Assessment of Stress Salivary Markers, Perceived Stress, and Shift Work in a Cohort of Fishermen: A Preliminary Work
Abstract
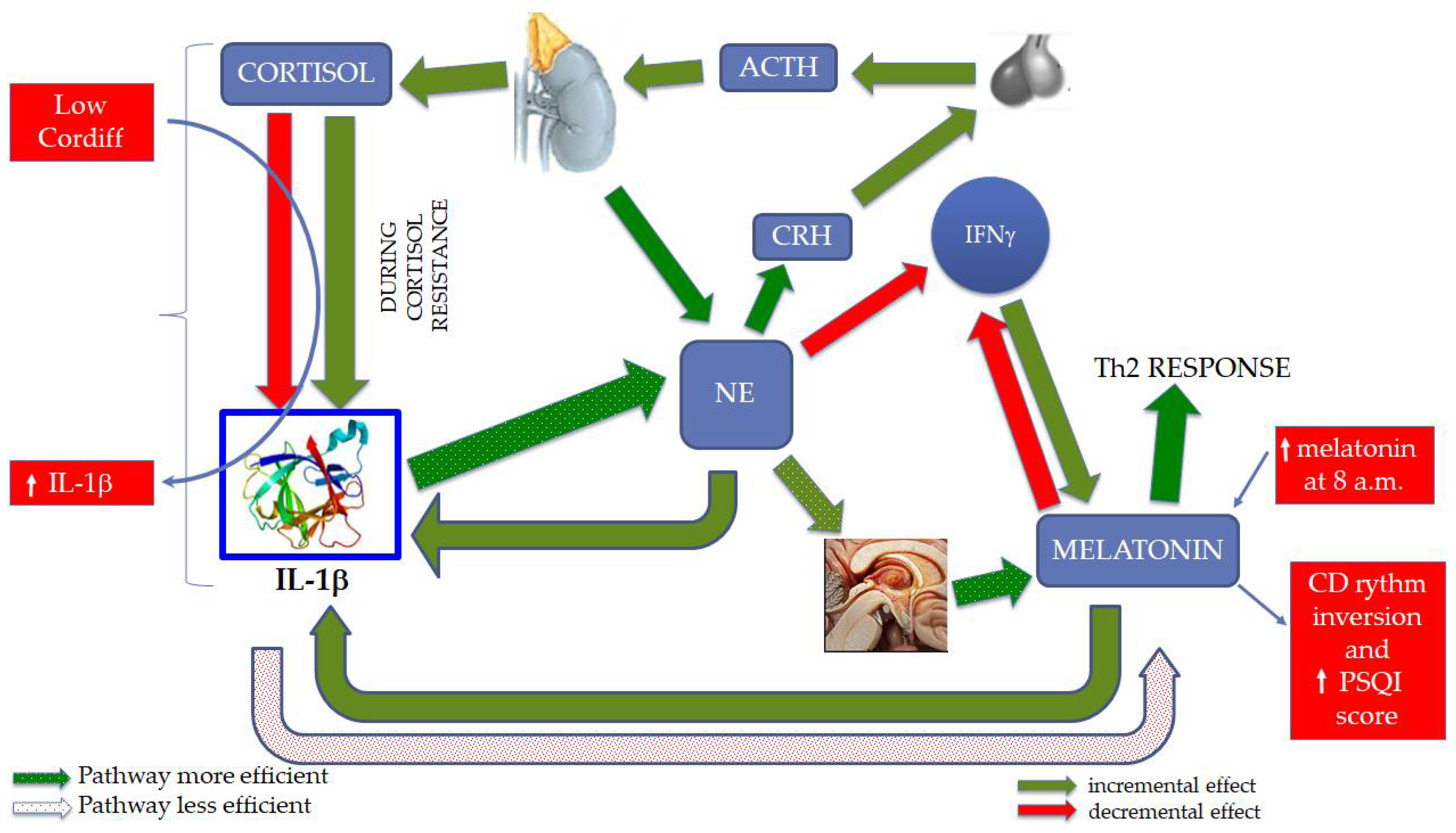
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Evaluation of Perceived Stress
2.3. Saliva Samples Collection
2.4. ELISA Assays
2.5. Statistical Analysis
3. Results
3.1. Salivary Marker Levels
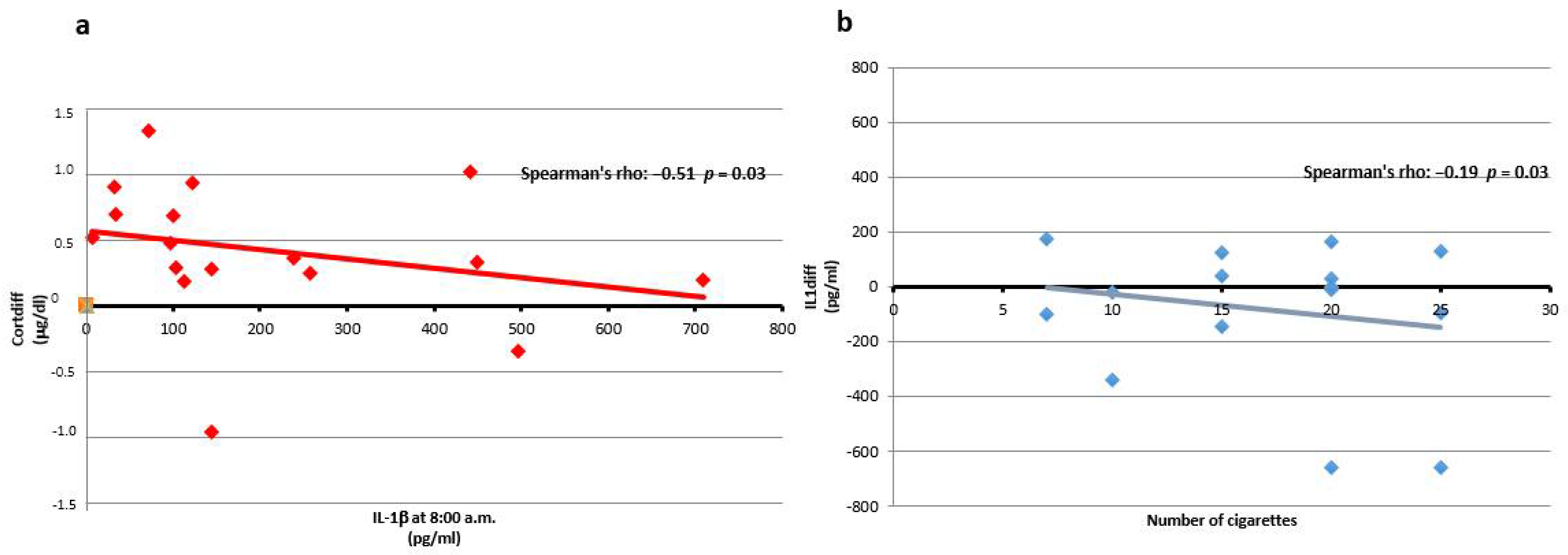
3.2. IL-1β
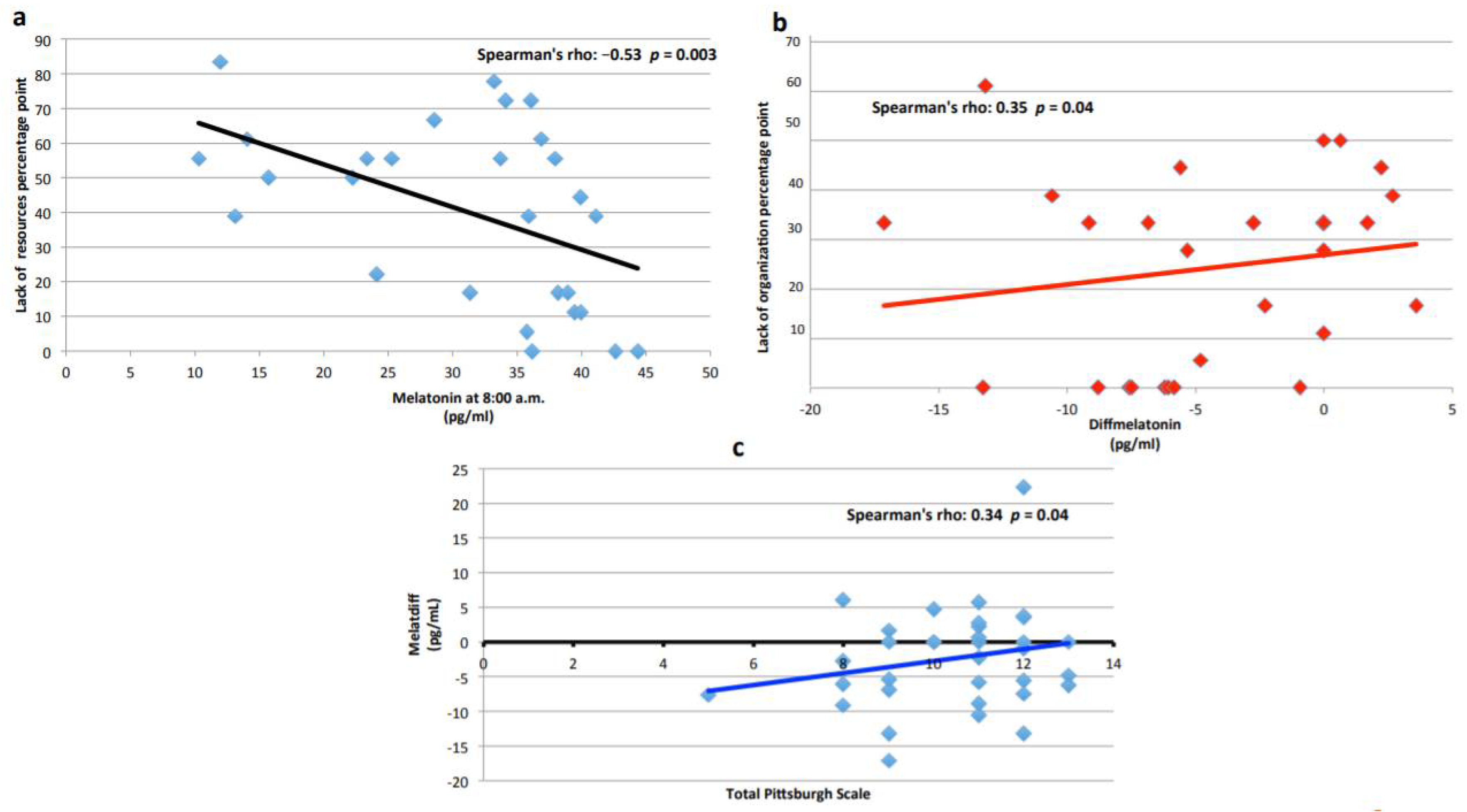
3.3. Melatonin
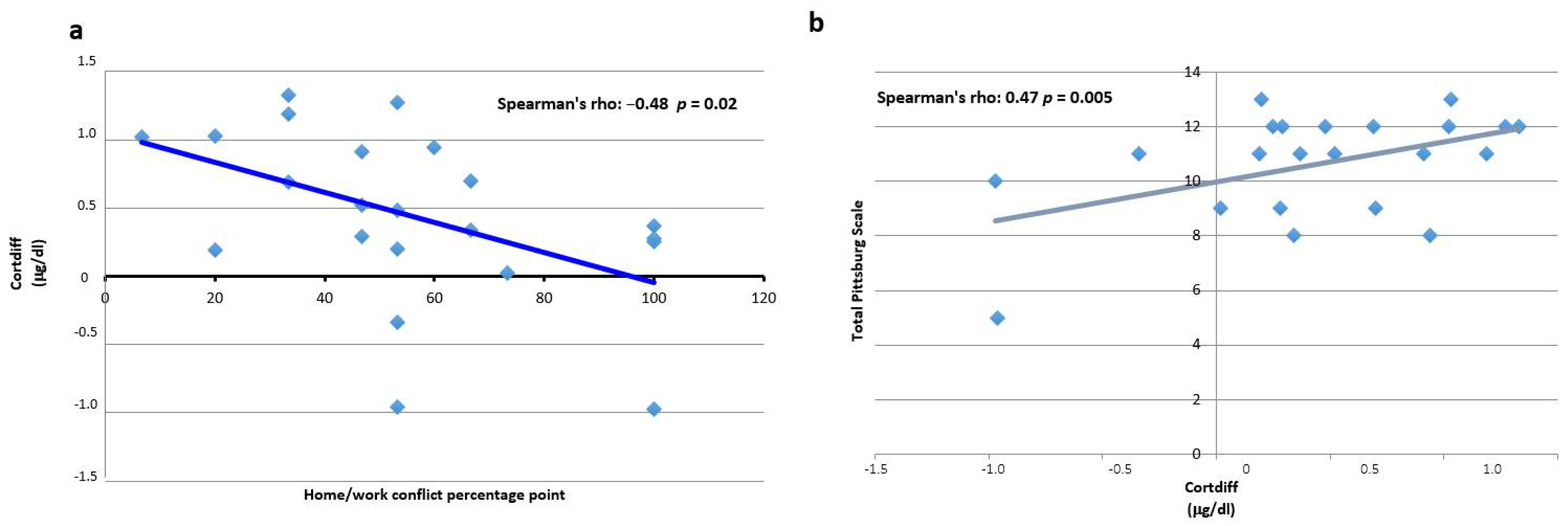
3.4. Cortisol
3.5. Shift Work
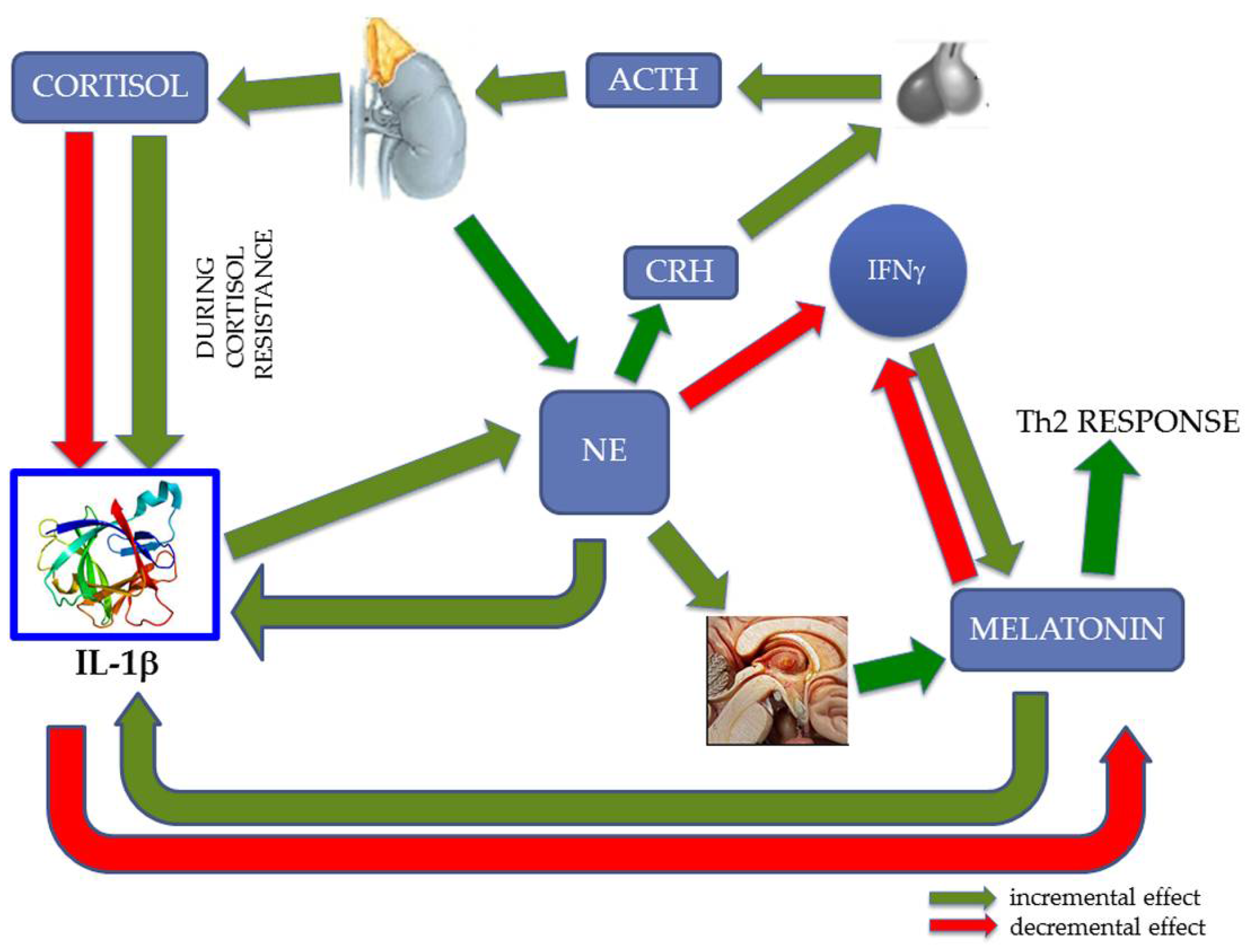
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seyle, H. The Stress of Life; McGraw-Hill Book Company: New York, NY, USA, 1956. [Google Scholar]
- Cohen, S.; Miller, G.E.; Rabin, B.S. Psychological stress and antibody response to immunization: A critical review of the human literature. Psychosom. Med. 2001, 63, 7–18. [Google Scholar] [CrossRef]
- Karasek, R.; Brisson, C.; Kawakami, N.; Houtman, I.; Bongers, P.; Amick, B. The job content questionnaire (JCQ): An instrument for internationally comparative assessments of psychosocial job characteristics. J. Occup. Health Psychol. 1998, 3, 322–355. [Google Scholar] [CrossRef]
- De Lange, A.H.; Taris, T.W.; Kompier, M.A.; Houtman, I.L.; Bongers, P.M. Different mechanisms to explain the reversed effects of mental health on work characteristics. Scand. J. Work Environ. Health 2005, 31, 3–14. [Google Scholar] [CrossRef]
- Dikkers, J.S.E.; Geurts, S.A.E.; Kinnunen, U.; Kompier, M.A.J.; Taris, T.W. Crossover between work and home in dyadic partner relationships. Scand. J. Psychol. 2007, 48, 529–538. [Google Scholar] [CrossRef]
- Perrewé, P.L.; Zellars, K.L. An examination of attributions and emotions in the transactional approach to the organizational stress process. J. Organ. Behav. 1999, 20, 739–752. [Google Scholar] [CrossRef]
- Dewe, P. Coping and appraisal in a work setting: A closer examination of the relationship. In Handbook of Managerial Behaviour and Occupational Health; Antoniou, A.S.G., Cooper, C.L., Chrousos, G.P., Spielberger, C.D., Eysenck, M.W., Eds.; Edward Elgar: Cheltenham, UK, 2009; pp. 62–76. [Google Scholar]
- Siegrist, J. Adverse health effects of high-effort/low-reward conditions. J. Occup. Health Psychol. 1996, 1, 27–41. [Google Scholar] [CrossRef]
- Siegrist, J.; Li, J. Associations of extrinsic and intrinsic components of work stress with health: A systematic review of evidence on the effort-reward imbalance model. Int. J. Environ. Res. Public Health 2016, 13, 432. [Google Scholar] [CrossRef]
