Factors Related to Hospitalisation-Associated Disability in Patients after Surgery for Acute Type A Aortic Dissection: A Retrospective Study
Abstract
1. Introduction
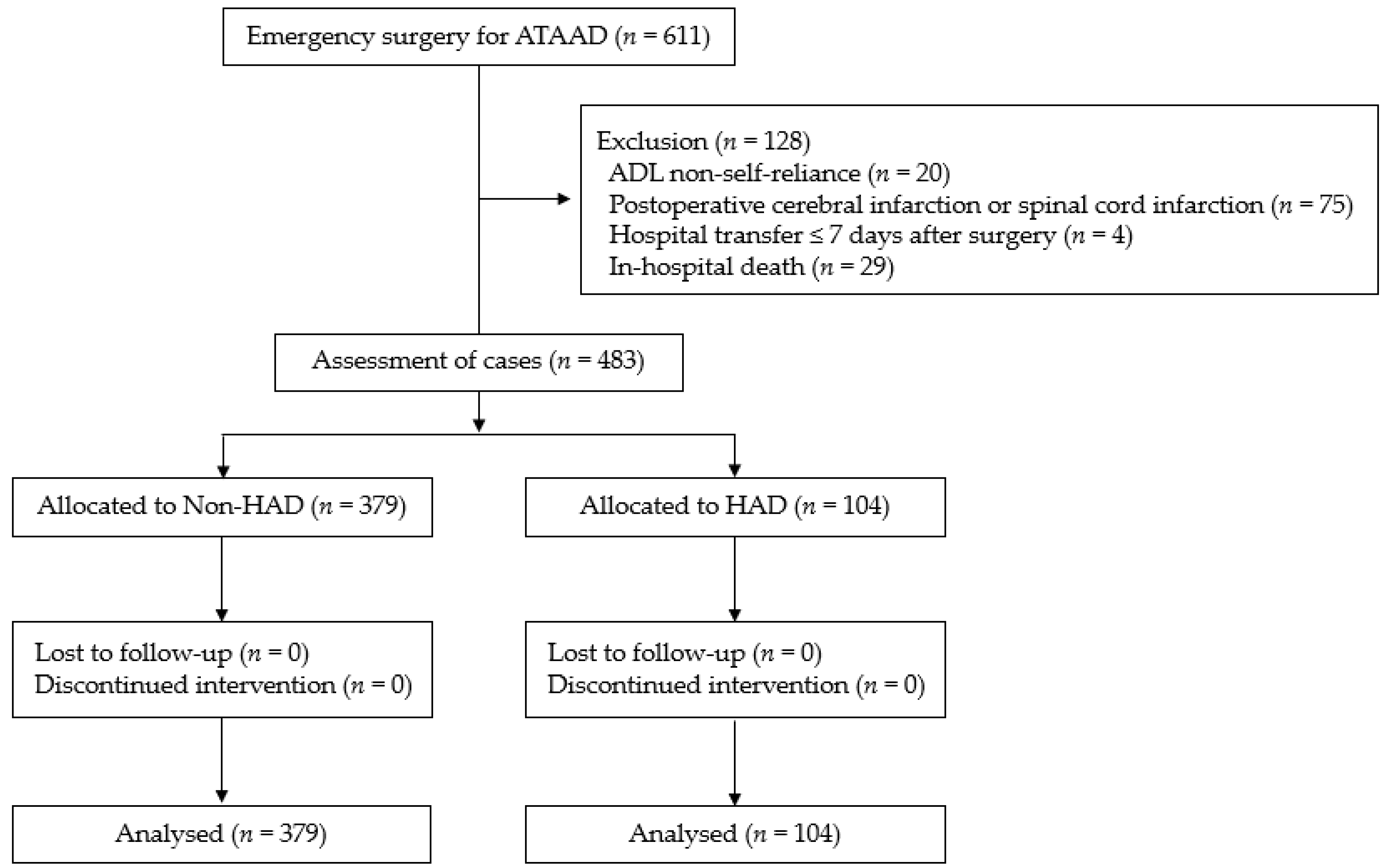
2. Materials and Methods
2.1. Participants
2.2. Definition of HAD
2.3. Postoperative Cardiac Rehabilitation
2.4. Additional Assessments
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. The Progression of Cardiac Rehabilitation after ATAAD Surgery
4.2. Factors Associated with HAD
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [PubMed]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends from the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.; Ahlsson, A.; Fuglsang, S.; Geirsson, A.; Gunn, J.; Hansson, E.C.; Hjortdal, V.; Jarvela, K.; Jeppsson, A.; Mennander, A.; et al. Medium-term survival after surgery for acute Type A aortic dissection is improving. Eur. J. Cardiothorac. Surg. 2017, 52, 852–857. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Briegel, I.; Dolch, M.; Irlbeck, M.; Hauer, D.; Kaufmann, I.; Schelling, G. Quality of results of therapy of acute respiratory failure: Changes over a period of two decades. Anaesthesist 2013, 62, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Zisberg, A.; Shadmi, E.; Gur-Yaish, N.; Tonkikh, O.; Sinoff, G. Hospital-associated functional decline: The role of hospitalization processes beyond individual risk factors. J. Am. Geriatr. Soc. 2015, 63, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; von Heymann, C.; von Dossow, V.; Spaethe, C.; Konertz, W.F.; Jain, U.; Spies, C.D. Increased interleukin-6 after cardiac surgery predicts infection. Anesth. Analg. 2006, 102, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Files, D.C.; Sanchez, M.A.; Morris, P.E. A conceptual framework: The early and late phases of skeletal muscle dysfunction in the acute respiratory distress syndrome. Crit. Care 2015, 19, 266. [Google Scholar] [CrossRef]
- Okada, Y.; Unoki, T.; Matsuishi, Y.; Egawa, Y.; Hayashida, K.; Inoue, S. Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: A systematic review and meta-analysis. J. Intensive Care 2019, 7, 57. [Google Scholar] [CrossRef]
- Homepage of Sakakibara Heart Institute. Available online: https://www.hp.heart.or.jp/ (accessed on 6 April 2022).
