Exposition of Intermediate Hosts of Schistosomes to Niclosamide (Bayluscide WP 70) Revealed Significant Variations in Mortality Rates: Implications for Vector Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System
2.2. Collection and Rearing of Snails
2.3. Susceptibility of Snail Egg Embryos to Niclosamide
2.4. Susceptibility of Adult Snails to Niclosamide
2.5. Data Analyses
3. Results
3.1. Susceptibility of Egg Embryos to Niclosamide
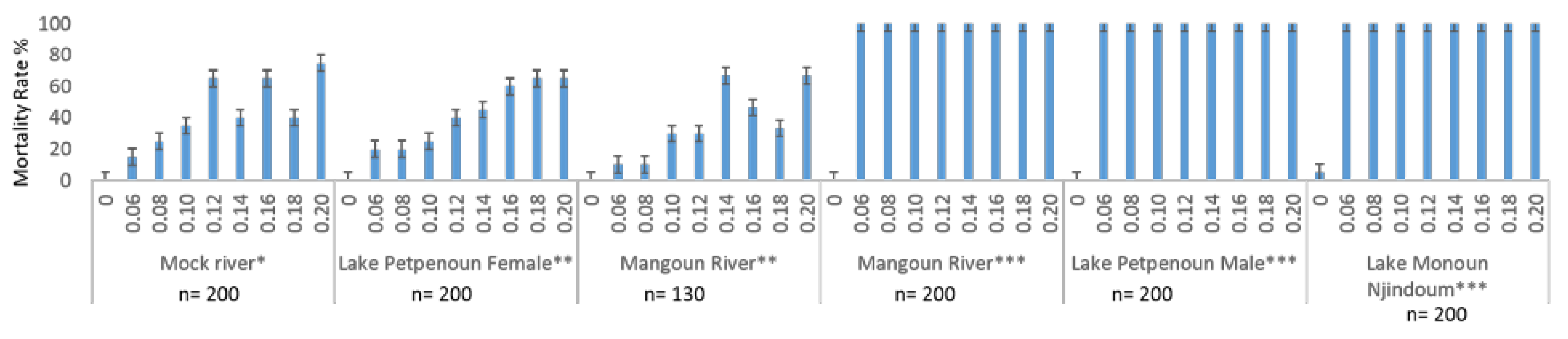
3.2. Susceptibility of Adult Snails to Niclosamide and Avoidance Behaviour
3.3. Avoidance Behaviour of Adult Snails Exposed to Niclosamide
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: One Health: Approach for Action against Neglected Tropical Diseases 2021–2030. 2022. Available online: https://apps.who.int/iris/handle/10665/351193 (accessed on 21 May 2022).
- Tchuem Tchuenté, L.A.; Kamwa Ngassam, R.I.; Sumo, L.; Ngassam, P.; Dongmo Noumedem, C.; Nzu, D.D.O.L.; Dankoni, E.; Kenfack, C.M.; Gipwe, N.F.; Akame, J.; et al. Mapping of Schistosomiasis and Soil-Transmitted Helminthiasis in the Regions of Centre, East and West Cameroon. PLoS Negl. Trop. Dis. 2012, 6, e1553. [Google Scholar] [CrossRef] [PubMed]
- Tchuem Tchuenté, L.A.; Dongmo Noumedem, C.; Ngassam, P.; Kenfack, C.M.; Feussom Gipwe, N.; Dankoni, E.; Tarini, A.; Zhang, Y. Mapping of schistosomiasis and soil-transmitted helminthiasis in the regions of Littoral, North-West, South and South-West Cameroon and recommendations for treatment. BMC Infect. Dis. 2013, 13, 602. [Google Scholar] [CrossRef] [Green Version]
- PNLSHI. Rapport d’Avancement 2003–2019. Programme National de Lutte contre la Schistosomiase et les Helminthes Intestinales (PNLSHI); PNLSHI: Yaounde, Cameroon, 2021; p. 165. [Google Scholar]
- Awono-Ambene, H.P.; Djieukap Njieyap, L.; Akono Ntonga, P.; Etang, J.D.; Antonio-Nkondjio, C.; Ndo, C.; Etame, J.; Njiokou, F. Soil-Transmitted Protozoans and Helminths from Market Gardening Sites of Yaounde, Cameroon. J. Environ. Sci. Public Health 2020, 4, 61–70. [Google Scholar] [CrossRef]
- Tchuem Tchuenté, L.A.; Rollinson, D.; Stothard, J.R.; Molyneux, D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: Time to change and adapt strategies. Infect. Dis. Poverty 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutuku, M.W.; Dweni, C.K.; Mwangi, M.; Kinuthia, J.M.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Zhang, S.M.; Maranga, R.; Loker, E.S.; et al. Field-derived Schistosoma mansoni and Biomphalaria pfeifferi in Kenya: A compatible association characterized by lack of strong local adaptation, and presence of some snails able to persistently produce cercariae for over a year. Parasites Vectors 2014, 7, 533. [Google Scholar] [CrossRef] [PubMed]
- Kengne, A.C. Biologie et Compatibilité à Schistosoma mansoni Sambon, 1909 de Biomphalaria pfeifferi (Krauss, 1848, Planorbis) et Biomphalaria camerunensis (Boettger, 1941, Australorbis) au Cameroun; Université de Yaoundé: Yaounde, Cameroon, 2019. [Google Scholar]
