Abstract
Gastric cancer (GC) patients with peritoneal metastasis tend to achieve poor clinical outcomes. Until recently, the treatment options were limited mainly to either palliative chemotherapy or radiation therapy in exceptional cases. Currently, these patients benefit from multimodal treatment, such as cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC). Despite good overall results, this treatment modality is still widely debated. The following study is designed to assess the papers about the possible application and utility of HIPEC in GC. A search in the PubMed, Web of Science, and Scopus databases was performed to assess the papers devoted to the role of HIPEC in GC treatment; a literature search was performed until March 21st; and, finally, 50 studies with a total number of 3946 patients were analyzed. According to the most recent data, it seems to be reasonable to limit the duration of HIPEC to the shortest effective time. Moreover, the drugs used in HIPEC need to have equal concentrations and the same solvent. Perioperative chemotherapy needs to be reported in detail and, furthermore, the term “morbidity” should be defined more clearly by the authors.
1. Introduction
Hyperthermic intraperitoneal chemotherapy (HIPEC) is a surgical procedure that aims to deliver heated chemotherapy directly to the abdomen after surgery; the procedure was invented and firstly used more than 20 years ago [1]. Currently, HIPEC is an emerging procedure aimed to treat peritoneal metastasis of gastric cancer (GC); unfortunately, data about HIPEC application in locally advanced GC is still scarce. This treatment modality is considered to provide beneficial results in the management of several clinical syndromes within the peritoneum, such as peritoneal mesothelioma, pseudomyxoma peritonei, and peritoneal metastasis (PM), as a result of the metastatic properties mainly of several cancers, including colorectal, ovarian, or gastric cancer [2,3,4,5,6,7]. However, in GC patients, HIPEC is still considered as an innovative way to both prevent and treat PM, which is diagnosed in approximately 30% of patients with advanced GC. PM is characterized by a very poor prognosis; even though it is just a regional condition restricted only to the peritoneum, it is usually fatal with a maximal 3-month-long prognosis surgery (CRS) in a natural course, prolonged operation, as well as great intraoperative hemorrhages [8,9].
HIPEC, as a potential treatment modality for GC patients, is not included as a part of the current national guidelines, despite reported effectiveness as well as long-term survival rates. Researchers consistently question the potential benefits of HIPEC in terms of GC treatment and, so far, they have tested many modalities of the procedure over time. Granieri et al., in their meta-analysis of randomized controlled trials (2021), reported that a combination of CRS with HIPEC seems to be beneficial for patients with locally advanced GC, in prophylactic as well as curative settings [10]. Despite the extensive experience and the multitude of studies, along with the usage of HIPEC in the leading oncological centers all over the world, there are still no direct recommendations regarding its application as a treatment modality in GC patients. Moreover, it appears that, since the first attempts of HIPEC in GC, the median survival, which is the most important parameter, has not significantly changed (Figure 1).
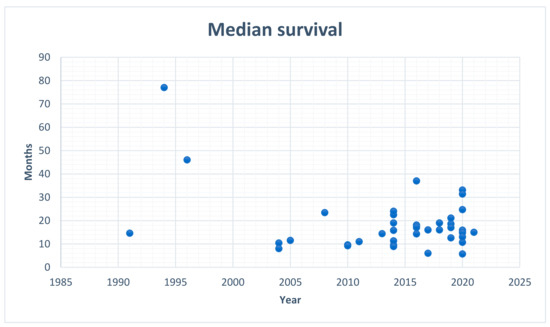
Figure 1.
Median survival in patients with gastric cancer treated with HIPEC; data from the analyzed papers.
This observation leads to a further discussion on the possible modifications that can be implemented to improve the utility of this procedure, and this should be primarily based on the results of the independent clinical studies. One of the most prevalent conundra regarding HIPEC therapy worldwide, includes the not yet established proper doses of drugs intraperitoneally administered, which is currently non-evidence-based due to the lack of proper recommendations [11]. However, even a brief analysis of the reports devoted to the application of HIPEC in GC patients shows that they present very difficult material for a comparative analysis, due to a wide spectrum of methodological differences applied in those studies. In order to draw credible and clinically useful conclusions, clinical trials need to be comparably reported. Moreover, the role of HIPEC in the treatment of PM in GC is still evolving and continually modified at all stages of treatment. Firstly, it is more frequently used in neoadjuvant therapy, such as neoadjuvant laparoscopic, heated intraperitoneal chemotherapy (NLHIPEC) after systemic chemotherapy, or neoadjuvant intraperitoneal and systemic chemotherapy (NIPS) in a bidirectional manner (BIPSC), used for both the intraperitoneal (IP) and intravenous (IV) routes of chemotherapy administration before the CRS. Secondly, it is used in the prophylactic treatment (P-HIPEC) and palliative treatment to minimize the risk and reduce the number of the ascites [2,12,13,14,15]. Apart from HIPEC, other forms of intraperitoneal chemotherapy, such as the early postoperative chemotherapy (EPIC) along with normothermic intra-operative intraperitoneal chemotherapy (NIIC), normothermic intraperitoneal chemotherapy long-term (NIPEC-LT), repeated intraperitoneal chemotherapy (RIPEC) [13,15,16,17], or pressurized intraperitoneal aerosol chemotherapy (PIPAC), with the relative benefits of delivering aerosolized chemotherapy under pressure into the abdominal cavity, are more frequently used [12,13,16].
The present study assesses the papers about the possible application of HIPEC in the treatment of GC patients, including those with GC and concomitant PM, with regard to the details, guidelines, and recommendations described by the researchers in the studies chosen for this review. Furthermore, we summarize the current state of knowledge regarding the discrepancies of the HIPEC technique applied in GC patients, with an emphasis on the inaccuracies concerning the technique duration as well as the agents, doses, and solvents used in different medical centers. This review will provide an insight into a broad spectrum of potential modifications regarding HIPEC itself, enabling a further exploration of this technique and its possible standardization for a specific group of GC patients who would potentially benefit from this technique.
2. Methods and Materials
2.1. Search Strategy
The PubMed, Web of Science, and Scopus databases were searched to assess the papers devoted to the role of HIPEC in the treatment of GC with a particular emphasis on the patients with PM present. The search string was as follows: “(gastric cancer) AND (HIPEC) OR (hyperthermic intraperitoneal chemotherapy) AND (peritoneal metastasis)”. The time period was restricted to 1 August 1989 and 21 March 2021 (32 years). The search was only restricted to the English language. During the first identification, which was the primary research conducted in March 2021, a total number of 552 papers was retrieved. After the removal of the duplicates, a total of 236 articles was included in the first analysis. Due to the disqualification of case reports, comments to other papers, letters to Editors, and papers devoted to other topics, a total number of 143 papers were assessed for eligibility. Due to the inaccessibility of several papers, and not considering the articles devoted to the tumors other than GC, the final analysis was based on a total number of 50 papers (Appendix A).
In every studied article, information was sought based on the following data: the number of patients; their medium age; agents and doses used in the HIPEC treatment of GC patients with or without PM; volume and kind of solvent for perfusate; duration of HIPEC; the modality of the procedure (open/closed); information about perioperative chemotherapy; the value of mortality and morbidity and their definition and interpretation, according to the authors of the paper; a detailed list of complications; as well as the median survival rates. The results are presented in Table A1 (Appendix A).
2.2. Details of the Studied Population
The analyzed reports varied with regards to the population of patients. The largest study included 249 patients, while the smallest was restricted to only 9 patients. The age range of patients was between 47 and 61 years; however, at least 5 papers did not mention this parameter at all, which is one of the limitations of this study.
3. Results
3.1. Intraperitoneal Chemotherapy
Intraperitoneal chemotherapy provides significantly higher local concentrations, which results in a direct anti-tumor effect on free peritoneal cancer cells. Therefore, intraperitoneal chemotherapy facilitates its uptake by cancer cells, by the enhanced drug penetration into them. In terms of the drugs used in HIPEC, mainly four variables differed in the analyzed reports: agents, doses, kind, and volume of the solvents. The most common chemotherapeutic agents used were Mitomycin C and Cisplatin, which were applied in 31 and 32 regimens, respectively. The doses of the applied agents were reported either as the whole dose for the procedure in milligrams (mg) (19 papers) [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], or in milligrams for the body surface area (mg/m2) (26 papers) [11,35,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. One of the latter units was additionally expressed in terms of the volume of solution (mg/m2/L) [48]. Two authors did not report the doses of the drugs [61,62,63]; in older studies, the doses were once reported in either µq/mL [64] or once in mg/kg [65]. Several articles lacked information about the volume of perfusate; those were mainly newer reports. In most of the cases, drugs were administered in the saline solution; however, there were single cases where the saline was replaced with 5% dextrose in water (D5W) or dialysis solution.