- Hirakawa, K.; Nakayama, A.; Saitoh, M.; Arimitsu, T.; Iwai, K.; Hori, K.; Shimokawa, T.; Takanashi, S.; Haraguchi, G.; Isobe, M. Physical function examination at intensive care unit as predictive indicators for hospitalization-associated disability in patients after cardiovascular surgery. Rev. Cardiovasc. Med. 2022, 23, 77. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The barthel index. Md. State Med. J. 1995, 14, 61–65. [Google Scholar]
- JCS Joint Working Group. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef]
- KDIGO AKI Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Ely, E.W.; Inouye, S.K.; Bernard, G.R.; Gordon, S.; Francis, J.; May, L.; Truman, B.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). J. Am. Med. Assoc. 2001, 286, 2703–2710. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for Diagnosis and Treatment of Aortic Aneurysm and Aortic Dissection (JCS 2011): Digest Version. Circ. J. 2013, 77, 789–828. [Google Scholar]
- Girdauskas, E.; Kuntze, T.; Borger, M.A.; Röhrich, K.; Schmitt, D.; Fassl, J.; Falk, V.; Mohr, F.W. Acute respiratory dysfunction after surgery for acute type A aortic dissection. Eur. J. Cardiothorac. Surg. 2010, 37, 691–696. [Google Scholar] [CrossRef]
- Di Eusanio, M.; Trimarchi, S.; Patel, H.J.; Hutchison, S.; Suzuki, T.; Peterson, M.D.; Di Bartolomeo, R.; Folesani, G.; Pyeritz, R.E.; Braverman, A.C.; et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: Observations from the International Registry of Acute Aortic Dissection. J. Thorac. Cardiovasc. Surg. 2013, 145, 385–390. [Google Scholar] [CrossRef]
- Pan, E.; Gudbjartsson, T.; Ahlsson, A.; Fuglsang, S.; Geirsson, A.; Hansson, E.C.; Hjortdal, V.; Jeppsson, A.; Järvelä, K.; Mennander, A.; et al. Low rate of reoperations after acute type A aortic dissection repair from The Nordic Consortium Registry. J. Thorac. Cardiovasc. Surg. 2018, 156, 939–948. [Google Scholar] [CrossRef]
- Balzi, D.; Lauretani, F.; Barchielli, A.; Ferrucci, L.; Bandinelli, S.; Buiatti, E.; Milaneschi, Y.; Guralnik, J.M. Risk factors for disability in older persons over 3-year follow-up. Age Ageing 2010, 39, 92–98. [Google Scholar] [CrossRef]
- Itagaki, A.; Saitoh, M.; Okamura, D.; Kawamura, T.; Otsuka, S.; Tahara, M.; Mori, Y.; Kamisaka, K.; Ochi, Y.; Yuguchi, S.; et al. Factors related to physical functioning decline after cardiac surgery in older patients: A multicenter retrospective study. J. Cardiol. 2019, 74, 279–283. [Google Scholar] [CrossRef]
- Loyd, C.; Markland, A.D.; Zhang, Y.; Fowler, M.; Harper, S.; Wright, N.C.; Carter, C.S.; Buford, T.W.; Smith, C.H.; Kennedy, R.; et al. Prevalence of Hospital-Associated Disability in Older Adults: A Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 455–461. [Google Scholar] [CrossRef]