- King, C.H.; Sutherland, L.J.; Bertsch, D. Systematic Review and Meta-analysis of the Impact of Chemical-Based Mollusciciding for Control of Schistosoma mansoni and S. haematobium Transmission. PLoS Negl. Trop. Dis. 2015, 9, e0004290. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Swartz, S.J.; Lopez, M.; Hsieh, M.H.; Lafferty, K.D.; Kuris, A.M.; Rickards, C.; De Leo, G.A. Global Assessment of Schistosomiasis Control Over the Past Century Shows Targeting the Snail Intermediate Host Works Best. PLoS Negl. Trop. Dis. 2016, 10, e0004794. [Google Scholar] [CrossRef] [Green Version]
- Greer, G.J.; Mimpfoundi, R.; Malek, E.A.; Joky, A.; Ngonseu, E.; Ratard, R.C. Human Schistosomiasis in Cameroon: II. Distribution of the Snail Hosts. Am. J. Trop. Med. Hyg. 1990, 42, 573–580. [Google Scholar] [CrossRef]
- Organisation Mondiale de la Santé. Lignes Directrices pour les Essais en Laboratoire et sur le Terrain de Molluscicides Destinés à la Lutte contre la Schistosomiase. 2020. Available online: https://apps.who.int/iris/handle/10665/330919 (accessed on 21 May 2022).
- Sarquis, O.; Pieri, O.S.; dos Santos, J.A.A. Effects of Bayluscide WP 70® on the Survival and Water-leaving Behaviour of Biomphalaria straminea, Snail Host of Schistosomiasis in Northeast Brazil. Memórias Inst. Oswaldo Cruz 1997, 92, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Tchami Mbagnia, M.C.; Melachio Tanekou, T.T.; Kengne Fokam, A.C.; Nguiffo Nguete, D.; Wondji, C.S.; Njiokou, F. PCR-based molecular identification of two intermediate snail hosts of Schistosoma mansoni in Cameroon. Parasites Vectors 2020, 13, 158. [Google Scholar] [CrossRef]
- Chappell, L.H. Freshwater snails of Africa and their medical importance (second edition). Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 717. [Google Scholar] [CrossRef]
- Kengne-Fokam, A.C.; Nana-Djeunga, H.C.; Djuikwo-Teukeng, F.F.; Njiokou, F. Analysis of mating system, fecundity, hatching and survival rates in two Schistosoma mansoni intermediate hosts (Biomphalaria pfeifferi and Biomphalaria camerunensis) in Cameroon. Parasites Vectors 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adenusi, A.; Odaibo, A. Effects of varying concentrations of the crude aqueous and ethanolic extracts of Dalbergia sissoo plant parts on Biomphalaria pfeifferi egg masses. Afr. J. Tradit. Complement. Altern. Med. 2010, 6. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.F.; Ferraz, P.A.; Pinto, A.V.; do Pinto, M.C.; Goulart, M.O.; Sant’Ana, A.E. Molluscicidal activity of 2-hydroxy-3-alkyl-1,4-naphthoquinones and derivatives. Int. J. Parasitol. 2000, 30, 1199–1202. [Google Scholar] [CrossRef]
- He, P.; Wang, W.; Sanogo, B.; Zeng, X.; Sun, X.; Lv, Z.; Yuan, D.; Duan, L.; Wu, Z. Molluscicidal activity and mechanism of toxicity of a novel salicylanilide ester derivative against Biomphalaria species. Parasites Vectors 2017, 10, 383. [Google Scholar] [CrossRef]
- Pieri, O.S.; Gonçalves, J.F.; Sarquis, O. Repeated focal mollusciciding for snail control in a sugar-cane area of northeast Brazil. Mem.-Inst. Oswaldo Cruz 1995, 90, 535–536. [Google Scholar] [CrossRef] [Green Version]