3.2. Duration of HIPEC
In the studied reports, there was insufficient information about the duration of the HIPEC procedure. Generally, the procedure lasted for approximately 60 or 90 min [18,19,20,21,23,24,25,26,29,32,33,35,36,37,38,42,43,44,46,47,48,49,50,51,52,57,58,59,61,64,65,66]. De Roover et al. reported that, in half of their cases (n = 8), there was a need to shorten the duration of HIPEC due to central hyperthermia [37]. Some researchers (mainly in newer reports) shortened the HIPEC duration to 30 or 45 min [11,45,53,54,55,56,67]. The others did not seem to value and stick to the time frames, and applied HIPEC for a time frame that was not directly specified; they preferred to choose a time range somewhere within the 30–120 min time frame [22,27,28,30,31,34,39,40,41,60,62,63].
3.3. Perioperative Systemic Chemotherapy
Most of the analyzed papers presented very modest information about the chemotherapy administered before and following the CRS + HIPEC. In 32 of the analyzed articles [11,18,19,20,21,22,23,30,31,34,36,38,39,40,41,42,43,44,45,46,47,50,51,52,54,55,58,60,61,62,63,66], the issue of the perioperative systemic treatment was at least mentioned (in the last analysis, the authors perceived it as an important factor), and in 19 papers it was not discussed at all [24,25,26,27,28,29,32,33,35,37,48,49,53,56,57,59,64,65,67].
3.4. Mortality
Ultimately, the analysis showed that mortality due to the application of HIPEC in GC patients is low. Only in 7 of the analyzed papers, the mortality rate exceeded 6%, but in 4 of them, these results were associated with a small number of the studied population (n = 9, 12, 16, and 17 respectively) [19,37,39,45,48,60,66]; only one postoperative death was reported. In fifteen of the analyzed papers, the mortality rate was 0% [11,22,24,25,26,28,30,38,44,46,49,51,52,63,65].
3.5. Morbidity
In the majority of cases, the term “morbidity” was defined as the occurrence of major postoperative complications, and it was reported in a wide range of percentages (from 5.6% to 72%). In the analyzed papers, some authors precisely listed the morbid events related to treatment or even made a brief comment (27 articles) [21,22,23,24,25,27,29,30,32,34,35,38,42,44,45,46,47,50,51,54,55,56,57,58,60,61,65], the others reported only numbers (13 articles) [11,18,19,20,26,28,39,40,41,48,49,52,62], and, in several papers, the morbidity was not discussed in the results (11 articles) [31,33,36,37,43,53,59,63,64,66,67]. In three papers, the complications were divided into surgery and HIPEC-related.
3.6. Median Survival
In some analyses, the patients were divided into the following groups: 1) curative, adjuvant, palliative, 2) patients who underwent CRS and HIPEC or only CRS, 3) patients with PCI < 6, and PCI > 6, and 4) complete cytoreduction, or not complete (Appendix A).
4. Discussion
HIPEC is a treatment strategy that, combined with surgery, aims to treat advanced cancers within the abdomen, such as colorectal cancer, gastric cancer, ovarian cancer, or peritoneal mesothelioma. Even though its usefulness was reported in several types of advanced cancers within the abdomen [68,69,70], it should be considered that the application of HIPEC is associated with a risk of several complications, including hematological toxicity, kidney failure, venous thromboembolism, and infections within the venous accesses and urinary tract [71]. Typical side effects include nausea, vomiting, fatigue, or weight loss, but those usually persist up to 3 months after surgery. The other most common complications include fatigue, disturbed sleep pattern, bloating, diarrhea, or constipation; depression is also reported as a side effect. Generally, the occurrence of any adverse events reflects the risks of the whole operation, but distinguishing between surgical and systemic complications can be additionally relevant in further analysis concerning the safest combination of agents and their doses. However, recently, the contemporary safety of HIPEC has significantly improved. Even though the morbidity and mortality rates remain relatively high in both HIPEC and CRS, the associated learning curve is steep and numerous well-structured tutor-based training programs have so far been implemented in Europe, to progressively overcome those drawbacks [72].
The authors of the reports that were directly devoted to HIPEC, usually discussed PM as an independent diagnosis, which, regardless of the origin, has a similar course and prognosis; therefore, it should be treated with the same means. According to the papers included in this narrative review, there are no clear indications as well as recommendations regarding the details of HIPEC, such as the types of agents and solvents (as well as their doses) used, along with the time of HIPEC duration. There are also discrepancies concerning the perioperative systemic chemotherapy applied in GC patients. For these reasons, it can be assumed that even though HIPEC seems to be beneficial for some of the GC patients, this type of therapy should be evaluated further and more standardized amongst the clinical centers; the group of patients who would be most beneficial to this therapy should also be investigated. In Poland, so far, there are only eight medical centers in which HIPEC is applied in GC patients; however, due to a high number of inconsistent data regarding the HIPEC procedure itself, there is no publication on this subject yet.
In our paper, 41 reports were excluded from further analysis, although they contained the required information about HIPEC in GC patients with PM. The reason was that the information about the patients’ characteristics (age and profile of chemotherapy, as well as outcomes data), rates of mortality, morbidity, and median survival, were reported together for the patients with PM from tumors of other origins.
Regarding the role of HIPEC in peritoneal carcinomatosis, more randomized trials still need to be conducted in order to select those patients who would constitute good candidates for such therapeutic approaches. In addition to the clinical features, the molecular and pathological features should be investigated in order to select patients for whom HIPEC would be beneficial. Apart from gastric cancer, minimally invasive secondary cytoreduction combined with HIPEC is currently under investigation to be applied in other peritoneal cancers, especially in the case of patients with ovarian cancer [73,74].
4.1. Intraperitoneal Chemotherapy
The diversity of regimens used for HIPEC in patients with GC and PM is understandable as, for the time being, the role of this treatment modality is still unsettled and there are no strict restrictions for this matter. The most common chemotherapeutic drugs used in HIPEC include Mitomycin-C and Cisplatin [75]—there were also reports about the potential application of oxaliplatin and doxorubicin; however, those are less common drugs [13]. However, the variability in the reports about the doses of the drugs and the lack of designation of the volume of perfusate during planning procedures, resulted in the situation that, even in the same institutions, the patients are treated with solutions of different concentrations of the drugs. It seems that the rules of treatment with HIPEC can be similar to dialysis therapy. Treating patients incomparably implies that the solutions of the drugs used during treatment need to have equal concentrations, expressed in the unit of mass to the volume of the solution [mg/mL]. The solvent needs to be universal—a saline solution, as in the majority of cases. Then, the volume of the applied drug solution should depend on the body surface area of the patient.
4.2. Duration of HIPEC
The duration of HIPEC depends on the used protocol and significantly varies depending on the type of chemotherapeutic agent used along with its pharmacokinetics features. One of the questions that have appeared in this study is whether the duration accuracy of the applied procedure matters. From the pharmacokinetic point of view, the depth of drug penetration is very minimal (up to 1–3 mm), and the prolongation of the treatment time would not increase this [76]. On the other hand, hyperthermia increases blood circulation and the longer the drugs solution remains in the peritoneal cavity, the more the drugs are prone to penetrate the vessels and enter into the blood. However, even if this course of events occurs, it is very limited, as the concentration of drugs in the blood after the application of HIPEC is far below the toxicity threshold [65]. However, the real problem exists from surgical and anesthesiological points of view, as a prolonged operation course increases the rate of postoperative morbidity, blood loss, as well as infection risk [8]. Therefore, some studies suggest that it would be reasonable to limit the duration of HIPEC to the shortest effective time, which means that the desired effects (favorable clinical outcomes with a minimization of the above-mentioned side effects) would be performed in the shortest time possible, without the additional risk of potential intraoperative side effects (either surgical or anesthesia).