- Morisawa, T.; Saitoh, M.; Otsuka, S.; Takamura, G.; Tahara, M.; Ochi, Y.; Takahashi, Y.; Iwata, K.; Oura, K.; Sakurada, K.; et al. Hospital-Acquired Functional Decline and Clinical Outcomes in Older Cardiac Surgical Patients: A Multicenter Prospective Cohort Study. J. Clin. Med. 2022, 11, 640. [Google Scholar] [CrossRef]
- National Survey on Physical and Motor Abilities by The Japan Sports Agency. 2021. Available online: https://www.mext.go.jp/sports/b_menu/toukei/chousa04/tairyoku/kekka/k_detail/1421920_00003.htm (accessed on 18 May 2022).
- Bossone, E.; Carbone, A.; Eagle, K.A. Gender Differences in Acute Aortic Dissection. J. Pers. Med. 2022, 12, 1148. [Google Scholar] [CrossRef]
- Peter, J.V.; Moran, J.L.; Phillips-Hughes, J.; Graham, P.; Bersten, A.D. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: A meta-analysis. Lancet 2006, 367, 1155–1163. [Google Scholar] [CrossRef]
- Rudolph, J.L.; Inouye, S.K.; Jones, R.N.; Yang, F.M.; Fong, T.G.; Levkoff, S.E.; Marcantonio, E.R. Delirium: An independent predictor of functional decline after cardiac surgery. J. Am. Geriatr. Soc. 2010, 58, 643–649. [Google Scholar] [CrossRef]
- Abelha, F.J.; Luís, C.; Veiga, D.; Parente, D.; Fernandes, V.; Santos, P.; Botelho, M.; Santos, A.; Santos, C. Outcome and quality of life in patients with postoperative delirium during an ICU stay following major surgery. Crit. Care 2013, 17, R257. [Google Scholar] [CrossRef]
- Schweickert, W.D.; Pohlman, M.C.; Pohlman, A.S.; Nigos, C.; Pawlik, A.J.; Esbrook, C.L.; Spears, L.; Miller, M.; Franczyk, M.; Deprizio, D.; et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet 2009, 373, 1874–1882. [Google Scholar] [CrossRef]
- Kayambu, G.; Boots, R.; Paratz, J. Early physical rehabilitation in intensive care patients with sepsis syndromes: A pilot randomised controlled trial. Intensive Care Med. 2015, 41, 865–874. [Google Scholar] [CrossRef]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef]
- Friedrich, O.; Reid, M.B.; Van den Berghe, G.; Vanhorebeek, I.; Hermans, G.; Rich, M.M.; Larsson, L. The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill. Physiol. Rev. 2015, 95, 1025–1109. [Google Scholar] [CrossRef]
- Marra, A.; Ely, E.W.; Pandharipande, P.P.; Patel, M.B. The ABCDEF Bundle in Critical Care. Crit. Care Clin. 2017, 33, 225–243. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, 825–873. [Google Scholar] [CrossRef]


