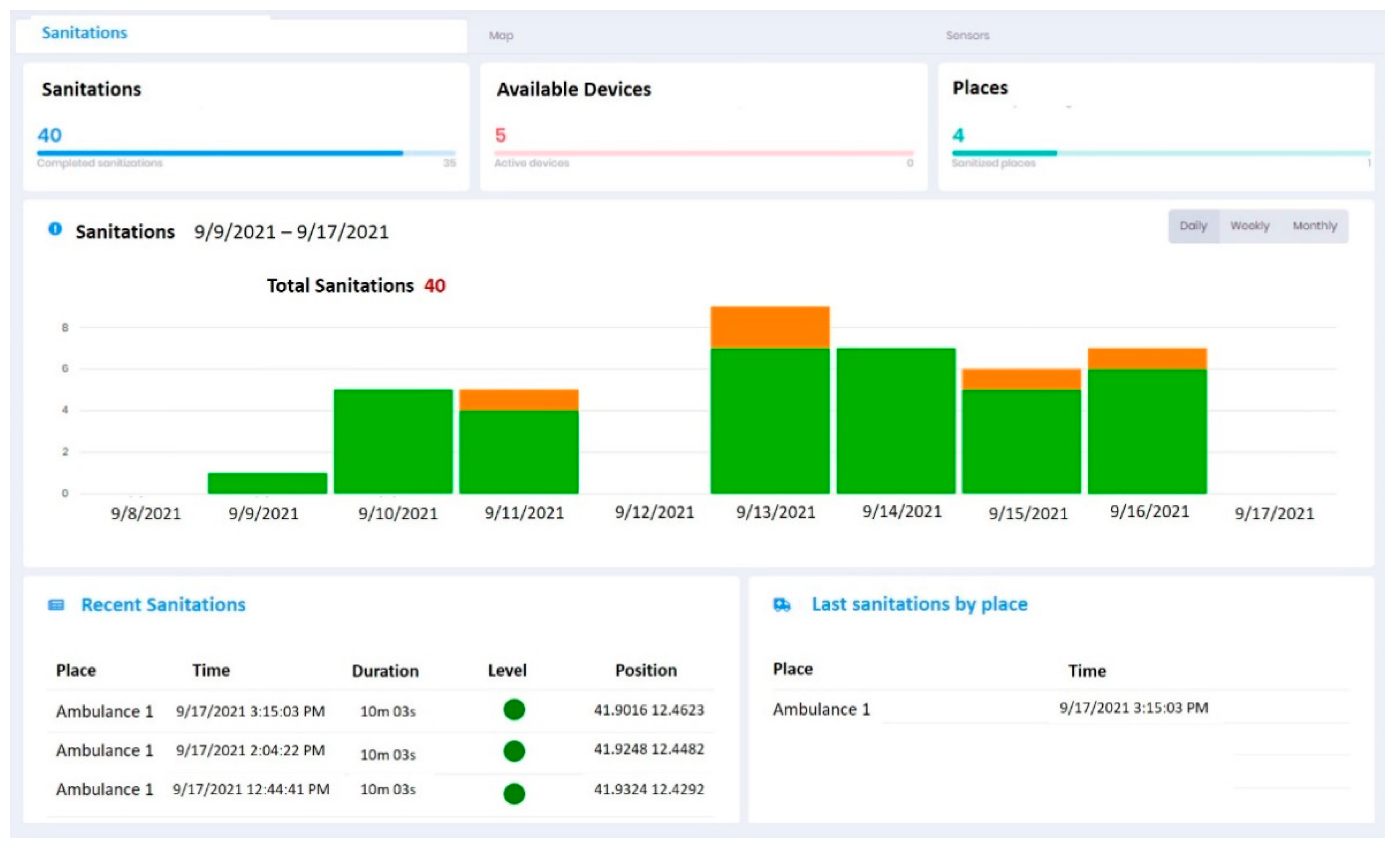
3.3. Trial Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BASS | Business Applications and Space Solutions |
| BSL-3 | BioSafety Level-3 |
| CCS | Central Control System |
| CFU | Colony Forming Unit |
| COVID-19 | COronaVIrus Disease 19 |
| DB | Data Base |
| DMEM | Dulbecco’s Modified Eagles |
| ESA | European Space Agency |
| FDA | Food and Drug Administration |
| GNSS | Global Navigation Satellite System |
| ISS | Istituto Superiore di Sanità |
| KPC | Carbapenemase-producing Klebsiella pneumoniae |
| KPIs | Key Performance Indicators |
| LSE | Lighting low pressure mercury vapor lamps |
| McF | McFarland standard |
| MDR | Multi Drug Resistant |
| MRCoNS | Methicillin-Resistant Coagulase-Negative Staphylococci |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| SARS-CoV-1 | Severe Acute Respiratory Syndrome CoronaVirus 1 |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome CoronaVirus 2 |
| SAT-COM | Satellite Communication System |
| SAT-NAV | Satellite Navigation System |
| SE | Sanitation Equipment |
| TCID50 | Tissue Culture Infectious Dose 50 |
| UVC | UltraViolet C |
| UV-SAN | UltraViolet SANitizing System |
| WHO | World Health Organization |
References
- Jarvis, M.C. Aerosol transmission of SARS-CoV-2: Physical principles and implications. Front. Public Health 2020, 8, 590041. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020, 144, 106039. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. The Proportion of SARS-CoV-2 Infections That Are Asymptomatic: A Systematic Review. Ann. Intern. Med. 2021, 174, 655–662. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases (U.S.). Division of Viral Diseases. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Coronavirus Disease 2019 (CoVID-19). 30 June 2020. Available online: https://stacks.cdc.gov/view/cdc/89980 (accessed on 22 December 2021).