- Pointier, J.P.; Giboda, M. The case for biological control of snail intermediate hosts of Schistosoma mansoni. Parasitol. Today Pers Ed. 1999, 15, 395–397. [Google Scholar] [CrossRef]
- Omobhude, M.E.; Morenikeji, O.A.; Oyeyemi, O.T. Molluscicidal activities of curcumin-nisin polylactic acid nanoparticle on Biomphalaria pfeifferi. PLoS Negl. Trop. Dis. 2017, 11, e0005855. [Google Scholar] [CrossRef] [Green Version]
- Parashar, B.D.; Kaushik, M.P.; Gupta, A.K.; Swamy, R.V.; Rao, K.M. Toxicity of some molluscicides to freshwater snail Lymnaea auricularia the vector of animal fasciolasis and to non-target organisms. Proc. Acad. Environ. Biol. 1995, 4, 183–187. [Google Scholar]
- Salawu, O.T.; Odaibo, A.B. The molluscicidal effects of Hyptis suaveolens on different stages of Bulinus globosus in the laboratory. Afr. J. Biotechnol. 2011, 10, 10241–10247. [Google Scholar] [CrossRef]
- Zhao, Q.P.; Xiong, T.; Xu, X.J.; Jiang, M.S.; Dong, H.F. De Novo Transcriptome Analysis of Oncomelania hupensis after Molluscicide Treatment by Next-Generation Sequencing: Implications for Biology and Future Snail Interventions. PLoS ONE 2015, 10, e0118673. [Google Scholar] [CrossRef] [PubMed]
- Webbe, G. The use of molluscicides in the control of human trematode infections. Toxicol. Molluscic. Int. Encycl. Pharmacol. Ther. Sect. 1987, 125, 1–11. [Google Scholar]
- Zhang, Y.; Guo, Y.H. Study on the effect of bromoacetamide upon the development of snail eggs. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 1992, 10, 258–262. [Google Scholar] [PubMed]
- Okeke, O.C.; Ubachukwu, P.O. Molluscicidal Effects of Talinum triangulare on Bulinus truncatus. Niger. J. Biotechnol. 2011, 22, 13–16. [Google Scholar]
- De Souza, C.P.; Mendes, N.M. Repopulation of breeding habitats of Biomphalaria glabrata after treatment with niclosamide. Rev. Inst. Med. Trop. São Paulo 1991, 33, 297–302. [Google Scholar] [PubMed]
- Takougang, I.; Meli, J.; Wabo Poné, J.; Angwafo, F. Community acceptability of the use of low-dose niclosamide (Bayluscide®), as a molluscicide in the control of human schistosomiasis in Sahelian Cameroon. Ann. Trop. Med. Parasitol. 2007, 101, 479–486. [Google Scholar] [CrossRef]
- Greer, G.J.; Tchounwou, P.B.; Takougang, I.; Monkiedje, A. Field tests of a village-based mollusciciding programme for the control of snail hosts of human schistosomes in Cameroon. Trop. Med. Int. Health 1996, 1, 320–327. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Englande, A.J., Jr.; Malek, E.A.; Anderson, A.C.; Abdelghani, A.A. The effects of bayluscide and malathion on the survival of Schistosoma mansoni miracidia. J. Environ. Sci. Health Part B 1991, 26, 69–82. [Google Scholar] [CrossRef]
- Takougang, I.; Meli, J.; Angwafo, F., III. Field trials of low dose Bayluscide on snail hosts of schistosome and selected non-target organisms in sahelian Cameroon. Mem. Inst. Oswaldo Cruz 2006, 101, 355–358. [Google Scholar] [CrossRef] [Green Version]
- WHO Expert Committee on the Control of Schistosomiasis. The Control of Schistosomiasis: Second Report of the WHO Expert Committee [Meeting Held in Geneva from 8–15 November 1991]; World Health Organization: Geneva, Switzerland, 1993; 86p.