| Overall | Non-HAD | HAD | p Value | |
|---|---|---|---|---|
| (n = 483) | (n = 379) | (n = 104) | ||
| Age, years | 69 (59–78) | 68 (58–75) | 78 (66–83) | <0.001 |
| Female, n (%) | 250 (51.8) | 187 (49.3) | 63 (60.6) | <0.05 |
| BMI, kg/m2 | 23.6 (21.3–26.3) | 23.7 (21.4–26.4) | 23.1 (21.2–26.0) | 0.62 |
| Comorbidity, n (%) | ||||
| Hypertension | 336 (69.6) | 260 (68.6) | 76 (73.1) | 0.38 |
| Dyslipidaemia | 93 (19.3) | 71 (18.7) | 22 (21.2) | 0.58 |
| Diabetes | 25 (5.2) | 17 (4.5) | 8 (7.7) | 0.19 |
| Chronic kidney disease | 24 (5.0) | 17 (4.5) | 7 (6.7) | 0.35 |
| Coronary artery disease | 21 (4.3) | 16 (4.2) | 5 (4.8) | 0.49 |
| Chronic obstructive pulmonary disease | 8 (1.7) | 6 (1.6) | 2 (1.9) | 0.54 |
| Blood biochemistry | ||||
| Haemoglobin, g/dL | 12.3 (11.1–13.8) | 12.5 (11.2–14.0) | 11.6 (10.2–12.7) | <0.001 |
| Platelet, 103/μL | 164 (132–200) | 165 (132–202) | 161 (133–194) | 0.53 |
| Creatinine, mg/dL | 0.87 (0.70–1.12) | 0.86 (0.69–1.12) | 0.91 (0.75–1.13) | 0.15 |
| Albumin, g/dL | 3.6 (3.3–4.0) | 3.7 (3.4–4.0) | 3.4 (3.0–3.7) | <0.001 |
| CRP, mg/dL | 0.15 (0.04–0.99) | 0.13 (0.04–0.90) | 0.18 (0.05–1.40) | 0.38 |
| Aortic surgery, n (%) | 0.40 | |||
| Ascending aortic replacement | 255 (51.6) | 187 (49.2) | 68 (59.6) | |
| Hemi arch replacement | 26 (5.3) | 18 (4.7) | 8 (7.0) | |
| Total arch replacement | 135 (27.3) | 112 (29.5) | 23 (20.2) | |
| Concomitant | 78 (15.8) | 63 (16.6) | 15 (13.2) | |
| Operation time, min | 225 (185–287) | 225 (182–285) | 229 (193–304) | 0.21 |
| Cardiopulmonary bypass time, min | 128 (105–168) | 128 (104–168) | 128 (110–169) | 0.54 |
| Circulatory arrest time, min | 27 (22–38) | 27 (21–39) | 28 (23–38) | 0.42 |
| IMV time, h | 22.9 (18.2–34.6) | 22.1 (17.7–30.9) | 31.6 (21.8–46.3) | <0.001 |
| Bleeding, ml | 220 (150–350) | 210 (150–320) | 250 (163–438) | <0.05 |
| APACHE II score, points | 15 (12–18) | 15 (12–18) | 16 (13–18) | <0.05 |
| NPPV, n (%) | 102 (21.1) | 67 (17.7) | 35 (33.7) | <0.001 |
| CRRT, n (%) | 12 (2.5) | 6 (1.6) | 6 (5.8) | <0.05 |
| Acute kidney injury, n (%) | 175 (36.2) | 130 (34.3) | 45 (43.3) | 0.09 |
| Delirium, n (%) | 123 (25.5) | 71 (18.7) | 52 (50.0) | <0.001 |
| Distal enlargement, n (%) | 34 (7.0) | 26 (6.7) | 8 (7.7) | 0.77 |
| ICU stay, days | 3 (2–5) | 3 (2–4) | 4 (3–7) | <0.001 |
| Hospital stay, days | 14 (11–21) | 14 (11–21) | 13 (10–22) | 0.50 |
| Cardiac rehabilitation, days | ||||