4.3. Perioperative Chemotherapy
Perioperative chemotherapy (high rates of systemic chemotherapy in the neoadjuvant and adjuvant settings) is a favorable prognostic factor with a positive effect on survival [2,18,19,38]. The aim of this report was not to analyze the details of perioperative chemotherapy, but rather to assess the frequency of reporting this parameter in studies. Based on our analysis, it appears that this issue is generally neglected. In only 17 papers, there was some information about the types of agents used, doses, schedule of regimens, several patients qualified for chemotherapy, and reasons of disqualification; however, in very few, the data was complex. The probable reason is that the analyzed reports were mainly addressed to the surgeons and too much data can lead to information noise. However, from a multidisciplinary point of view, the factor of perioperative chemotherapy is relevant, as it diversifies the treated population.
4.4. Mortality
HIPEC is always the complement to severe and extensive CRS and; therefore, “the mortality rate” must be considered throughout the procedure. In most of the studied articles, “mortality” was defined as the number of deaths in 30 postoperative days. However, sometimes the authors redefined this entry and extended the mortality-free period to emphasize the safety of the procedure. In the literature, the careful and rigorous selection of patients qualified for CRS and HIPEC was often underlined as the crucial factor contributing to the effectiveness of treatment [2,38,77].
4.5. Morbidity
While “mortality” is an easy and unambiguous event to be defined, and the term “morbidity” is interpreted variously by different authors. It seems that one of the best options is to grade the adverse events related to CRS and HIPEC, according to the “common terminology criteria for adverse events” valid for the time of publication [78]. However, the authors did not always consider the same grades in the final analysis. Most often, they reported grade III–IV, but, in some papers, the less severe grades along with the fatal grade V were also included. Eventually, when analyzing a single paper, the way of reporting a matter of morbidity seemed satisfactory; however, in a wider perspective, the percentage numbers were very misleading, as they could not be compared. Complications are usually graded using the Clavien–Dindo classification system [79]. CRS combined with HIPEC presents significantly lower mortality and morbidity rates, compared with other major gastrointestinal surgical procedures [80].
4.6. Median Survival
The estimated median survival rate of patients with GC and concomitant PM is about 6–18 months [81]. Of the analyzed papers, the lowest median survival was mentioned in a study [28] by Hall et al. (2004), while the highest median survival rate was reported by Hamazoe et al. (1994) [64] with the values being equal to 8 months and 77 months, respectively. The appropriate selection of patients using the Peritoneal Carcinomatosis Index (PCI) < 6 and complete cytoreduction, showed promising results in improving overall survival (OS) rates [12,16,82]. The implementation of HIPEC in the case of patients with GC and PM seems to be reasonable, since, even though the median survival rates differ among single-center or prospective registry studies, they are continually improving, not only due to the favorable modifications of the technique itself, but also because of proper surgical training [72].
5. Conclusions
According to the studies devoted to the application of HIPEC in GC patients, it seems that at least the selected patients can benefit from this type of therapy. Even though similar, the technique itself is continually modified and differs between clinics in terms of the solvents and agents used, as well as the duration of the whole procedure. Further studies, with long-term evaluations, are of major importance to identify the prognostic factors that either positively or negatively affect the overall survival rate of GC patients treated with HIPEC. Numerous studies regarding this matter are currently ongoing, as researchers worldwide try to investigate those factors; e.g., Graziosi et al. indicate the patient-related parameters (pre-operative serum albumin level or platelets-to-lymphocytes ratio) as well as the tumor-related factors (such as the primary tumor site or PCI) as factors strongly associated with the survival of operated patients [83]. Patients with low CC scores present a significant survival advantage [10]. What is crucial, while considering the outcome of HIPEC, is the proper patient selection. Cocollini et al. suggest that the morbidity rate of patients is incremented by intraperitoneal chemotherapy [84]. In the papers devoted to HIPEC in the treatment of PM of any origin, it would be beneficial to distinguish the detailed data of patients and results of the procedures for populations of the same primary neoplasm. To draw credible conclusions and finally settle the role of HIPEC in GC, the reports need to fulfill several conditions:
- Solutions of the drugs used in HIPEC need to have equal concentrations, expressed in the unit of mass to the volume of solution [mg/mL]; the solvent needs to be universal, and the volume of the solution should depend on the body surface area of the patient, as well as the optimal doses of intraperitoneal administrated chemotherapy agents’ doses.
- The information about perioperative chemotherapy needs to be reported and should contain details about the chemotherapeutic agents used, their doses, the schedule of regimens, how many patients qualified for chemotherapy, and the reasons for disqualification.
The term “morbidity” should be clearly defined. We suggest applying to the observations in the majority of reports the consideration of “morbidity” as grade III–IV adverse events that comes from too common terminology criteria for adverse events [78]. Moreover, it is not enough to specify the number; however, morbid events should be listed. Currently, several randomized clinical trials (RCTs) have been conducted; however, with quite different conclusions regarding the usage of CRS and HIPEC in patients with gastric cancer. The Italian Association of Medical Oncology (AIOM) strongly advises against the application of CRS and HIPEC in patients with PM, while the Peritoneal Surface Oncology Group International (PSOGI) suggests that patients with gastric cancer and PM can strongly benefit from such treatment [85,86]. Such discrepancies once again suggest that RCT, as well as seeking a potential standardization, are of a major necessity.
Author Contributions
Conceptualization, M.M. and R.S.; methodology, M.M., M.S. and A.F.; formal analysis, J.B., R.M., G.R., L.M. and F.R.; investigation, M.M., M.S., A.F., K.P. and R.S.; writing—original draft preparation, M.M., M.S., A.F., J.B., R.M., G.R., L.M., F.R., K.P. and R.S.; writing—review and editing, M.M., A.F., K.P. and R.S.; visualization, M.M., K.P. and R.S.; supervision, K.P., R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CDD | cisplatin |
| CRS | cytoreductive surgery |
| EPIC | early post-operative chemotherapy |
| GC | gastric cancer |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| IP | intraperitoneal |
| IV | intravenous |
| LDG | laparoscopy distal gastrectomy |
| NIIC | normothermic intra-operative intraperitoneal chemotherapy |
| NIPEC-LT | normothermic intraperitoneal chemotherapy long-term |
| NIPS | neoadjuvant intraperitoneal and systemic chemotherapy |
| NLHIPEC | neoadjuvant laparoscopic, heated intraperitoneal chemotherapy |
| MMC | mitomycin C |
| PM | peritoneal metastasis |
| P-HIPEC | prophylactic heated intraperitoneal chemotherapy |
| PIPAC | pressurized intraperitoneal aerosol chemotherapy |
| RIPEC | repeated intraperitoneal chemotherapy |
Appendix A

Table A1.
Content of the analyzed papers.
Table A1.
Content of the analyzed papers.