- Magurano, F.; Baggieri, M.; Marchi, A.; Rezza, G.; Nicoletti, L. COVID-19 Study Group. SARS-CoV-2 infection: The environmental endurance of the virus can be influenced by the increase of temperature. Clin. Microbiol. Infect. 2021, 27, 289.e5–289.e7. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J.; The Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Update: May 2019. CDC. Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ (accessed on 22 December 2021).
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 10285, Erratum in Sci. Rep. 2021, 11, 19569. [Google Scholar] [CrossRef]
- Yang, J.H.; Wu, U.I.; Tai, H.M.; Sheng, W.H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef]
- Budowsky, E.I.; Bresler, S.E.; Friedman, E.A.; Zheleznova, N.V. Principles of selective inactivation of viral genome. I. UV-induced inactivation of influenza virus. Arch. Virol. 1981, 68, 239–247. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Singh, H.; Deep, A.; Khatri, M.; Bhaumik, J.; Kim, K.H.; Bhardwaj, N. UVC-based photoinactivation as an efficient tool to control the transmission of coronaviruses. Sci. Total Environ. 2021, 792, 148548. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, A.H.; Underbrink, A.G.; Sparrow, R.C. Chromosomes and cellular radiosensitivity. I. The relationship of D0 to chromosome volume and complexity in seventy-nine different organisms. Radiat. Res. 1967, 32, 915–945. [Google Scholar] [CrossRef]
- Noh, H.; Shin, S.D.; Kim, N.J.; Ro, Y.S.; Oh, H.S.; Joo, S.I.; Kim, J.I.; Ong, M.E. Risk stratification-based surveillance of bacterial contamination in metropolitan ambulances. J. Korean Med. Sci. 2011, 26, 124–130. [Google Scholar] [CrossRef][Green Version]
- Roline, C.E.; Crumpecker, C.; Dunn, T.M. Can methicillin-resistant Staphylococcus aureus be found in an ambulance fleet? Prehospital Emerg. Care. 2007, 11, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Galtelli, M.; Deschamp, C.; Rogers, J. An assessment of the prevalence of pathogenic microorganisms in the rotor wing air ambulance: One program’s findings. Air Med. J. 2006, 25, 81–84. [Google Scholar] [CrossRef]
- Makiela, S.; Taylor-Robinson, A.W.; Weber, A.; Maguire, B.J. A preliminary assessment of contamination of emergency service helicopters with MRSA and multi-resistant Staphylococcus aureus. Emerg. Med. Open Access 2016, 6, 304–310. [Google Scholar] [CrossRef]
- Alves, D.W.; Bissell, R.A. Bacterial pathogens in ambulances: Results of unannounced sample collection. Prehospital Emerg. Care 2008, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.; Minnon, J.; Schneider, S.; Vaughn, J. Prevalence of methicillin-resistant Staphylococcus aureus in ambulances in southern Maine. Prehospital Emerg. Care 2010, 14, 176–181. [Google Scholar] [CrossRef]
- Nigam, Y.; Cutter, J. A preliminary investigation into bacterial contamination of Welsh emergency ambulances. Emerg. Med. J. 2003, 20, 479–482. [Google Scholar] [CrossRef]