- Sun, R.C.; Lan, Y.J. The correlation between worker-occupation fit and occupational stress in nurses. Chin. J. Prev. Med. 2020, 54, 1197–1201. [Google Scholar]
- Fischer, D.; Lombardi, D.A.; Folkard, S.; Willetts, J.; Christiani, D.C. Updating the “Risk Index”: A systematic review and meta-analysis of occupational injuries and work schedule characteristics. Chronobiol. Int. 2017, 34, 1423–1438. [Google Scholar] [CrossRef]
- Åstrand, I.; Fugelli, P.; Karlsson, C.G.; Rodahl, K.; Vokac, Z. Energy output and work stress in coastal fishing. Scand. J. Clin. Lab. Investig. 1973, 31, 105–113. [Google Scholar] [CrossRef]
- Allen, P.; Wellens, B.; Smith, A. Fatigue in British fishermen. Int. Marit. Health 2010, 62, 154–158. [Google Scholar]
- Hovdanum, A.S.A.; Jensen, O.C.; Petursdottir, G.; Holmen, I.M. A review of fatigue in fishermen: A complicated and under-prioritised area of research. Int. Marit. Health 2014, 65, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gander, P.; van den Berg, M.; Signal, L. Sleep and sleepiness of fishermen on rotating schedules. Chronobiol. Int. 2008, 25, 389–398. [Google Scholar] [CrossRef]
- Allegri, F.; Passarello, B.; Orrù, G.; Coppola, A.; Antona, A.; Cannizzaro, E.; Gagliano, M. Effects of prolonged work on “deep-sea” fishermen: Influence of blood cortisol, prolactinemia and urinary catecholamines. G. Ital. Med. Lav. 1996, 18, 101–105. [Google Scholar] [PubMed]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear and Rage; D. Appleton & Company: New York, NY, USA, 1915. [Google Scholar]
- Karlson, B.; Lindfors, P.; Riva, R.; Mellner, C.; Theorell, T.; Lundberg, U. Psychosocial work stressors and salivary cortisol. In The Role of Saliva Cortisol Measurement in Health and Disease; Kristenson, M., Garvin, P., Lundberg, U., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 43–66. [Google Scholar]
- Hodes, G.E.; Pfau, M.L.; Leboeuf, M.; Golden, S.A.; Christoffel, D.J.; Bregman, D.; Rebusi, N.; Heshmati, M.; Aleyasin, H.; Warren, B.L.; et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA 2014, 111, 16136–16141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, J.M.; Rector, D.M.; Churchill, L. Sleep and cytokines. Sleep Med. Clin. 2007, 2, 161–169. [Google Scholar] [CrossRef]
- Obal, F., Jr.; Krueger, J.M. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003, 8, d520–d550. [Google Scholar]
- Kapsimalis, F.; Richardson, G.; Opp, M.R.; Kryger, M. Cytokines and normal sleep. Curr. Opin. Pulm. Med. 2005, 11, 481–484. [Google Scholar] [CrossRef]
- Berkenbosch, F.; van Oers, J.; del Rey, A.; Tilders, F.; Besedovsky, H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science 1987, 238, 524–526. [Google Scholar] [CrossRef]