| Ref. | Authors | Year | No. Patients | Mean Age (Years) | Agent | Dose | Solvent | Time | Technique | Perioperative Chemotherapy | Mortality | Morbidity | Median Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [53] | Zhu et al. | 2020 | 43 (22 CHIP treatment, 21 chemotherapy) | 51.0 (CHIP group) 55.0 (chemotherapy group) | CDDP | 75 mg/m2 | Saline | 30 | ND | ND | ND | ND definition—complications listed | Not reached (CHIP group); 33.1 months (group with chemotherapy alone) |
| [54,55] | Koemans et al. | 2021 | 25 (gastrectomy, CRS, HIPEC) | 60.0 | Oxaliplatin | 460 mg/m2 | ND | 30 min 90 min | ND | Yes | ND | Serious adverse events—68.0% | 15 months |
| Docetaxel | 0, 50, 75 mg/m2 (escalating doses) | ||||||||||||
| [35] | Ji et al. | 2020 | 125 (CRS + HIPEC) | 51.0 | CDDP, MMC (CDDP + MMC) | 120 mg (CDDP) 30 mg (MMC) | Saline | 60 min or 90 min | ND | ND | 30 day perioperative mortality—0.9%; 90 day postoperative mortality—3.2% | Serious adverse events—8.8% | 10.7 months; 1 year—43.8%, 2 years—24.7%, 3 years—18.6%, 5 years—15.7% |
| CDDP, Docetaxel (CDDP + DOC) | 120 mg 120 mg | ||||||||||||
| Lobaplatin, Docetaxel (LP + DOC) | 50 mg/m2 60 mg/m2 | ||||||||||||
| [36] | Blumenthaler et al. | 2020 | 52 (25 LS-HIPEC, 27 standard care (SC)) | 57 (LS-HIPEC group), 64 (SC group) | MMC, CDDP | 30 mg (MMC) 200 mg (CDDP) | ND | 60 min | Closed | Yes | ND | ND | 24.7 months (LS-HIPEC group), 21.3 months (SC group); 1 year—95.5% (LS-HIPEC group), 76.9% (SC group); 2 years—57.2% (LS-HIPEC group), 19.1% (SC group); 3 years—19.1% (LS-HIPEC group), 9.6% (SC group) |
| [56] | Yin et al. | 2021 | 138 (92 LDG , 46 LDG + HIPEC) | 53.3 (LDG group), 52.5 (LDG + HIPEC group) | CDDP | 75 mg/m2 | 6 L of heated saline | 45 min | Closed | ND | ND | Complications: 11.96% (LDG group), 13.04% (LDG + HIPEC group) Abdominal recurrence after 2 years from operation: 10.87% (LDG group), 4.35% (LDG + HIPEC group) | ND |
| [67] | Fan et al. | 2021 | 50 (33 HIPEC with CDDP, 17 adjuvant chemotherapy with SOX regime) | 61.0 | CDDP | 50 mg/L | 0.9% sodium chloride | 30 min | ND | ND | ND | ND definition-Complications listed | 3 years—92.0% (87.9% in HIPEC group, 100% in adjuvant chemotherapy group) |
| [57] | Rosa et al. | 2021 | 85 (39 CRS, 23 gastrectomy + curative HIPEC, 23 gastrectomy + prophylactic HIPEC) | 61.0 (68 CRS, 52 gastrectomy + curative HIPEC, 58 gastrectomy + prophylactic HIPEC) | MMC, CDDP | 15 mg/m2 (MMC) 75 mg/m2 (CDDP) | 2 L/m2 0.9% NaCl solution | 90 min | Open | ND | Death 30 days from surgery—5% (CRS), 4% (gastrectomy + curative HIPEC), 0% (gastrectomy + prophylactic HIPEC) | Postoperative complications within 30 days from surgery—46% (CRS), 39% (gastrectomy + curative HIPEC), 39% (gastrectomy + prophylactic HIPEC) | 5 years—9% (CRS), 27% (gastrectomy + curative HIPEC), 33% (gastrectomy + prophylactic HIPEC) |
| [66] | Xie et al. | 2020 | 113 (51 HIPEC + adjuvant chemotherapy, 62 adjuvant chemotherapy) | 60.9 (HIPEC + adjuvant chemotherapy), 61.5 (adjuvant chemotherapy) | CDDP | 50 mg/L | saline | 60 min | Open | No | 17.6% (HIPEC group), 38.7% (conventional adjuvant chemotherapy group) | ND definition—complications listed | 1 year—96.1% (HIPEC group), 95.2% (conventional adjuvant chemotherapy group). 3 years—68.6% (HIPEC group), 66.3% (conventional adjuvant chemotherapy group) |
| [58] | Yu et al. | 2020 | 38 (18: neoadjuvant systemic chemotherapy + HIPEC + CRS; 20: chemotherapy + HIPEC) | 52.0 (49.8 neoadjuvant systemic chemotherapy + HIPEC + CRS; 53.5: chemotherapy + HIPEC) | Paclitaxel | 75 mg/m2 | 3 L of heated 0.9% saline | 60 min | ND | Yes | ND | 28.9% adverse events (grade 3 or 4) | 15.1 months (21.1 months (neoadjuvant systemic chemotherapy + HIPEC + CRS), 10.8 months (chemotherapy + HIPEC)) |
| [59] | Lei et al. | 2020 | 498 (249 HIPEC + chemotherapy, 249 chemotherapy) | 55.3 (54.6 HIPEC + chemotherapy, 56.0 chemotherapy) | Paclitaxel or platinum (oxaliplatin: or CDDP) | 75−100 mg/m2 100−130 mg/m2 50−75 mg/m2 | ND | 60 min | Closed | ND | ND | ND definition—complications listed | 15.9 months (HIPEC + chemotherapy), 10.8 months (chemotherapy) |
| [63] | White et al. | 2020 | 70 (LS-HIPEC) | 54.3 | 43 patients (MMC + CDDP) | ND | ND | ND | ND | Yes | Death 30 days after LS-HIPEC—0% Death 30 days after LS-HIPEC—4% | ND | 31.4 months (patients without gross carcinomatosis PCI = 0); 14.8 months (patients with PCI scores of 1–7); 5.7 months (patients with PCI > 7) |
| 55.6 | 27 patients (MMC + CDDP + Paclitaxel) | ||||||||||||
| [39] | Bonnot et al. | 2019 | 277 (180 CRS-HIPEC, 90 CRSa) | 59.8 (CRS-HIPEC), 51.1 (CRSa) | Monochemotherapy (77.2%) MMC CDDP Oxaliplatin 22,8 % drug combination | 30–50 mg/m2 50–100 mg/m2 300–460 mg/m2 | ND | 30–120 min | Open (40.6%), closed (59.4%) | Yes | 3.2% (30 days), 8.4% (90 days) | 54.3 %, 53.7% (CRS-HIPEC), 55.3% (CRSa) | 18.6 months (CRS-HIPEC), 11.4 months (CRSa) |
| [11] | Hotopp et al. | 2019 | 26 | 50 | Taxotere | 80 mg/m2 | 4 L rinse solution | 45 min | Open | Yes | 0% (30 days) | 26.9% | 17 months |
| Oxaliplatin | 200 mg/m2 | ||||||||||||
| [40] | Yarema et al. | 2019 | 117 | 54.1 | MMC + CDDP | 12.5 mg/m2 + 75 mg/m2 460 mg/m2 | 30–90 min | Open | Yes | 5.1% | 29.1% | 12.6 months survivals 1 year—53.8% (curative group); 34 months survival 1 year—91.7% (adjuvant group); 3.5 months survival 1 year—0% (palliative group) | |
| Oxaliplatin | |||||||||||||
| MMC | 10–15 mg/m2 | ||||||||||||
| CDDP + Doxorubicin | 75 mg/m2 + 15 mg/m2 | ||||||||||||
| CDDP | 75 mg/m2 | ||||||||||||
| [41] | Rau et al. | 2020 | 235 | 53.4 | CDDP, Doxorubicin, MMC, Oxaliplatin with combination CDDP + DoX, CDDP + MMC | 75 mg/m2, 15 mg/m2, 30 mg/m2, 300 mg/m2 | ND | 30–90 min | Closed (184), open (51) | Yes | 5.1% | 17,0% | 13 months |
| [62] | Manzanedo et al. | 2019 | 88 | 53 | CDDP +Doxorubicin, MMC + CDDP, MMC, Oxaliplatin | ND | ND | ND | Open (63) closed withCO2 (22) | Yes | 3.4% | 31,0% | 21.2 months survivals 1 year—79.9%; 3 year—30.9%; and 5 year—27.5% |
| [42] | Rihuete et al. | 2018 | 35 | 53 | CDDP | 100 mg/m2 | ND | 90 min | Open | Yes | 5.7% (90 day) | 25.7% serious adverse events (grade IIIb–V) | 16 months survivals 1 year—70.8%; 3 year—21.3%; 5 year—21.3% |
| Doxorubicin | 15 mg/m2 | ||||||||||||
| [18] | Kim et al. | 2018 | 38 | 45.8 | MMC + CDDP | 30 mg 90 mg | ND | 90 min | Closed | Yes | 5.7% | 42.1% | 19 months |
| [38] | Topal et al. | 2017 | 32 | 58 | CDDP | 100 mg/m2 | 3–4 L saline | 60 min | Open | Yes | 0% | 72%—postoperative including 16%—nephrotoxity | 16 months survivals 1 year—71.9%; 3 year—14.1%; 5 year—3.5% |