- Gidari, A.; Sabbatini, S.; Bastianelli, S.; Pierucci, S.; Busti, C.; Bartolini, D.; Stabile, A.M.; Monari, C.; Galli, F.; Rende, M.; et al. SARS-CoV-2 Survival on Surfaces and the Effect of UV-C Light. Viruses 2021, 13, 408. [Google Scholar] [CrossRef]
- Meselson, M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2063. [Google Scholar] [CrossRef] [PubMed]
- Bueckert, M.; Gupta, R.; Gupta, A.; Garg, M.; Mazumder, A. Infectivity of SARS-CoV-2 and other coronaviruses on dry surfaces: Potential for indirect transmission. Materials 2020, 13, 5211. [Google Scholar] [CrossRef]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rashid, R.A.R.; Palanisamy, S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef]
- Pastorino, B.; Touret, F.; Gilles, M.; de Lamballerie, X.; Charrel, R.N. Prolonged Infectivity of SARS-CoV-2 in Fomites. Emerg. Infect. Dis. 2020, 26, 2256–2257. [Google Scholar] [CrossRef] [PubMed]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, e00441-20. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Smith, K.C. Physical and chemical changes induced in nucleic acids by ultraviolet light. Radiat. Res. Suppl. 1966, 6, 54–79. [Google Scholar] [CrossRef]
- Biasin, M.; Bianco, A.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, C.; Galli, P.; Lessio, L.; Lualdi, M.; Tombetti, E.; et al. UV-C irradiation is highly effective in inactivating SARS-CoV-2 replication. Sci. Rep. 2021, 11, 6260. [Google Scholar] [CrossRef]
- Beggs, C.B.; Avital, E.J. Upper-room ultraviolet air disinfection might help to reduce COVID-19 transmission in buildings: A feasibility study. PeerJ 2020, 8, e10196. [Google Scholar] [CrossRef] [PubMed]
- Sabino, C.P.; Sellera, F.P.; Sales-Medina, D.F.; Machado, R.R.G.; Durigon, E.L.; Freitas-Junior, L.H.; Ribeiro, M.S. UV-C (254 nm) lethal doses for SARS-CoV-2. Photodiagn. Photodyn. Ther. 2020, 32, 101995. [Google Scholar] [CrossRef]
- Inagaki, H.; Saito, A.; Sugiyama, H.; Okabayashi, T.; Fujimoto, S. Rapid inactivation of SARS-CoV-2 with Deep-UV LED irradiation. Emerg. Microbes Infect. 2020, 9, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Ozog, D.M.; Sexton, J.Z.; Narla, S.; Pretto-Kernahan, C.D.; Mirabelli, C.; Lim, H.W.; Hamzavi, I.H.; Tibbetts, R.J.; Mi, Q.S. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int. J. Infect. Dis. 2020, 100, 224–229. [Google Scholar] [CrossRef]
- Fischer, R.J.; Morris, D.H.; Van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Effectiveness of N95 respirator decontamination and reuse against SARS-CoV-2 Virus. Emerg. Infect. Dis. 2020, 26, 2253–2255. [Google Scholar] [CrossRef]
- Claus, H. Ozone Generation by Ultraviolet Lamps. Photochem. Photobiol. 2021, 97, 471–476. [Google Scholar] [CrossRef]
