1. Introduction
Pregnant women are among the most vulnerable populations to malaria infection. In 2020, approximately 34 million pregnant women were at risk of exposure to the
Plasmodium falciparum parasite.[
1] For pregnant women, especially, malaria infection is life-threatening and accounts for over 10,000 maternal and 200,000 neonatal deaths per year.[
1,
2] Other adverse outcomes include increased risks of maternal anemia, prematurity, and low birth weight. In 2020, it was estimated that malaria infection during pregnancy accounted for 819,000 low birthweight newborns in sub-Saharan Africa [
1].
Intermittent preventive treatment in pregnancy (IPTp) is a core intervention recommended by the World Health Organization (WHO) for the prevention of malaria in pregnancy [
1]. IPTp with sulfadoxine-pyrimethamine (SP) is safe in pregnancy and effective in reducing the risk of clinical malaria during pregnancy, maternal anemia, placental parasitemia, and low birthweight [
3,
4,
5,
6,
7,
8]. The WHO currently recommends that women receive at least three doses of IPTp-SP during pregnancy [
1]. IPTp-SP can be administered as early as possible in the second trimester and given at monthly intervals during scheduled antenatal care visits (ANC) up to the time of delivery.
While 33 moderate-to-high transmission Sub-Saharan Africa (SSA) countries have adopted IPTp into their national malaria control guidelines, the gap between the actual implementation of IPTp and target coverage levels remains large. According to the WHO Malaria World Report (2021), 74% of pregnant women report using ANC at least once; yet, only 57% of pregnant women receive at least one dose of IPTp, which is far below the recommended target level (90%) [
1]. It has been estimated that if 90% of pregnant women received at least three doses of IPTp, an additional 206,000 low birth weights would have been averted in these 33 countries [
1]. Understanding how IPTp is delivered at health facilities is crucial to closing the gap between ANC utilization and IPTp coverage.
Countries such as Senegal have made great strides in strengthening their health care infrastructure and reducing malaria transmission. Senegal has an annual malaria incidence rate of 50.5 cases per 1000, ranking it in the lowest 33rd percentile among malaria-endemic countries in the African region [
2]. The national malaria control policy in Senegal focuses on achieving universal coverage of IPTp, insecticide-treated nets (ITNs), indoor residual spraying (IRS), malaria diagnostic testing, and prompt treatment of malaria with artemisinin-based combination therapies (ACTs) [
9,
10]. IPTp is one of the country’s earliest malaria interventions, adopted in 2005 [
11]. According to the 2013 Senegal National Strategic Plan for Malaria Control, all pregnant women shall receive at least two doses of SP after the onset of active fetal movements, with the first dose given from the 16th week of amenorrhea or the perception of active movements of the fetus and the second at least one month after the first dose [
12,
13]. In 2016, about 50% of pregnant women in Senegal received two or more SP doses (IPTp-SP2+), above the 44% average among African countries [
2,
14]. However, compared to its 96% ANC utilization rate (2016), Senegal still experienced a substantial gap between ANC service and IPTp provision [
15]. In the 2016–2020 National Strategic Plan, the recommended IPTp-SP treatment was updated to at least three doses per the most recent WHO recommendation. As Senegal sets a goal to increase the coverage of IPTp to at least three doses of SP (IPTp-SP3+) (In 2012, WHO changed the recommended SP doses from two to three, but Senegal did not complete the new IPTp training on the new recommendation until 2016 [
14]. It also did not update the national malaria treatment and prevention guideline based on the new recommendation until 2016 [
16].) to ≥80%, understanding how IPTp is provided at ANC visits and the roles that health care providers play are important for timely receipt of IPTp [
15].