- Clark, T.E.; Appleton, C.C.; Drewes, S.E. A semi-quantitative approach to the selection of appropriate candidate plant molluscicides—A South African application. J. Ethnopharmacol. 1997, 56, 1–13. [Google Scholar] [CrossRef]
- Yuan, H.; Jiagang, G.; Bergquist, R.; Tanner, M.; Xianyi, C.; Huanzeng, W. The 1992–1999 World Bank schistosomiasis research initiative in China: Outcome and perspectives. Parasitol. Int. 2000, 49, 195–207. [Google Scholar] [CrossRef]
- Schall, V.T.; Vasconcellos, M.C.; Rocha, R.S.; Souza, C.P.; Mendes, N.M. The control of the schistosome-transmitting snail Biomphalaria glabrata by the plant Molluscicide Euphorbia splendens var.hislopii (syn milli Des. Moul): A longitudinal field study in an endemic area in Brazil. Acta Trop. 2001, 79, 165–170. [Google Scholar] [CrossRef]
| Snail Sampling Sites | Niclosamide Dose (mg/L) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | |
| Mock River * | ||||||
| No. egg masses | 55 | 12 | 12 | 12 | 12 | |
| No. egg embryos | 483 | 106 | 129 | 134 | 116 | 12 |
| % egg embryos hatched (95% CI) | 90.5 (87.5–92.8) | 66.6 (56.6–74.4) | 0 (0.0–3.5) | 0 (0.0–2.8) | 0 (0.0–3.2) | 134 |
| Lake Petpenoun Female ** | ||||||
| No. egg masses | 34 | 10 | 11 | 10 | 11 | 0 (0–2.8) |
| No. egg embryos | 766 | 210 | 233 | 248 | 277 | 11 |
| % egg embryos hatched (95% CI) | 99.0 (98.0–99.5) | 91.9 (87.4–94.9) | 86.3 (81.3–90.1) | 0.0 (0.0–1.5) | 0.0 (0.0–1.4) | 232 |
| Mangoun River * | ||||||
| No. egg masses | 37 | 4 | 4 | 4 | 5 | 0.0 (0.0–1.6) |
| No. egg embryos | 478 | 83 | 59 | 85 | 68 | 5 |
| % egg embryos hatched (95% CI) | 96.7 (94.6–97.9) | 65.1 (54.3–74.4) | 3.4 (0.9–11.5) | 0.0 (0.0–4.3) | 0.0 (0.0–5.3) | 111 |
| Mangoun River *** | ||||||
| No. egg masses | 42 | 15 | 15 | 16 | 16 | 0.0 (0.0–3.3) |
| No. egg embryos | 621 | 141 | 159 | 186 | 198 | 16 |
| % egg embryos hatched (95% CI) | 97.9 (96.5–98.8) | 83.0 (75.9–88.3) | 66.7 (59.0–73.5) | 0.0 (0.0–2.0) | 0.0 (0.0–1.9) | 218 |
| Lake Petpenoun Male *** | ||||||
| No. egg masses | 40 | 9 | 12 | 10 | 10 | 0.0 (0.0–1.7) |
| No. egg embryos | 422 | 119 | 146 | 121 | 141 | 10 |
| % egg embryos hatched (95% CI) | 89.3 (86.0–91.9) | 54.6 (45.7–63.3) | 89.7 (83.7–93.7) | 0.0 (0.0–3.1) | 0.0 (0.0–2.7) | 153 |
| Lake Monoun Njindoum *** | ||||||
| No. egg masses | 48 | 9 | 8 | 8 | 9 | 0.0 (0.0–2.4) |
| No. egg embryos | 483 | 124 | 109 | 86 | 94 | 8 |
| % egg embryos hatched (95% CI) | 93.4 (90.8–95.3) | 75.0 (66.7–81.8) | 63.3 (53.9–71.8) | 0.0 (0.0–4.3) | 0.0 (0.0–3.9) | 89 |
| Snail Sampling Sites | LC50 (95% CI) | LC95 (95% CI) |