- Sapolsky, R.; Rivier, C.; Yamamoto, G.; Plotsky, P.; Vale, W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 1987, 238, 522–524. [Google Scholar] [CrossRef]
- Sirivelu, M.P.; MohanKumar, P.; MohanKumar, S.M. Interleukin-1 beta simultaneously affects the stress and reproductive axes by modulating norepinephrine levels in different brain areas. Life Sci. 2012, 91, 878–884. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, T.M.; O’Halloran, D.J.; Shanahan, F. The stress response and the hypothalamic-pituitary-adrenal axis: From molecule to melancholia. QJM 2000, 93, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Pierpaoli, W.; Maestroni, G.J. Melatonin: A principal neuroimmunoregulatory and anti-stress hormone: Its anti-aging effects. Immunol. Lett. 1987, 16, 355–361. [Google Scholar] [CrossRef]
- Terenina, E.E.; Cavigelli, S.; Mormede, P.; Zhao, W.; Parks, C.; Lu, L.; Jones, B.C.; Mulligan, M.K. Genetic factors mediate the impact of chronic stress and subsequent response to novel acute stress. Front. Neurosci. 2019, 13, 438. [Google Scholar] [CrossRef] [Green Version]
- Mattina, G.F.; Van Lieshout, R.J.; Steiner, M. Inflammation, depression and cardiovascular disease in women: The role of the immune system across critical reproductive events. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719851950. [Google Scholar] [CrossRef]
- Jeżewska, M.; Grubman-Nowak, M.; Leszczyñska, I.; Jaremin, B. Occupational hazards for fishermen in the workplace in Polish coastal and beach fishing—A point of view. Int. Marit. Health 2012, 63, 40–48. [Google Scholar]
- Cushway, D.; Tyler, P.A.; Nolan, P. Development of a stress scale for mental health professionals. Br. J. Clin. Psychol. 1996, 35, 279–295. [Google Scholar] [CrossRef]
- Curcio, G.; Tempesta, D.; Scarlata, S.; Marzano, C.; Moroni, F.; Rossini, P.M.; Ferrara, M.; De Gennaro, L. Validity of the Italian version of the Pittsburgh sleep quality index (PSQI). Neurol. Sci. 2013, 34, 511–519. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, D.-M.; Li, C.-B.; Tao, M.-F. Influencing factors for sleep quality among shift-working nurses: A cross-sectional study in China using 3-factor Pittsburgh sleep quality index. Asian Nurs. Res. 2016, 10, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Tsai, P.S.; Wang, S.Y.; Wang, M.Y.; Su, C.T.; Yang, T.T.; Huang, C.J.; Fang, S.C. Psychometric evaluation of the Chinese version of the Pittsburgh sleep quality index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005, 14, 1943–1952. [Google Scholar] [CrossRef]