- Stefanelli, P.; Faggioni, G.; Lo Presti, A.; Fiore, S.; Marchi, A.; Benedetti, E.; Fabiani, C.; Anselmo, A.; Ciammaruconi, A.; Fortunato, A.; et al. Whole genome and phylogenetic analysis of two SARS-CoV-2 strains isolated in Italy in January and February 2020: Additional clues on multiple introductions and further circulation in Europe. Eurosurveillance 2020, 25, 2000305. [Google Scholar] [CrossRef] [PubMed]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Spearman, C. The Method of “Right and Wrong Cases” (Constant Stimuli) without Gauss’s Formula. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar]
- Pereira, R.V.; Bicalho, M.L.; Machado, V.S.; Lima, S.; Teixeira, A.G.; Warnick, L.D.; Bicalho, R.C. Evaluation of the effects of ultraviolet light on bacterial contaminants inoculated into whole milk and colostrum, and on colostrum immunoglobulin G. J. Dairy Sci. 2014, 97, 2866–2875. [Google Scholar] [CrossRef]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of Antibiotic-Resistance by MALDI-TOF Mass Spectrometry: An Expanding Area. Front. Cell. Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef]
- Anderson, D.J.; Chen, L.F.; Weber, D.J.; Moehring, R.W.; Lewis, S.L.; Triplett, P.F.; Blocker, M.; Becherer, P.; Schwab, J.C.; Knelson, L.P.; et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): A cluster-randomised, multicentre, crossover study. Lancet 2017, 389, 805–814. [Google Scholar] [CrossRef]
- Giese, N.; Darby, J. Sensitivity of microorganisms to different wavelengths of UV light: Implications on modeling of medium pressure UV systems. Water Res. 2000, 34, 4007–4013. [Google Scholar] [CrossRef]
- Shoults, D.C.; Ashbolt, N.J. Decreased efficacy of UV inactivation of Staphylococcus aureus after multiple exposure and growth cycles. Int. J. Hyg. Environ. Health 2019, 222, 111–116. [Google Scholar] [CrossRef]
- Williams, P.D.; Eichstadt, S.L.; Kokjohn, T.A.; Martin, E. Effects of Ultraviolet Radiation on the Gram-Positive Marine Bacterium Microbacterium maritypicum. Curr. Microbiol. 2007, 55, 1–7. [Google Scholar] [CrossRef]
- Ronald, G.R.; Wagnera, M.; Veerasubramaniana, P.; Payment, P. Disinfection efficiency of peracetic acid, UV and ozone after enhanced primary treatment of municipal wastewater. Water Res. 2003, 37, 4573–4586. [Google Scholar] [CrossRef]
- Raeiszadeh, M.; Adeli, B. A Critical Review on Ultraviolet Disinfection Systems against COVID-19 Outbreak: Applicability, Validation, and Safety Considerations. ACS Photonics 2020, 7, 2941–2951. [Google Scholar] [CrossRef]
- Kowalsky, W. Ultraviolet Germicidal Irradiation Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–117. [Google Scholar]


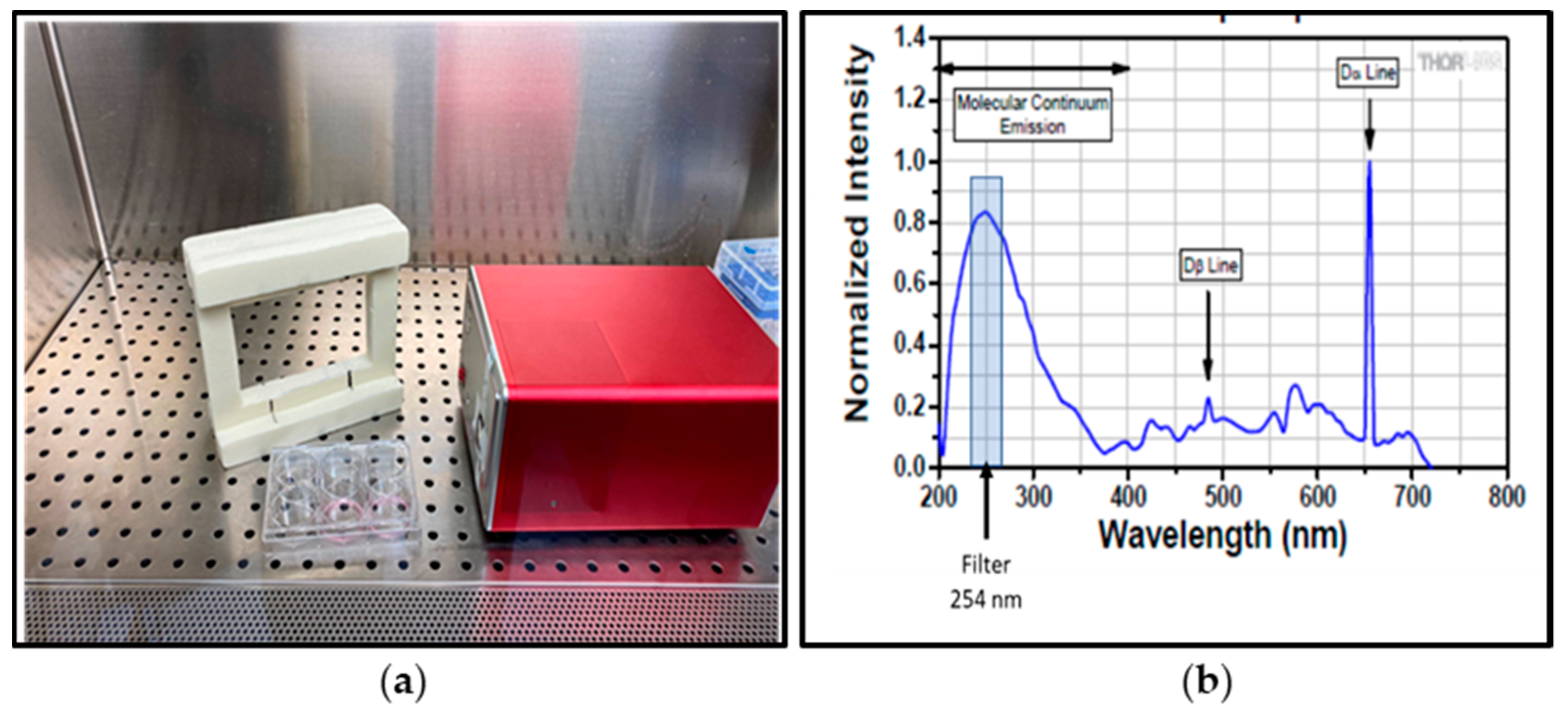



| Material | UVC Dose for Log3 Reduction [J/m2] 95% Confidence Level | p Value | R2 | ||
|---|---|---|---|---|---|
| Lower | Mean | Upper | |||
| Polystyrene | 135 | 184 | 226 | 5.3 × 10−3 | 0.944 |
| Metal | 92 | 129 | 164 | 5.7 × 10−3 | 0.947 |