| [19] | Fugazzolaet al. | 2017 | 17 | 53 | CDDP + Paclitaxel | (150.3 mg -175.9 mg) + (263 - 302.8 mg) | 90 min | Open | Yes | 8%S PC, 50% MPC | 61% SPC, 100 % MPC | 16 months (SPC), 6 months (MPC) | |
| MMC + CDDP | (26–27.5 mg) + (163–173 mg)—mean dosage | ||||||||||||
| [20] | Geng et al. | 2016 | 312 (40 HIPEC) | 53,9 | Docetaxel | 120 mg | 3.5 L normal saline | 60 min | Closed | Yes | ND | 11,2% | 17 months |
| [21] | Tu et al. | 2016 | 231 | 55.1 | 5FU | 1500 mg | 4.5L ofsaline | 60 min | Closed | Yes | 0.9% postoperative | 6.9% postoperative (grade I–IV)—complications listed | 37 months |
| CDDP | 100 mg | ||||||||||||
| [43] | Boerner et al. | 2016 | 38 | 52.6 | CDDP | 75 mg/m2 | ND | 60 min | Closed | Yes | ND | ND | 18.1 months |
| Doxorubicin | 15 mg/m2 | ||||||||||||
| [44] | Wu et al. | 2016 | 50 | ND | Lobaplatin | 50 mg/m2 | 6 L of saline | 60 min | Open | Yes | 0% postoperative | 23.1%* postoperative 30 days (grade III–V) | 14.3 months |
| Docetaxel | 60 mg/m2 | ||||||||||||
| [61] | Chia et al. | 2016 | 81 | ND | MMC CDDP/OX | ND ND | ND | 90 min | Closed/ Open | Yes | 2.5% 30 days | 44% postoperative (grade III–IV) | 17.3 months |
| [22] | Magge et al. | 2014 | 23 | 51.5 | MMC | 40 mg | Saline | 100 min | Open | Yes | 0 % 60 days | 52.2% ND period (grade III–IV) | 9.5 months |
| [45] | Rudloff et al. | 2014 | 9 | ND | OX i.v. 5FU + leucovorin | 460 mg/m2 400 mg/m2 | 5% dextrose in water (D5W) | 30 min | Open | Yes | 11.1% 90 days | 90 days (grade III–V) | 11.3 months |
| [46] | Königsrainer et al. | 2014 | 18 | 56 | CDDP | 50 mg/m2 | ND | 90 min | Open | Yes | 0% 30 days | 46% ND period (grade I–IV) | 8.9 months |
| [47] | Yarema et al. | 2014 | 49 | ND | MMC | 12.5 mg/m2 | ND | 90 min | Closed | Yes | 4.1% postoperative | 26.5% postoperative (grade III–IV)—distinguished between surgical and HIPEC | 22.5 months |
| CDDP | 75 mg/m2 | ||||||||||||
| [48] | Saladino et al. | 2014 | 12 | ND | MMC | 25 mg/m2/L | ND | 90 min | Closed | ND | 8.3% ND ND | 33.3% ND ND | 24 months |
| CDDP | 3.3 mg/m2/L | ||||||||||||
| [49] | Muller et al. | 2014 | 26 | 53 | OX | 200 mg/m2 | ND | 90 min | Closed | ND | 0% 30 days | 23% postoperative ND definition | 19 months |
| Docetaxel | 80 mg/m2 | ||||||||||||
| [50] | Canbay et al. | 2014 | 152 | 51.5 | Docetaxel | 30 mg/m2 | ND | 90 min | Open | Yes | 3.9% postoperative 30 days | 23% perioperative (grade I–V)—complications listed | 15.8 months |
| [23] | Glehen et al. | 2004 | 49 | 53,7 | MMC | 40–60 mg | 4–6 L | 90 min | Closed | Yes | 4% 30 days | 27% postoperative 30 days ND definition—complications listed | 10.4 months |
| [51] | Hultman et al. | 2013 | 8 | ND | Doxorubicin | 15 mg/m2 | ND | 90 min | Open | Yes | 0% postoperative 30 days | 62.5% perioperative (grade II–IV) | 14.4 months |
| CDDP | 50 mg/m2 | ||||||||||||
| ND | (3 patients) OX i.v. 5FU Leucovorin | 460 mg/m2 500 mg/m2 60 mg/m2 | 30 min | Complications listed | |||||||||
| [24] | Mizumoto et al. | 2012 | 13 | 48 | MMC | 20 mg | Saline | 60 min | Open | ND | 0% 30 days | 19% 38% postoperative (grade I–II and III–IV) | ND |
| CDDP | 1000 g | ||||||||||||
| [52] | Costa et al. | 2012 | 10 | 47 | MMC | 34 mg/m2 | 3–4 L of dialysis solution | 90 min | Closed | Yes | 0% postoperative | 50% postoperative ND definition | ND |
| [25] | Yang et al. | 2011 | 34 | 50 | MMC | 30 mg | 6 L of saline | 60–90 min | Open | ND | 0% ND | 14.7% ND period (serious adverse events) | 11 months |
| CDDP | 120 mg | ||||||||||||
| [26] | Yang et al. | 2010 | 30 | 50 | HCPT | 20 mg | 12 L of saline | 90 min | Open | ND | 0% postoperative 30 days | 14.3% ND period ND definition | 9.6 months |
| MMC | 30 mg | ||||||||||||
| [60] | Glehen et al. | 2010 | 150 | 53.4 | MMC | 30–50 mg/m2 | ND | 60 – 120 min | Closed/ Open | Yes | 6.5% postoperative | 27.8% postoperative (grade III–IV) | 9.2 month |
| CDDP | 50–100 mg/m2 | ||||||||||||
| OX IR 5FU | 360–460 mg/m2 100–200 mg/m2 ND | ||||||||||||
| [34] | Scaringi et al. | 2008 | 37 | 53,7 | MMC | 120 mg | 12 L of saline | 90–120 min | Open | Yes | 5.4% 30 days | 27% postoperative ND definition—complications listed | 23.4 months |
| CDDP | 200/m2 | ||||||||||||
| [37] | De Roover et al. | 2006 | 16 | ND | MMC | 15 mg/m2 | ND | 73 min | ND | ND | 6.25% postoperative | ND postoperative ND definition—complications listed | ND |
| [27] | Yonemura et al. | 2005 | 107 | 52 | MMC | 30 mg | 8 L of saline | ND | Open | ND | 2.8% postoperative | 21.5% postoperative ND definition—complications listed | 11.5 months |
| CDDP | 300 mg | ||||||||||||
| Etoposide | 150 mg | ||||||||||||
| [28] | Hall et al. | 2004 | 34 | 54,5 | MMC | 40 mg | 4 L | 120 min | Closed | ND | 0% 30 days | 35% ND period ND definition | 8 months |
| [29] | Yonemura et al. | 2001 | 48 | ND | MMC | 30 mg | 8–10 L | 60 min | Open | ND | 4% ND | 19% ND period (major operative complications) | ND |
| CDDP | 300 mg | ||||||||||||
| [30] | Fujimoto et al. | 1999 | 71 | 58.5 | MMC | ̴ 40 mg | 3–4 L | 120 min | Closed | Yes | 0% ND | 11,4% Postoperative ND period—complications listed | Survivals 2 year—88% 4 year—76% 8 year—62% |
| [31] | Fujimoto et al. | 1997 | 48 | Group 1–56.9 Group 2–48 Group 3–47.4 | MMC | ̴ 40 mg | 3–4 L of dialysis solution | 120 min | Closed | Yes | ND | ND | ND |
| [32] | Yonemura et al. | 1996 | 83 | 60 | MMC | 30 mg | 60 min | Open | ND | 1,2% ND | 5,8% ND period ND definition—complications listed | 46 months | |
| CDDP | 120 mg | ||||||||||||
| Etoposide | 150 mg | ||||||||||||
| [64] | Hamazoe et al. | 1994 | 42 | 56.5 | MMC | 10 μg/mL | 8–12 L | 50–60 min | Closed | ND | ND | ND | 77 months |
| [65] | Fujimura et al. | 1994 | 22 | 60.3 | MMC | 30 mg/kg | 10 L | 60 min | Open | ND | 0% Postoperative | 36% ND period morbid events associated to HIPEC—complications listed | Survivals:1 year—95% 2 year—89% 3 year—68% |
| CDDP | 300 mg/kg | ||||||||||||
| [33] | Yonemura et al. | 1991 | 41 | 56 | MMC | 50 mg | 8–10 L | 40–60 min | Open | ND | NDND | ND | 14.6 months |
| CDDP | 300 mg | ||||||||||||
Papers studied on abstract base only, lacking some information; ND: not discussed; 5FU: 5-fluorouracil; CDDP: cisplatin; OX: oxaliplatin; MMC: mitomicyn C; SPC: synchronous peritoneal carcinomatosis; MPC: metachronous peritoneal carcinomatosis; IR: irinotecan; CRS: cytoreductive surgery; PCI: peritoneal carcinomatosis index, CHIP: chemotherapeutic hyperthermic intraperitoneal perfusion, HIPEC: hyperthermic intraperitoneal chemotherapy, DOC: docetaxel, LP: lobaplatin, LS-HIPEC: laparoscopic hyperthermic intraperitoneal chemotherapy, LDG: laparoscopy distal gastrectomy, i.v.: intravenous. 1 Authors divided the population of patients into subgroups.