- Gardner, A.; Carpenter, G.; So, P.-W. Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Okuma, N.; Saita, M.; Hoshi, N.; Soga, T.; Tomita, M.; Sugimoto, M.; Kimoto, K. Effect of masticatory stimulation on the quantity and quality of saliva and the salivary metabolomic profile. PLoS ONE 2017, 12, e0183109. [Google Scholar] [CrossRef] [Green Version]
- Cole, S.W.; Korin, Y.D.; Fahey, J.L.; Zack, J.A. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J. Immunol. 1998, 161, 610–616. [Google Scholar]
- Grebe, K.M.; Takeda, K.; Hickman, H.D.; Bailey, A.L.; Embry, A.C.; Bennink, J.R.; Yewdell, J.W. Cutting edge: Sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J. Immunol. 2010, 184, 540–544. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid re-ceptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [Green Version]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S.; Varghese, F.P.; McEwen, B.S. Association of depression with medical illness: Does cortisol play a role? Biol. Psychiatry 2004, 55, 1–9. [Google Scholar] [CrossRef]
- Marques, A.H.; Silverman, M.N.; Sternberg, E.M. Glucocorticoid dysregulations and their clinical correlates. In Glucocorticoids and Mood: Clinical Manifestations, Risk Factors and Molecular Mechanisms; Judd, L.L., Sternberg, E.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 1–18. [Google Scholar]
- McEwen, B.S. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998, 338, 171–179. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008, 583, 174–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Seeman, T. Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Russo, S.J. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 2017, 42, 62–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, E.; Schumm, L.P.; McClintock, M.; Waite, L.; Lauderdale, D.S. Sleep characteristics and daytime cortisol levels in older adults. Sleep 2017, 40, zsx043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spath-Schwalbe, E.; Gofferje, M.; Kern, W.; Born, J.; Fehm, H.L. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol. Psychiatry 1991, 29, 575–584. [Google Scholar] [CrossRef]
- Schüssler, P.; Uhr, M.; Ising, M.; Weikel, J.C.; Schmid, D.A.; Held, K.; Mathias, S.; Steiger, A. Nocturnal ghrelin, ACTH, GH and cortisol secretion after sleep deprivation in humans. Psychoneuroendocrinology 2006, 31, 915–923. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Cyprus, G.; Overlin, J.; Hotchkiss, K.; Kandalam, S.; Olivares-Navarrete, R. Cigarette smoke increases pro-inflammatory markers and inhibits osteogenic differentiation in experimental exposure model. Acta Biomater. 2018, 76, 308–318. [Google Scholar] [CrossRef]