| Rubber | 29.9 | 54.4 | 98.3 | 9.3 × 10−2 | 0.66 |
| Log Reduction | % Reduction | UVC Dose [J/m2] | ||
|---|---|---|---|---|
| Polystyrene | Metal | Rubber | ||
| Log1 | 90% | 57.3 | 46.2 | 7.8 |
| Log2 | 99% | 121 | 87.5 | 23.4 |
| Log3 | 99.9% | 184 | 129 | 54.4 |
| Log4 | 99.99% | 248 | 170 | 55.8 |
| Position | Pathogen and Concentration | Exposure Time [min] | Irradiation [mJ/cm2] | Growth after Incubation | |
|---|---|---|---|---|---|
| 24 h | 48 h | ||||
| HeadRest | Kleb. Pne. ESBL+ 0.5, 1, 2 McF * | 10 | 76 | None | None |
| 20 | 152 | None | None | ||
| 30 | 228 | None | None | ||
| Staph. aur. MRSA 0.5, 1, 2 McF * | 10 | 76 | None | None | |
| 20 | 152 | None | None | ||
| 30 | 228 | None | None | ||
| Armrest | Kleb. Pne. ESBL+ 0.5, 1, 2 McF * | 10 | 44 | None | None |
| 20 | 88 | None | None | ||
| 30 | 132 | None | None | ||
| Staph. aur. MRSA 0.5, 1, 2 McF * | 10 | 44 | None | None | |
| 20 | 88 | None | None | ||
| 30 | 132 | None | None | ||
| Chair | Kleb. Pne. ESBL+ 0.5, 1, 2 McF * | 10 | 45 | None | None |
| 20 | 90 | None | None | ||
| 30 | 135 | None | None | ||
| Staph. aur. MRSA 0.5, 1, 2 McF * | 10 | 45 | None | None | |
| 20 | 90 | None | None | ||
| 30 | 135 | None | None | ||
| Urinary catheter zone | Kleb. Pne. ESBL+ 0.5, 1, 2 McF * | 10 | 18 | None | None |
| 20 | 36 | None | None | ||
| 30 | 54 | None | None | ||
| Staph. aur. MRSA 0.5 McF | 10 | 18 | 2 | 2 | |
| 20 | 36 | 2 | 2 | ||
| 30 | 54 | 2 | 2 | ||
| Staph. aur. MRSA 1 McF | 10 | 18 | >100 | >100 | |
| 20 | 36 | 3 | 3 | ||
| 30 | 54 | 2 | 2 | ||
| Staph. aur. MRSA 2 McF | 10 | 18 | >100 | >100 | |
| 20 | 36 | 3 | 3 | ||
| 30 | 54 | 2 | 2 | ||
| KPI | Definition | Type of Measure | Expected Result | Result |
|---|---|---|---|---|
| KPI 1 | SARS-CoV-2 and MDR pathogen inactivation | Laboratory Test On-Site Test | Reduction >99% | Confirmed in about 10 min |
| KPI 2 | Monitoring & Traceability | Analysis of DB data | <1% loss of data | <0.1% |
| KPI 3 | Real Time Monitoring | Analysis of DB data transmitted with standard comms link | <1 min | <30 s |
| KPI 4 | SAT-COM data transmission | Analysis of DB data of data transmitted with SAT-COM | <3 min | <150 s |
| KPI 5 | Safety Check (ozone, human presence) | Reports from Pilot User | <1% | No problem detected |
| KPI6 | Time & Work-load Reduction | Report from Pilot User | No downtime of ambulance service | Confirmed |
| Sanitation Procedure for Transportation of COVID-19 Patients | |||
|---|---|---|---|
| Pandemic Period 2020 | Pandemic Period 2021 | UV-SAN | |
| Procedure | Sanitation performed after the transportation of each COVID-19 patient:
| Sanitation performed only at the end of the shift:
| Sanitation performed at any time when the compartment is empty |
| Time needed for the sanitation | 2 h | Time needed for the sanitation | |