Previous studies have shown that the coverage of IPTp was affected by factors at the health system level, facility level, provider level, and individual level. Key health system issues affecting the provision and uptake of IPTp included the discordance between malaria control and maternal health policies, human resource shortages, and medication stock-outs [
17,
18]. At the facility level, private facilities were less likely to administer IPTp than facilities managed by public authorities [
19]. Women’s individual factors, such as older age, higher education level, more ANC visits, receiving health education information and HIV counseling during ANC, previous pregnancy complications, and history of pregnancy, were reported to be positively associated with the uptake of IPTp, while never being married, long distance to ANC facility, young age, and limited ANC visits, were found to be negatively associated with the uptake of IPTp [
20,
21,
22]. However, fewer studies have focused on factors influencing the uptake of IPTp among women attending ANC [
19,
20]. To our knowledge, no previous study has examined factors affecting the uptake of IPTp in ANC settings in Senegal [
23,
24]. This study aims to investigate the key factors influencing IPTp-SP administration in Senegal. Data from the Service Provision Assessment (SPA), a comprehensive survey implemented by the Demographic Health Survey (DHS Program) to determine the quality of health service delivery [
25], is used to collect information from health care workers and women utilizing ANC and IPTp services.
3. Results
On average, pregnant women in our final sample were 27 years old and 27 gestational weeks (see
Table 1). Among them, 42.8% had never received any SP dose before the ANC visit (see
Appendix A Table A2), and 76% were pregnant for the first time. In addition, approximately 50% of pregnant women received some form of formal education. About 46% of visits took place in health facilities located in urban areas. Of the health facilities included in this study, 6% were hospitals, 13% were health centers, and 81% were health clinics. Eighty-eight percent of ANC visits in this study were conducted in government-owned facilities. About 92% of visits occurred in facilities equipped with malaria treatment and prevention guidelines. In comparison, only about 64% of visits occurred in facilities with unexpired SP in stock. Lastly, the proportion of staff trained on malaria IPTp guidelines that were on-site at the time of the survey averaged 31%. The regional distribution of ANC visits is presented in
Appendix A Table A4.
For the conditional probability of IPTp-SP prescription for each ANC visit category along with gestational age, we collapsed the ANC visits at the 4-week interval to compute the mean likelihood and 95% CI (see
Figure 1). In addition to the color-coded probability curve of each IPTp-SP history category, we plot the distribution density of the unconditional ANC visits along the week of pregnancy at the time of the interview. Contrary to the national guideline, we find that pregnant women who previously never received IPTp-SP (red line) were not always prescribed SP. Specifically, only 37% (95% CI = [23%, 51%]) of pregnant women of 4-month pregnancy who needed the first IPTp-SP received the prescription. The probability increases to 83% (95% CI = [74%, 92%]) for women of 5-month pregnancy with the same IPTp-SP history but is still statistically significantly different from 100%. Although women further along in pregnancy had SP prescription probability not statistically different from 100%, the overall probability curve rejects the hypothesis that the first dose of IPTp-SP prescription is accordant to the national malaria treatment and prevention guideline.
The blue line visualizes the probability curve for ANC visits that required the second dose of IPTp-SP. Unlike the previous category, women of 4-month pregnancy or longer all had SP prescription probability that was not statistically different from 100%. However, this result is primarily due to the wide CIs of the sample means. The probability ranges from 71% in the fourth month of pregnancy to 96% in the ninth month.
Lastly, the green line depicts the probability curve for ANC visits that IPTp-SP prescription was optional according to the national guideline. Interestingly, ANC visits in this category had a high chance of receiving SP prescriptions. For instance, women who were 4–6 months pregnant in this category all received the optional SP prescriptions. Furthermore, among all women who already had the required 2 IPTp-SP doses, 60.8% received the SP prescriptions at the ANC visits.
We predict the probabilities that a pregnant woman receives an IPTp-SP prescription using our logit regression results (see
Table 2; logit regression coefficients are reported in
Appendix A Table A3, predicted probabilities). We use the IPTp-SP history of never having any SP dose before (IPTp-SP0) and its interaction term, when applicable, as the reference group. Without the interaction between IPTp-SP history and the week of pregnancy (Specification 1), the probability that IPTp-SP1 women receive SP prescriptions is not statistically different from IPTp-SP0 women. In comparison, IPTp-SP2+ women whose SP prescription is optional at the time of ANC visits are 0.08–0.48 times as likely to be prescribed with SP. The differences across IPTp-SP types are statistically significant. In Specification 2, we added the interaction terms between IPTp-SP types and weeks of pregnancy. An IPTp-SP0 patient has an 84% chance of receiving an SP prescription at mean gestational age and other covariates. The probability is similar for IPTp-SP1 women (85%) but reduces to 70% for IPTp-SP2 women, 61% for IPTp-SP3 women, and 42% for IPTp-SP4 women.