- Theorell, T.; Hasselhorn, H.-M.; Vingård, E.; Andersson, B. Interleukin 6 and cortisol in acute musculoskeletal disorders: Results from a case-referent study in Sweden. Stress Med. 2000, 16, 27–35. [Google Scholar] [CrossRef]
- Salín-Pascual, R.J.; Ortega-Soto, H.; Huerto-Delgadillo, L.; Camacho-Arroyo, I.; Roldán-Roldán, G.; Tamarkin, L. The effect of total sleep deprivation on plasma melatonin and cortisol in healthy human volunteers. Sleep 1988, 11, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitzer, J.M.; Duffy, J.F.; Lockley, S.W.; Dijk, D.J.; Czeisler, C.A. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep 2007, 30, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Burgess, H.J. Evening ambient light exposure can reduce circadian phase advances to morning light independent of sleep deprivation. J. Sleep Res. 2013, 22, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.; Etheredge, C.E.; Marcus, L.; Bellar, D. Prolonged sleep deprivation and continuous exercise: Effects on melatonin, tympanic temperature, and cognitive function. BioMed Res. Int. 2014, 2014, 781863. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, J.J.; Díaz-Castro, J.; Kajarabille, N.; García, C.; Guisado, I.M.; De Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011, 51, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Veneroso, C.; Tuñón, M.J.; González-Gallego, J.; Collado, P.S. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J. Pineal Res. 2009, 47, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, T.; Fröberg, J.E.; Friberg, Y.; Wetterberg, L. Melatonin excretion, body temperature and subjective arousal during 64 hours of sleep deprivation. Psychoneuroendocrinology 1979, 4, 219–225. [Google Scholar] [CrossRef]
- Arias, J.; Melean, E.; Valero, N.; Pons, H.; Chacin-Bonilla, L.; Larreal, Y.; Bonilla, E. Effect of melatonin on lymphocyte proliferation and production of interleukin-2 (IL-2) and interleukin-1 beta (IL-1 beta) in mice splenocytes. Investig. Clin. 2003, 44, 41–50. [Google Scholar]
- Wichmann, M.W.; Zellweger, R.; DeMaso, C.M.; Ayala, A.; Chaudry, I.H. Melatonin administration attenuates depressed immune functions trauma-hemorrhage. J. Surg. Res. 1996, 63, 256–262. [Google Scholar] [CrossRef]
- Lin, X.J.; Mei, G.P.; Liu, J.; Li, Y.L.; Zuo, D.; Liu, S.J.; Zhao, T.B.; Lin, M.T. Therapeutic effects of melatonin on heat-stroke-induced multiple organ dysfunction syndrome in rats. J. Pineal Res. 2011, 50, 436–444. [Google Scholar] [CrossRef]