| Service down-time | 2 h for each transport | None (sanitation performed at the end of the shift) | None |
| Location | Depot | Depot | Everywhere |
| Staff needed | 1 person fully employed | 1 person fully employed | 1 person partially employed (start and stop sanitation) |
| Traceability | Manually recorded | Manually recorded | Automatic |
| Surface Characteristics | Time for Log Reduction [s] | |||||
|---|---|---|---|---|---|---|
| Position | Material | Irradiance [W/cm2] | Log1 | Log2 | Log3 | Log4 |
| Instruments shelf | Plastic | 1 | 46 | 88 | 129 | 170 |
| Stretcher base | Metal | 0.45 | 127 | 269 | 410 | 551 |
| Back Door | Plastic | 0.24 | 193 | 365 | 537 | 709 |
| Lateral seat | Plastic | 1 | 46 | 88 | 129 | 170 |
| Seat | Plastic | 0.35 | 132 | 250 | 368 | 486 |
| Floor 1 | Rubber | 0.65 | 12 | 36 | 84 | 132 |
| Headrest | Plastic | 1.27 | 36 | 69 | 101 | 134 |
| Floor 2 | Rubber | 0.65 | 12 | 36 | 84 | 132 |
| Stretcher Armrest (right) | Metal | 0.74 | 77 | 163 | 249 | 335 |
| Stretcher Armrest (left) | Metal | 0.72 | 80 | 168 | 256 | 344 |
| Seat Armrest (right) | Plastic | 0.75 | 62 | 117 | 172 | 227 |
| Seat Armrest (left) | Plastic | 1.34 | 34 | 65 | 96 | 127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michelini, Z.; Mazzei, C.; Magurano, F.; Baggieri, M.; Marchi, A.; Andreotti, M.; Cara, A.; Gaudino, A.; Mazzalupi, M.; Antonelli, F.; et al. UltraViolet SANitizing System for Sterilization of Ambulances Fleets and for Real-Time Monitoring of Their Sterilization Level. Int. J. Environ. Res. Public Health 2022, 19, 331. https://doi.org/10.3390/ijerph19010331
Michelini Z, Mazzei C, Magurano F, Baggieri M, Marchi A, Andreotti M, Cara A, Gaudino A, Mazzalupi M, Antonelli F, et al. UltraViolet SANitizing System for Sterilization of Ambulances Fleets and for Real-Time Monitoring of Their Sterilization Level. International Journal of Environmental Research and Public Health. 2022; 19(1):331. https://doi.org/10.3390/ijerph19010331
Chicago/Turabian StyleMichelini, Zuleika, Chiara Mazzei, Fabio Magurano, Melissa Baggieri, Antonella Marchi, Mauro Andreotti, Andrea Cara, Alessandro Gaudino, Marco Mazzalupi, Francesca Antonelli, and et al. 2022. "UltraViolet SANitizing System for Sterilization of Ambulances Fleets and for Real-Time Monitoring of Their Sterilization Level" International Journal of Environmental Research and Public Health 19, no. 1: 331. https://doi.org/10.3390/ijerph19010331
APA StyleMichelini, Z., Mazzei, C., Magurano, F., Baggieri, M., Marchi, A., Andreotti, M., Cara, A., Gaudino, A., Mazzalupi, M., Antonelli, F., Sommella, L., Angeletti, S., Razzano, E., Runge, A., & Petrinca, P. (2022). UltraViolet SANitizing System for Sterilization of Ambulances Fleets and for Real-Time Monitoring of Their Sterilization Level. International Journal of Environmental Research and Public Health, 19(1), 331. https://doi.org/10.3390/ijerph19010331