| Sitting | 2 (1–2) | 2 (1–2) | 2 (2–3) | <0.001 |
| Standing | 2 (1–3) | 2 (1–2) | 2 (2–3) | <0.001 |
| Walking | 3 (3–4) | 3 (2–4) | 5 (3–7) | <0.001 |
| Barthel index, points | ||||
| Preoperative | 100 (100–100) | 100 (100–100) | 100 (100–100) | 0.40 |
| Discharge | 100 (100–100) | 100 (100–100) | 80 (55–90) | <0.001 |
| Home discharge, n (%) | 276 (57.1) | 255 (67.3) | 21 (20.2) | <0.001 |
| n = 131 | |
|---|---|
| Not awakening | 51 (38.9) |
| Respiratory-related | 26 (19.8) |
| Sedation | 14 (10.7) |
| Uncontrolled blood pressure | 10 (7.6) |
| Arrhythmia | 8 (6.1) |
| Acute kidney injury | 6 (4.6) |
| Pain distress | 5 (3.8) |
| Active bleeding | 4 (3.1) |
| Acute limb ischemia | 2 (1.5) |
| Others | 5 (3.8) |
| Walking Onset Category | |||
|---|---|---|---|
| Early | Usual | Delayed | |
| (n = 111) | (n = 243) | (n = 129) | |
| Age, years | 66 (55–75) | 71 (62–79) 1 | 69 (57–81) |
| Female, n (%) | 48 (43.2) | 137 (56.4) | 65 (50.4) |
| BMI, kg/m2 | 23.4 (21.3–25.6) | 23.7 (21.5–26.4) | 23.1 (20.9–26.6) |
| Comorbidity, n (%) | |||
| Hypertension | 79 (71.2) | 170 (70.0) | 87 (67.4) |
| Dyslipidemia | 22 (19.8) | 50 (20.6) | 21 (16.3) |
| Diabetes | 4 (3.6) | 15 (6.2) | 6 (4.7) |
| Chronic kidney disease | 3 (2.7) | 11 (4.5) | 10 (7.8) |
| Coronary artery disease | 2 (1.8) | 12 (4.9) | 7 (5.4) |
| Chronic obstructive pulmonary disease | 3 (2.7) | 3 (1.2) | 2 (1.6) |
| Operation time, min | 215 (184–280) | 218 (180–268) | 263 (197–324) 1,2 |
| Cardiopulmonary bypass time, min | 123 (102–165) | 125 (103–157) | 149 (110–192) 1,2 |
| IMV time, h | 19.3 (16.4–23.7) | 22.6 (18.0–30.5) 1 | 38.1 (22.2–50.7) 1,2 |
| Bleeding, ml | 200 (130–270) | 220 (150–350) | 280 (165–453) 1,2 |
| APACHE II score, points | 14 (12–17) | 15 (13–17) | 15 (13–19) 1 |
| NPPV, n (%) | 8 (7.2) | 42 (17.3) | 52 (40.3) 3 |
| CRRT, n (%) | 1 (0.9) | 2 (0.8) | 9 (7.0) 3 |
| Delirium, n (%) | 11 (9.9) | 58 (23.9) | 54 (41.9) 3 |
| ICU stay, days | 2 (1–2) | 3 (2–3) 1 | 5 (4–8) 1,2 |
| Hospital stay, days | 13 (10–18) | 14 (11–20) | 19 (13–28) 1,2 |
| Preoperative BI, points | 100 (100–100) | 100 (100–100) | 100 (100–100) |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age [every 1-year increase] | 1.05 | 1.03–1.08 | <0.001 | 1.05 | 1.02–1.09 | <0.01 |
| <60 years | 1.00 | 1.00 | ||||
| 60–69 years | 0.75 | 0.36–1.57 | 0.45 | |||
| 70–79 years | 1.38 | 0.71–2.67 | 0.35 | |||
| ≥80 years | 4.83 | 2.56–9.12 | <0.001 | 4.28 | 1.62–11.35 | <0.01 |