- Chen, C.F.; Wang, D.; Reiter, R.J.; Yeh, D.Y. Oral melatonin attenuates lung inflammation and airway hyperreactivity induced by inhalation of aerosolized pancreatic fluid in rats. J. Pineal Res. 2011, 50, 46–53. [Google Scholar] [CrossRef]
- Mei, Q.; Yu, J.-P.; Xu, J.-M.; Wei, W.; Xiang, L.; Yue, L. Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacol. Sin. 2002, 23, 882–886. [Google Scholar]
- Agil, A.; Reiter, R.J.; Jiménez-Aranda, A.; Ibán-Arias, R.; Navarro-Alarcón, M.; Marchal, J.A.; Adem, A.; Fernández-Vázquez, G. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 2012, 54, 381–388. [Google Scholar] [CrossRef]
- Li, J.H.; Yu, J.P.; Yu, H.G.; Xu, X.M.; Yu, L.L.; Liu, J.; Luo, H.S. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediat. Inflamm. 2005, 2005, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Kara, A.; Akman, S.; Ozkanlar, S.; Tozoglu, U.; Kalkan, Y.; Canakci, C.F.; Tozoglu, S. Immune modulatory and antioxidant effects of melatonin in experimental periodontitis in rats. Free Radic. Biol. Med. 2013, 55, 21–26. [Google Scholar] [CrossRef]
- Yip, H.-K.; Chang, Y.-C.; Wallace, C.G.; Chang, L.-T.; Tsai, T.-H.; Chen, Y.-L.; Chang, H.-W.; Leu, S.; Zhen, Y.-Y.; Tsai, C.-Y.; et al. Melatonin treatment improves adipose-derived mesenchymal stem cell therapy for acute lung ischemia-reperfusion injury. J. Pineal Res. 2013, 54, 207–221. [Google Scholar] [CrossRef]
- Kaur, C.; Sivakumar, V.; Robinson, R.; Foulds, W.S.; Luu, C.D.; Ling, E.A. Neuroprotective effect of melatonin against hypoxia-induced retinal ganglion cell death in neonatal rats. J. Pineal Res. 2013, 54, 190–206. [Google Scholar] [CrossRef]
- Kunak, Z.I.; Macit, E.; Yaren, H.; Yaman, H.; Cakir, E.; Aydin, I.; Turker, T.; Kurt, Y.G.; Ozcan, A.; Uysal, B.; et al. Protective Effects of melatonin and S-methylisothiourea on mechlorethamine induced nephrotoxicity. J. Surg. Res. 2012, 175, e17–e23. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, W.; Zhang, S.-Y.; Shen, Y.-X.; Yue, L.; Wang, N.-P.; Xu, S.-Y. Melatonin-selenium nanoparticles inhibit oxidative stress and protect against hepatic injury induced by Bacillus Calmette-Guérin/lipopolysaccharide in mice. J. Pineal Res. 2005, 39, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.S.; Kim, H.G.; Lee, J.S.; Han, J.M.; Park, H.J.; Huh, G.J.; Son, C.G. Melatonin reduces X-ray radiation-induced lung injury in mice by modulating oxidative stress and cytokine expression. Int. J. Radiat. Biol. 2012, 89, 97–105. [Google Scholar] [CrossRef]
- Gitto, E.; Aversa, S.; Salpietro, C.D.; Barberi, I.; Arrigo, T.; Trimarchi, G.; Reiter, R.J.; Pellegrino, S. Pain in neonatal intensive care: Role of melatonin as an analgesic antioxidant. J. Pineal Res. 2012, 52, 291–295. [Google Scholar] [CrossRef]