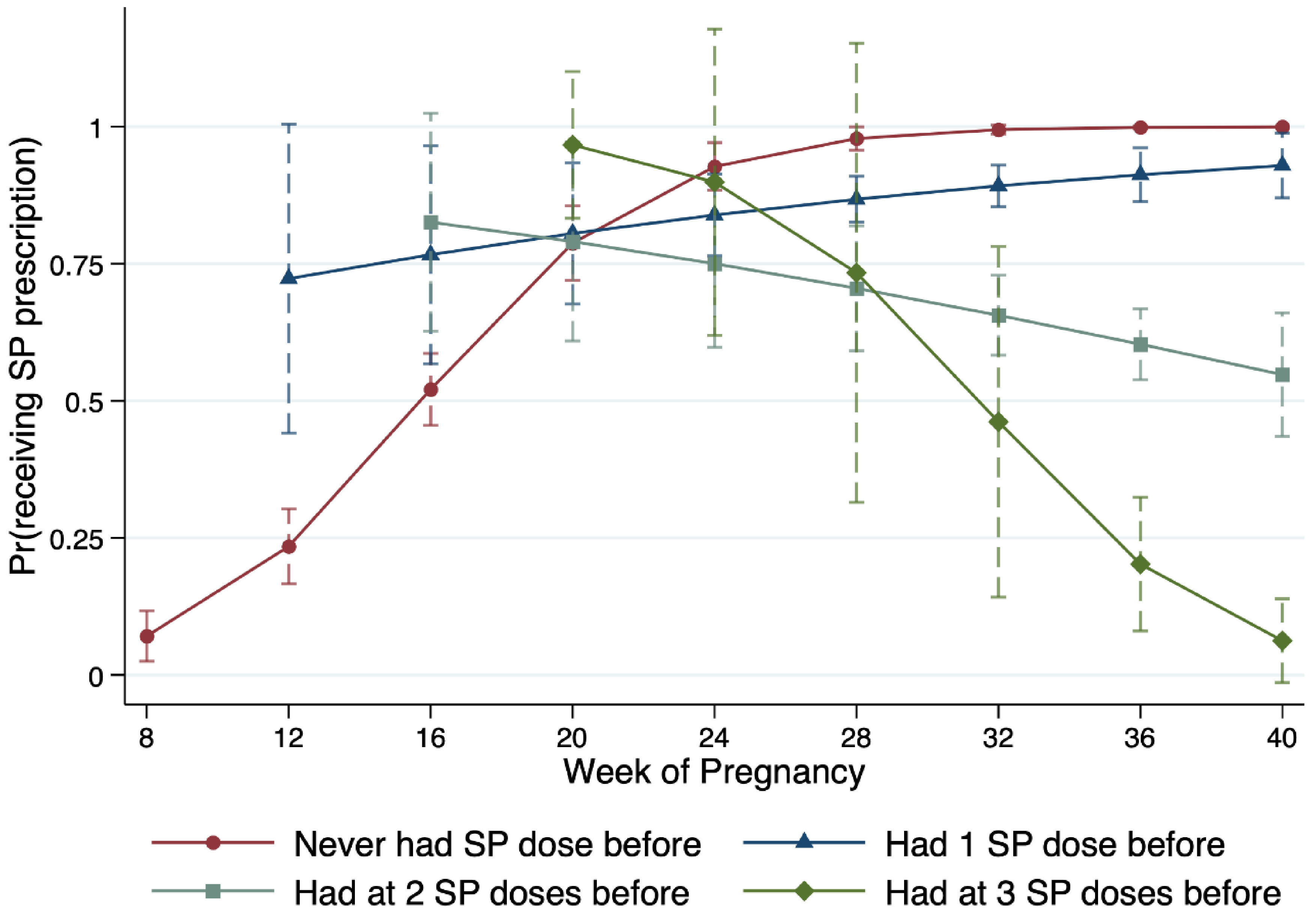
To further unpack the relationship between SP prescription, IPTp-SP history, and women’s gestational age,
Figure 2 presents the marginal mean probability of SP prescription along the week of pregnancy. For illustration simplicity, we use the same color codes as in
Figure 1 to distinguish between IPTp-SP types. For IPTp-SP0 (red circle) and IPTp-SP1 (blue triangle) women, the marginal mean probability of SP prescription increases the further along in pregnancy. The probability of a 16-week pregnant IPTp-SP0 patient who needs an SP prescription the most is only about 52%. This number is not only statistically significantly lower than the national recommendation but also statistically significantly lower than that of an IPTp-SP1 patient with a similar or longer pregnant length, as well as that of women whose IPTp-SP prescription is optional. For pregnant women whose IPTp-SP treatment is optional (IPTp-SP2+), the marginal mean probability of receiving an SP prescription decreases with gestational age.
Pregnant women’s characteristics also have a strong correlation with the probability of receiving IPTp-SP. Older women tend to have a lower probability of receiving IPTp-SP treatment or prescriptions. Women who are pregnant for the first time are more likely to receive SP but only significant at a 10% level. Among facility characteristics, we find facility location (rural vs. urban) does not significantly impact IPTp-SP provision. Different types of hospitals also have similar effects, except that providers in private for-profit facilities shrink the likelihood of treating with or prescribing IPTp-SP by a factor of 0.05 compared to providers in other types of facilities (Odds ratio is calculated using logit regression coefficients.). Lastly, a higher share of care providers who ever received IPTp training is positively correlated with the likelihood of IPTp-SP treatment and prescription.
The sensitivity analyses with clustered standard errors at the regional level showed similar results to the main findings (results not shown).
4. Discussion