| Female | 1.58 | 1.01–2.45 | <0.05 | |||
| Albumin [every 1 g/dL increase] | 0.37 | 0.23–0.58 | <0.001 | |||
| Haemoglobin [every 1 g/dL increase] | 0.76 | 0.67–0.86 | <0.001 | |||
| APACHE II score [every 1-point increase] | 1.05 | 1.00–1.11 | 0.07 | |||
| IMV time [every 1-h increase] | 1.03 | 1.02–1.05 | <0.001 | |||
| NPPV | 2.36 | 1.46–3.84 | <0.01 | 2.15 | 1.10–4.19 | <0.05 |
| CRRT | 3.81 | 1.20–12.1 | <0.05 | |||
| Delirium | 4.34 | 2.73–6.89 | <0.001 | 2.93 | 1.60–−5.37 | <0.01 |
| ICU stay (every 1-day increase) | 1.28 | 1.18–1.39 | <0.001 | |||
| Walking (every 1-day increase) | 1.37 | 1.23–1.54 | <0.001 | 1.29 | 1.07–1.56 | <0.01 |
| Univariate | Multivariate * | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Walking start category | ||||||
| Early | 1.00 | 1.00 | ||||
| Usual | 1.97 | 0.91–4.25 | 0.08 | 0.97 | 0.42–2.26 | 0.94 |
| Delayed | 9.55 | 4.45–20.52 | <0.001 | 2.76 | 1.05–7.21 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirakawa, K.; Nakayama, A.; Saitoh, M.; Hori, K.; Shimokawa, T.; Iwakura, T.; Haraguchi, G.; Isobe, M. Factors Related to Hospitalisation-Associated Disability in Patients after Surgery for Acute Type A Aortic Dissection: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 12918. https://doi.org/10.3390/ijerph191912918
Hirakawa K, Nakayama A, Saitoh M, Hori K, Shimokawa T, Iwakura T, Haraguchi G, Isobe M. Factors Related to Hospitalisation-Associated Disability in Patients after Surgery for Acute Type A Aortic Dissection: A Retrospective Study. International Journal of Environmental Research and Public Health. 2022; 19(19):12918. https://doi.org/10.3390/ijerph191912918
Chicago/Turabian StyleHirakawa, Kotaro, Atsuko Nakayama, Masakazu Saitoh, Kentaro Hori, Tomoki Shimokawa, Tomohiro Iwakura, Go Haraguchi, and Mitsuaki Isobe. 2022. "Factors Related to Hospitalisation-Associated Disability in Patients after Surgery for Acute Type A Aortic Dissection: A Retrospective Study" International Journal of Environmental Research and Public Health 19, no. 19: 12918. https://doi.org/10.3390/ijerph191912918
APA StyleHirakawa, K., Nakayama, A., Saitoh, M., Hori, K., Shimokawa, T., Iwakura, T., Haraguchi, G., & Isobe, M. (2022). Factors Related to Hospitalisation-Associated Disability in Patients after Surgery for Acute Type A Aortic Dissection: A Retrospective Study. International Journal of Environmental Research and Public Health, 19(19), 12918. https://doi.org/10.3390/ijerph191912918