References
- Sugarbaker, P.H.; Cunliffe, W.J.; Belliveau, J.; de Bruijn, E.A.; Graves, T.; Mullins, R.E.; Schlag, P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin. Oncol. 1989, 16, 83–97. [Google Scholar]
- Yonemura, Y.; Canbay, E.; Li, Y.; Coccolini, F.; Glehen, O.; Sugarbaker, P.H.; Morris, D.; Moran, B.; Gonzaletz-Moreno, S.; Deraco, M.; et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent. Eur. J. Surg. Oncol. 2016, 42, 1123–1131. [Google Scholar] [CrossRef]
- Dehal, A.; Smith, J.J.; Nash, G.M. Cytoreductive surgery and intraperitoneal chemotherapy: An evidence-based review-past, present and future. J. Gastrointest. Oncol. 2016, 7, 143–157. [Google Scholar]
- Dewhirst, M.W.; Kirsch, D. Technological Advances, Biologic Rationales, and the Associated Success of Chemotherapy with Hy-perthermia in Improved Outcomes in Patients with Sarcoma. JAMA Oncol. 2018, 4, 493. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Lee, C.-T.; Ashcraft, K.A. The future of biology in driving the field of hyperthermia. Int. J. Hyperth. 2016, 32, 4–13. [Google Scholar] [CrossRef]
- Jones, E.; Dewhirst, M.; Vujaskovic, Z.; Samulski, T.; Yu, D.; Sanders, L.; Prosnitz, L.R. Hyperthermia improves the complete response rate for superficial tumors treated with radiation: Results of a prospective randomized trial testing the thermal dose parameter CEM 43° T90. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, S253–S254. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The 30-year experience-A meta-analysis of random-ised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Hartgrink, H.H.; van de Velde, C.J.; Putter, H.; Bonenkamp, J.J.; Klein Kranenbarg, E.; Songun, I.; Welvaart, K.; van Krieken, J.H.J.M.; Meijer, S.; Plukker, J.T.M.; et al. Extended lymph node dissection for gastric cancer: Who may benefit? Final results of the randomized Dutch gastric cancer group trial. J. Clin. Oncol. 2004, 22, 2069–2077. [Google Scholar] [CrossRef]
- Paredes, A.Z.; Guzman-Pruneda, F.A.; Abdel-Misih, S.; Hays, J.; Dillhoff, M.E.; Pawlik, T.M.; Cloyd, J.M. Perioperative Morbidity of Gastrectomy during CRS-HIPEC: An ACS-NSQIP Analysis. J. Surg. Res. 2019, 241, 31–39. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef]
- Hotopp, T. HIPEC and CRS in peritoneal metastatic gastric cancer—Who really benefits? Surg. Oncol. 2019, 28, 159–166. [Google Scholar] [CrossRef]
- Leiting, J.L.; Grotz, T.E. Optimizing outcomes for patients with gastric cancer peritoneal carcinomatosis. World J. Gastrointest. Oncol. 2018, 10, 282–289. [Google Scholar] [CrossRef]
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Drugs, doses, and durations of intraperitoneal chemotherapy: Standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int. J. Hyperth. 2017, 33, 582–592. [Google Scholar] [CrossRef]
- Seshadri, R.A.; Glehen, O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J. Gastroenterol. 2016, 22, 1114–1130. [Google Scholar] [CrossRef]
- Seshadri, R.A.; Glehen, O. The Role of Hyperthermic Intraperitoneal Chemotherapy in Gastric Cancer. Indian J. Surg. Oncol. 2016, 7, 198–207. [Google Scholar] [CrossRef][Green Version]
- Kitayama, J.; Ishigami, H.; Yamaguchi, H.; Sakuma, Y.; Horie, H.; Hosoya, Y.; Lefor, A.K.; Sata, N. Treatment of patients with peritoneal metastases from gastric cancer. Ann. Gastroenterol. Surg. 2018, 2, 116–123. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Pharmacology of chemotherapy treatments for peritoneal metastases: Optimizing and augmenting HIPEC. Pleura Peritoneum. 2017, 2, 43–45. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, D.G.; Song, S.; Jee, Y.S. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy as Treatment Options for Peritoneal Metastasis of Advanced Gastric Cancer. J. Gastric Cancer 2018, 18, 296–304. [Google Scholar] [CrossRef]
- Fugazzola, P.; Coccolini, F.; Montori, G.; Ceresoli, M.; Baggi, P.; Costanzo, A.; Tomasoni, M.; Gregis, F.; Nozza, S.; Ansaloni, L. Overall and disease-free survival in patients treated with CRS + HIPEC with cisplatin and paclitaxel for gastric cancer with peritoneal carcinomatosis. J. Gastrointest. Oncol. 2017, 8, 572–582. [Google Scholar] [CrossRef]
- Geng, X.; Liu, H.; Lin, T.; Hu, Y.; Chen, H.; Zhao, L.; Mou, T.; Qi, X.; Yu, J.; Li, G. Survival benefit of gastrectomy for gastric cancer with peritoneal carcinomato-sis: A propensity score-matched analysis. Cancer Med. 2016, 5, 2781–2791. [Google Scholar] [CrossRef]
- Tu, Y.; Tian, Y.; Fang, Z.; Ruan, Q.; Tang, H.; Zhang, X.; Wu, Y.; Ding, Y.; Cui, S. Cytoreductive surgery combined with hyperthermic intraperitoneal chemoperfusion for the treatment of gastric cancer: A single-centre retrospective study. Int. J. Hyperth. 2016, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Magge, D.; Zenati, M.; Mavanur, A.; Winer, J.; Ramalingam, L.; Jones, H.; Zureikat, A.; Holtzman, M.; Lee, K.; Ahrendt, S.; et al. Aggressive locoregional surgical therapy for gastric peri-toneal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Schreiber, V.; Cotte, E.; Sayag-Beaujard, A.C.; Osinsky, D.; Freyer, G.; Francois, Y.; Vignal, J.; Gilly, F.N. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch. Surg. 2004, 139, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, A.; Canbay, E.; Hirano, M.; Takao, N.; Matsuda, T.; Ichinose, M.; Yonemura, Y. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan. Gastroenterol. Res. Pract. 2012, 139, 20–26. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemother-apy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, Y.; Yonemura, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J. Surg. Oncol. 2010, 101, 457–464. [Google Scholar] [CrossRef]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Takahashi, S.; Sawa, T.; Matsuki, N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br. J. Surg. 2005, 92, 370–375. [Google Scholar] [CrossRef]
- Hall, J.J.; Loggie, B.W.; Shen, P.; Beamer, S.; Douglas Case, L.; McQuellon, R.; Geisinger, K.R.; Levine, E.A. Cytoreductive surgery with intraperitoneal hyperther-mic chemotherapy for advanced gastric cancer. J. Gastrointest. Surg. 2004, 8, 454–463. [Google Scholar] [CrossRef]
- Yonemura, Y.; de Aretxabala, X.; Fujimura, T.; Fushida, S.; Katayama, K.; Bandou, E.; Sugiyama, K.; Kawamura, T.; Kinoshita, K.; Endou, Y.; et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomized controlled study. Hepatogastroenterology 2001, 48, 1776–1782. [Google Scholar]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer 1999, 85, 529–534. [Google Scholar] [CrossRef]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T.; Isawa, E.; Sumida, M.; Ohkubo, H. Improved mortality rate of gastric carcinoma pa-tients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997, 79, 884–891. [Google Scholar] [CrossRef]
- Yonemura, Y.; Fujimura, T.; Nishimura, G.; Falla, R.; Sawa, T.; Katayama, K.; Tsugawa, K.; Fushida, S.; Miyazaki, I.; Tanaka, M.; et al. Effects of intraoperative chemohyperthermia in pa-tients with gastric cancer with peritoneal dissemination. Surgery 1996, 119, 437–444. [Google Scholar] [CrossRef]