- Gitto, E.; Reiter, R.J.; Cordaro, S.P.; La Rosa, M.; Chiurazzi, P.; Trimarchi, G.; Gitto, P.; Calabró, M.P.; Barberi, I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: Beneficial effects of melatonin. Am. J. Perinatol. 2004, 21, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Harrison, L.C. Diurnal rhythmicity of human cytokine production: A dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J. Immunol. 1997, 158, 5163–5168. [Google Scholar] [PubMed]
- Wu, C.C.; Lu, K.C.; Lin, G.J.; Hsieh, H.Y.; Chu, P.; Lin, S.H.; Sytwu, H.K. Melatonin enhances endogenous heme oxygen-ase-1 and represses immune responses to ameliorate experimental murine membranous nephropathy. J. Pineal Res. 2012, 52, 460–469. [Google Scholar] [CrossRef]
- Dimitrov, S.; Lange, T.; Tieken, S.; Fehm, H.L.; Born, J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav. Immun. 2004, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kelestimur, H.; Sahin, Z.; Sandal, S.; Bulmus, O.; Ozdemir, G.; Yilmaz, B. Melatonin-related alterations in Th1/Th2 polarisation in primary thymocyte cultures of pinealectomized rats. Front. Neuroendocrinol. 2006, 27, 103–110. [Google Scholar] [CrossRef]
- Cagnacci, A. Melatonin in relation to physiology in adult humans. J. Pineal Res. 1996, 21, 200–213. [Google Scholar] [CrossRef]
| Characteristic | Mean | SD | CI (95%) | Range |
|---|---|---|---|---|
| age (years) | 49.6 | 11.4 | 45.6–53.5 | 27–71 |
| length of service (years) | 30.6 | 12.9 | 26.2–35.1 | 6–50 |
| systolic pressure (mm/Hg) | 129 | 11.9 | 124.9–133.1 | 100–160 |
| diastolic pressure (mm/Hg) | 87.7 | 8.2 | 84.7–90.5 | 70–105 |
| number of cigarettes | 17.8 | 5.9 | 14.1–19.4 | 5–25 |
| Marker | Mean | Mean Difference | SD | Median | CV (%) |
|---|---|---|---|---|---|
| cortisol at 8:00 a.m. | 1.23 | 0.48 | 1.39 | 39.0 | |
| cortisol at 2:00 p.m. | 0.71 | 0.52 | 0.50 | 0.56 | 70.4 |
| IL-1β at 8:00 a.m. | 239.4 | 229.3 | 145.0 | 6.4 | |
| IL-1β at 2:00 p.m. | 179.8 | −59.6 | 183.6 | 124.0 | 6.8 |
| melatonin at 8:00 a.m. | 30.9 | 10.2 | 34.9 | 33.1 | |
| melatonin at 2:00 p.m. | 28.0 | −2.9 | 9.6 | 29.9 | 34.3 |
| Never/Sometime | Often/Always | p | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Effort into falling asleep | |||||
| higher melatonin (8:00 a.m.) | 27.6 | 10.4 | 32.5 | 10.4 | 0.0351 |
| Premature awakening | |||||
| higher melatonin (8:00 a.m.) | 26.1 | 10.3 | 34.9 | 8.4 | 0.0093 |
| Melatdiff | 0.06 | 8.52 | −5.42 | 6.54 | 0.0454 |
| Night awakening | |||||
| Melatdiff | 0.22 | 6.05 | −5.02 | 9.19 | 0.0370 |