|---|---|---|
| Egg embryos | ||
| Mock River * | 0.10 (0.02–0.20) | 6.30 (2.50–15.60) |
| Lake Petpenoun Female ** | 0.84 (0.47–1.49) | 8.07 (4.52–14.39) |
| Mangoun River ** | 0.11 (0.05–0.22) | 2.64 (1.28–5.5) |
| Lake Petpenoun Male *** | 0.27 (0.18–0.42) | 2.10 (1.35–3.25) |
| Mangoun River *** | 0.81 (0.38–1.72) | 20.72 (9.76–44.01) |
| Lake Monoun Njindoum *** | 0.20 (0.03–1.29) | 1102.53 (174.27–6975.34) |
| Adult snails | ||
| River Mock * | 0.14 (0.11–0.18) | 0.60 (0.46–0.76) |
| Lake Petpenoun Female ** | 0.14 (0.11–1,18) | 0.56 (0.44–0.71) |
| Mangoun River ** | 0.20 (0.13–0.30) | 2.57 (1.68–3.94) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kengne Fokam, A.C.; Sumo, L.; Bagayan, M.; Nana-Djeunga, H.C.; Kuete, T.; Nganjou, G.S.O.; Tchami Mbagnia, M.C.; Djune-Yemeli, L.; Wondji, C.S.; Njiokou, F. Exposition of Intermediate Hosts of Schistosomes to Niclosamide (Bayluscide WP 70) Revealed Significant Variations in Mortality Rates: Implications for Vector Control. Int. J. Environ. Res. Public Health 2022, 19, 12873. https://doi.org/10.3390/ijerph191912873
Kengne Fokam AC, Sumo L, Bagayan M, Nana-Djeunga HC, Kuete T, Nganjou GSO, Tchami Mbagnia MC, Djune-Yemeli L, Wondji CS, Njiokou F. Exposition of Intermediate Hosts of Schistosomes to Niclosamide (Bayluscide WP 70) Revealed Significant Variations in Mortality Rates: Implications for Vector Control. International Journal of Environmental Research and Public Health. 2022; 19(19):12873. https://doi.org/10.3390/ijerph191912873
Chicago/Turabian StyleKengne Fokam, Alvine Christelle, Laurentine Sumo, Mohamed Bagayan, Hugues Clotaire Nana-Djeunga, Thomas Kuete, Gabriella S. Ondoua Nganjou, Murielle Carole Tchami Mbagnia, Linda Djune-Yemeli, Charles Sinclair Wondji, and Flobert Njiokou. 2022. "Exposition of Intermediate Hosts of Schistosomes to Niclosamide (Bayluscide WP 70) Revealed Significant Variations in Mortality Rates: Implications for Vector Control" International Journal of Environmental Research and Public Health 19, no. 19: 12873. https://doi.org/10.3390/ijerph191912873
APA StyleKengne Fokam, A. C., Sumo, L., Bagayan, M., Nana-Djeunga, H. C., Kuete, T., Nganjou, G. S. O., Tchami Mbagnia, M. C., Djune-Yemeli, L., Wondji, C. S., & Njiokou, F. (2022). Exposition of Intermediate Hosts of Schistosomes to Niclosamide (Bayluscide WP 70) Revealed Significant Variations in Mortality Rates: Implications for Vector Control. International Journal of Environmental Research and Public Health, 19(19), 12873. https://doi.org/10.3390/ijerph191912873