- Yonemura, Y.; Fujimura, T.; Fushida, S.; Takegawa, S.; Kamata, T.; Katayama, K.; Kosaka, T.; Yamaguchi, A.; Miwa, K.; Miyazaki, I. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J. Surg. 1991, 15, 530–535. [Google Scholar] [CrossRef]
- Scaringi, S.; Kianmanesh, R.; Sabate, J.M.; Facchiano, E.; Jouet, P.; Coffin, B.; Parmentier, G.; Hay, J.M.; Flamant, Y.; Msika, S. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: A single western center experience. Eur. J. Surg. Oncol. 2008, 34, 1246–1252. [Google Scholar] [CrossRef]
- Ji, Z.H.; Yu, Y.; Liu, G.; Zhang, Y.B.; An, S.L.; Li, B.; Li, X.B.; Yan, G.J.; Li, Y. Peritoneal cancer index (PCI) based patient selecting strategy for complete cytore-ductive surgery plus hyperthermic intraperitoneal chemotherapy in gastric cancer with peritoneal metastasis: A single center ret-rospective analysis of 125 patients. Eur. J. Surg. Oncol. 2020, 47, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Blumenthaler, A.N.; Allen, C.J.; Ikoma, N.; Blum, M.; Das, P.; Minsky, B.D.; Mansfield, P.F.; Ajani, J.A.; Badgwell, B.D. Laparoscopic HIPEC for Low-Volume Peritoneal Metas-tasis in Gastric and Gastroesophageal Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 5047–5056. [Google Scholar] [CrossRef] [PubMed]
- De Roover, A.; Detroz, B.; Detry, O.; Coimbra, C.; Polus, M.; Belaiche, J.; Meurisse, M.; Honore, P. Adjuvant hyperthermic intraperitoneal peroperative chem-otherapy (HIPEC) associated with curative surgery for locally advanced gastric carcinoma. An initial experience. Acta Chir. Belg. 2006, 106, 297–301. [Google Scholar] [CrossRef]
- Topal, B.; Demey, K.; Topal, H.; Jaekers, J.; Van Cutsem, E.; Vandecaveye, V.; Sagaert, X.; Prenen, H. Cytoreductive surgery and Hyperthermic intra-operative peritoneal chemotherapy with Cisplatin for gastric peritoneal Carcinomatosis Monocentric phase-2 nonrandomized prospective clinical trial. BMC Cancer 2017, 17, 771. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperther-mic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analy-sis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Yarema, R.; Mielko, J.; Fetsych, T.; Ohorchak, M.; Skorzewska, M.; Rawicz-Pruszynski, K.; Mashukov, A.; Maksimovsky, V.; Jastrzębski, T.; Polkowski, W.; et al. Hyperthermic intraperitoneal chemo-therapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: A retrospective cooperative Central-Eastern European study. Cancer Med. 2019, 8, 2877–2885. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Piso, P.; Pelz, J.; Busch, P.; Demtroder, C.; Schule, S.; Schlitt, H.J.; Roitman, M.; Tepel, J.; et al. Peritoneal metastasis in gastric cancer: Results from the German data-base. Gastric Cancer 2020, 23, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rihuete Caro, C.; Manzanedo, I.; Pereira, F.; Carrion-Alvarez, L.; Serrano, A.; Perez-Viejo, E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2018, 44, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Boerner, T.; Graichen, A.; Jeiter, T.; Zemann, F.; Renner, P.; Marz, L.; Soeder, Y.; Schlitt, H.J.; Piso, P.; Dahkle, M.H. CRS-HIPEC Prolongs Survival but is Not Curative for Patients with Peritoneal Carcinomatosis of Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 3972–3977. [Google Scholar] [CrossRef]
- Wu, H.T.; Peng, K.W.; Ji, Z.H.; Sun, J.H.; Zhang, Q.; Yang, X.J.; Huang, C.Q.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur. J. Surg. Oncol. 2016, 42, 1024–1034. [Google Scholar] [CrossRef]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Konigsrainer, I.; Horvath, P.; Struller, F.; Konigsrainer, A.; Beckert, S. Initial clinical experience with cytoreductive surgery and hyper-thermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J. Gastric Cancer 2014, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Yarema, R.R.; Ohorchak, M.A.; Zubarev, G.P.; Mylyan, Y.P.; Oliynyk, Y.Y.; Zubarev, M.G.; Gyrya, P.I.; Kovalchuk, Y.J.; Safiyan, V.I.; Fetsych, T.G. Hyperthermic intraperitoneal chemoperfu-sion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int. J. Hyperth. 2014, 30, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Saladino, E.; Fleres, F.; Mazzeo, C.; Pruiti, V.; Scollica, M.; Rossitto, M.; Cucinotta, E.; Macri, A. The role of prophylactic hyperthermic intraperitoneal chemotherapy in the management of serosal involved gastric cancer. Anticancer Res. 2014, 34, 2019–2022. [Google Scholar]
- Muller, H.; Hotopp, T.; Tofeili, A.; Wutke, K. Systemic Chemotherapy using FLOT—Regimen Combined with Cytoreductive Surgery plus HIPEC for Treatment of Peritoneal Metastasized Gastric Cancer. Hepatogastroenterology 2014, 61, 703–706. [Google Scholar]
- Canbay, E.; Yonemura, Y.; Brucher, B.; Baik, S.H.; Sugarbaker, P.H. Intraperitoneal chemotherapy and its evolving role in management of gastric cancer with peritoneal metastases. Chin. J. Cancer Res. 2014, 26, 1–3. [Google Scholar]
- Hultman, B.; Lind, P.; Glimelius, B.; Sundbom, M.; Nygren, P.; Haglund, U.; Mahteme, H. Phase II study of patients with peritoneal carcinomato-sis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemo-therapy. Acta Oncol. 2013, 52, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.L., Jr.; Coimbra, F.J.F.; Ribeiro, H.S.; Diniz, A.L.; de Godoy, A.L.; Begnami, M.; Silva, M.J.B.; Fanelli, M.F.; Mello, C.A.L. Safety and preliminary results of perioperative chemotherapy and hyperthermic intraperitoneal chemotherapy (HIPEC) for high-risk gastric cancer patients. World J. Surg. Oncol. 2012, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xu, Z.; Wu, Y.; Liu, P.; Qian, J.; Yu, S.; Xia, B.; Lai, J.; Ma, S.; Wu, Z. Prophylactic chemotherapeutic hyperthermic intraperitoneal perfusion reduces perito-neal metastasis in gastrin cancer: A retrospective clinical study. BMC Cancer 2020, 20, 827. [Google Scholar] [CrossRef] [PubMed]
- Koemans, W.J.; van der Kaaij, R.T.; Wassenaar, E.C.E.; Boerma, D.; Boot, H.; Sikorska, K.; Los, M.; Grootscholten, C.; Hartemink, K.J.; Veenhof, A.A.F.A.; et al. Tumor characteristics and clinical outcome of peritoneal metastasis of gastric origin treated with a hyperthermic intraperitoneal chemotherapy procedure in the PERISCOPE I trial. J. Surg. Oncol. 2021, 123, 904–910. [Google Scholar] [CrossRef]
- Koemans, W.J.; Houwink, A.; van der Kaaij, R.T.; Wassenaar, E.C.E.; Boerma, D.; Hahn, C.; Imhof, O.; Brandt, M.G.; Ariens, M.P.; Veenhof, A.A.F.A.; et al. Perioperative Management of Gastric Cancer Patients Treated With (Sub)Total Gastrectomy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy (HIPEC): Lessons Learned. Ann. Surg. Oncol. 2021, 28, 4647–4654. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, M.; Xie, S.; Zhou, S.; Zhang, B.; Gao, P.; Wu, T.; Qiao, Q.; Wang, N.; He, X. Laparoscopic distal gastrectomy and hyperthermic intraperitoneal chemother-apy in the treatment of advanced gastric cancer: A retrospective case-matched study on perioperative outcomes. J. Gastrointest. Oncol. 2021, 12, 133–141. [Google Scholar] [CrossRef]