| Home/Work Conflict | ||
|---|---|---|
| Mean ± SD | p | |
| melatonin (8:00 a.m.) | ||
| <median | 48.6 ± 16.2% | 0.0414 * |
| >median | 64.1 ± 29.6% | |
| Total PSQI ** | ||
| Mean | p | |
| melatonin (8:00 a.m.) | ||
| <median | 10.8 ± 1.8 | 0.0386 * |
| >median | 9.8 ± 1.7 |
| Cortdiff | Mean ± SD | p |
|---|---|---|
| sleep latency | ||
| <median | 15.0 ± 8.0 min | |
| >median | 8.7 ± 4.0 min | 0.04 * |
| home/work conflict | ||
| <median | 69.9 ± 27.0% | |
| >median | 52.5 ± 24.0% | 0.034 * |
| self-esteem | ||
| <median | 47.7 ± 28.2% | |
| >median | 25.3 ± 18.4% | 0.01 * |
| Shift System | BSW | NSW | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| Cortdiff (μg/dL) | 1.17 | 0.21 | 0.30 | 0.55 | 0.01 |
| length of sleep (hours) | 8.6 | 0.89 | 5.0 | 0.87 | <0.01 |
| home–work conflict | 25% | 11.92 | 63% | 23.62 | <0.01 |
| systolic blood pressure (mm Hg) | 121 | 16.73 | 130 | 10.82 | 0.05 |
| Effort into falling asleep | |||||
| often/always | 20% | 53% | 0.02 | ||
| never/sometime | 80% | 47% | |||
| 47% Night awakening | |||||
| often/always | 40% | 60% | 0.03 | ||
| never/sometime | 60% | 40% | |||
| 40% Premature awakening | |||||
| often/always | 20% | 63% | 0.02 | ||
| never/sometime | 80% | 37% | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zefferino, R.; Fortunato, F.; Arsa, A.; Di Gioia, S.; Tomei, G.; Conese, M. Assessment of Stress Salivary Markers, Perceived Stress, and Shift Work in a Cohort of Fishermen: A Preliminary Work. Int. J. Environ. Res. Public Health 2022, 19, 699. https://doi.org/10.3390/ijerph19020699
Zefferino R, Fortunato F, Arsa A, Di Gioia S, Tomei G, Conese M. Assessment of Stress Salivary Markers, Perceived Stress, and Shift Work in a Cohort of Fishermen: A Preliminary Work. International Journal of Environmental Research and Public Health. 2022; 19(2):699. https://doi.org/10.3390/ijerph19020699
Chicago/Turabian StyleZefferino, Roberto, Francesca Fortunato, Addolorata Arsa, Sante Di Gioia, Gianfranco Tomei, and Massimo Conese. 2022. "Assessment of Stress Salivary Markers, Perceived Stress, and Shift Work in a Cohort of Fishermen: A Preliminary Work" International Journal of Environmental Research and Public Health 19, no. 2: 699. https://doi.org/10.3390/ijerph19020699
APA StyleZefferino, R., Fortunato, F., Arsa, A., Di Gioia, S., Tomei, G., & Conese, M. (2022). Assessment of Stress Salivary Markers, Perceived Stress, and Shift Work in a Cohort of Fishermen: A Preliminary Work. International Journal of Environmental Research and Public Health, 19(2), 699. https://doi.org/10.3390/ijerph19020699