- Rosa, F.; Galiandro, F.; Ricci, R.; Di Miceli, D.; Longo, F.; Quero, G.; Tortorelli, A.P.; Alfieri, S. Survival advantage of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced gastric cancer: Experience from a Western tertiary referral center. Langenbeck’s Arch. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Ye, Z.; Dai, G.; Zhang, Y.; Huang, L.; Du, Y.; Cheng, X. Neoadjuvant systemic and hyperthermic intraperitoneal chemotherapy combined with cytoreductive surgery for gastrin cancer patients with limited peritoneal metastasis: A prospective cohort study. BMC Cancer 2020, 20, 1108. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, J.; Li, Z.; Li, B.; Luo, J.; Wang, X.; Wang, J.; Ba, M.; Tang, H.; He, Q.; et al. Hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal me-tastasis: A multicenter propensity scorematched cohort study. Chin. J. Cancer Res. 2020, 32, 794–803. [Google Scholar] [CrossRef]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.M.; Arvieux, C.; Boschetti, G.; Glehen, O.; et al. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Perez-Viejo, E.; Serrano, A.; Gutierrez Calvo, A.; Regueira, F.M.; Casado-Adam, A.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive Surgery and Hyper-thermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef]
- White, M.G.; Kothari, A.; Ikoma, N.; Murphy, M.B.; Song, S.; Ajani, J.; Mansfield, P.; Badgwell, B. Factors Associated with Resection and Survival After Laparo-scopic HIPEC for Peritoneal Gastric Cancer Metastasis. Ann. Surg. Oncol. 2020, 27, 4963–4969. [Google Scholar] [CrossRef] [PubMed]
- Hamazoe, R.; Maeta, M.; Kaibara, N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994, 73, 2048–2052. [Google Scholar] [CrossRef]
- Fujimura, T.; Yonemura, Y.; Muraoka, K.; Takamura, H.; Hirono, Y.; Sahara, H.; Ninomiya, I.; Matsumoto, H.; Tsugawa, K.; Nishimura, G.; et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: Randomized controlled study. World J. Surg. 1994, 18, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.Y.; Wu, D.; Li, S.; Qiu, Z.Y.; Song, Q.Y.; Guan, D.; Wang, L.P.; Li, X.G.; Duan, F.; Wang, X.X. Role of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer. World J. Gastrointest. Oncol. 2020, 12, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Bu, Z.; Zhang, J.; Zong, X.; Ji, X.; Fu, T.; Jia, Z.; Zhang, Y.; Wu, X. Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery. BMC Cancer 2021, 21, 216. [Google Scholar] [CrossRef]
- HTsuyoshi, D.; Inoue, T.; Kurokawa, Y. Yoshida Hyperthermic intraperitoneal chemotherapy (HIPEC) for gynecological cancer. J. Obstet. Gynaecol. Res. 2020, 46, 1661–1671. [Google Scholar] [CrossRef]
- Baratti, D.; Sammartino, P.; Kusamura, S.; Deraco, M.; Peritoneal Surface Malignancy Onco-team of the Italian Society of Surgical Oncology (SICO); Ansaloni, L.; Asero, S.; Baiocchi, G.; Bagnoli, P.; Cavaliere, D.; et al. Past, present and future of adjuvant HIPEC in patients at high risk for colorectal peritoneal metastases. Eur. J. Surg. Oncol. 2020, 46, 737–739. [Google Scholar] [CrossRef]
- Wang, X.; Li, T. Postoperative pain pathophysiology and treatment strategies after CRS + HIPEC for peritoneal cancer. World J. Surg. Oncol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Mehta, S.S.; Gelli, M.; Agarwal, D.; Goéré, D. Complications of Cytoreductive Surgery and HIPEC in the Treatment of Peritoneal Me-tastases. Indian J. Surg. Oncol. 2016, 7, 225–229. [Google Scholar] [CrossRef]
- Kusamura, S.; González-Moreno, S.; Nizri, E.; Baratti, D.; Guadagni, S.; Guaglio, M.; Battaglia, L.; Deraco, M. Learning Curve, Training Program, and Monitorization of Surgical Performance of Peritoneal Surface Malignancies Centers. Surg. Oncol. Clin. N. Am. 2018, 27, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Petrillo, M.; Costantini, B.; Fanfani, F.; Gallotta, V.; Chiantera, V.; Turco, L.C.; Bottoni, C.; Scambia, G. Minimally invasive secondary cytoreduction plus HIPEC for recurrent ovarian cancer: A case series. Gynecol. Oncol. 2014, 132, 303–306. [Google Scholar] [CrossRef]
- Petrillo, M.; Zucchetti, M.; Cianci, S.; Morosi, L.; Ronsini, C.; Colombo, A.; D’Incalci, M.; Scambia, G.; Fagotti, A. Pharmacokinetics of cisplatin during open and minimally-invasive secondary cytoreductive surgery plus HIPEC in women with platinum-sensitive recurrent ovarian cancer: A prospective study. J. Gynecol. Oncol. 2019, 30, e59. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.D.; McPartland, S.; Detelich, D.; Saif, M.W. Chemotherapy for intraperitoneal use: A review of hyperthermic intraperito-neal chemotherapy and early post-operative intraperitoneal chemotherapy. J. Gastrointest. Oncol. 2016, 7, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Elnemr, A.; Endou, Y.; Hirano, M.; Mizumoto, A.; Takao, N.; Ichinose, M.; Miura, M.; Li, Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J. Gastrointest. Oncol. 2010, 2, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Montori, G.; Coccolini, F.; Ceresoli, M.; Catena, F.; Colaianni, N.; Poletti, E.; Ansaloni, L. The treatment of peritoneal carcinomatosis in advanced gastric cancer: State of the art. Int. J. Surg. Oncol. 2014, 2014, 912418. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Common Terminology Criteria for Adverse Events v4.0; National Cancer Institute: Bethesda, MD, USA, 2009.
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Sleightholm, R.; Patel, A.; Shostrom, V.; Hall, B.; Neilsen, B.; Bartlett, D.; Smith, L. Morbidity and Mortality Rates Following Cytoreductive Surgery Combined With Hyperthermic Intraperitoneal Chemotherapy Compared With Other High-Risk Surgical Oncology Procedures. JAMA Netw. Open 2019, 2, e186847. [Google Scholar] [CrossRef]
- Badgwell, B.; Ikoma, N.; Murphy, M.B.; Wang, X.; Estrella, J.; Roy-Chowdhuri, S.; Das, P.; Minsky, B.D.; Lano, E.; Song, S.; et al. A Phase II Trial of Cytoreduction, Gastrectomy, and Hyperthermic Intraperitoneal Perfusion with Chemotherapy for Patients with Gastric Cancer and Carcinomatosis or Positive Cytology. Ann. Surg. Oncol. 2021, 28, 258–264. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Thuss-Patience, P.; Bergner, F.; Raue, W.; Arnold, A.; Hosrt, D.; Pratschke, J.; Biebl, M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer 2019, 22, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Graziosi, L.; Marino, E.; De Angelis, V.; Rebonato, A.; Donini, A. Survival prognostic factors in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy treatment: Analysis from a single oncological center. World J. Surg. Oncol. 2016, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Cotte, E.; Glehen, O.; Lotti, M.; Poiasina, E.; Catena, F.; Yonemura, Y.; Ansaloni, L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur. J. Surg. Oncol. 2014, 40, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana di Oncologia Medica. Linee Guida Tumori Peritoneali Primitivi E Secondari. 2019. Available online: https://www.aiom.it/wp-content/uploads/2019/10/2019_LG_AIOM_Peritoneo.pdf (accessed on 14 June 2021).
- PSOGI. International Recommendations for the Management of Peritoneal Metastases. Available online: http://www.psogi.com/psogi/international-recommendations-for-the-management-of-peritoneal-metastases/ (accessed on 14 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).