Despite the national guideline requiring care providers to prescribe at least two doses of IPTp-SP (Because Senegal issued the minimum three doses of IPTp-SP guideline and completed the corresponding training in 2016, we refer to the earlier IPTp-SP2+ requirement in the discussion, which was in effect during the data collection of our analysis sample (SPA 2014, 2016).) to pregnant women at scheduled ANC visits, a significant proportion of pregnant women in Senegal did not receive IPTp-SP prescriptions per the guideline. Specifically, IPTp-SP0 pregnant women who are the prime target of IPTp intervention have a chance as low as 52% to receive their first IPTp-SP dose at ANC visits in the 4th month of pregnancy (84% throughout the course of pregnancy). A similar gap also presents among the IPTp-SP1 women, who only have an 85% chance on average to be prescribed the second required SP dose by ANC providers throughout the course of pregnancy.
Notably, this gap could not be explained by the lack of proper infrastructure for malaria services at the facility level. The national malaria treatment and prevention guidelines are available to 92% of facilities (
Table 1). There is also evidence suggesting that the national guideline, is to some extent, binding for care providers. The probability of receiving the first two IPTp-SP doses at ANC visits is statistically higher than those of additional doses. The positive modification effect by gestational age among the IPTp-SP0 and IPTp-SP1 women also accredits the achievement of current interventions. Moreover, though only about 57.4% of facilities have unexpired SP in stock (
Table 1), the availability of SP does not significantly predict a woman’s chance of receiving an IPTp-SP at her ANC visit. Previous literature often cited medication stock-outs as a crucial factor affecting the provision and uptake of IPTp [
17,
18]. Our findings suggested that the mere presence of medication was not enough to promote the uptake of IPTp.
The positive association between a pregnant woman’s accumulative IPTp-SP history and her likelihood of receiving an IPTp-SP at an ANC visit during the second trimester highlights the importance of the interaction between care providers and pregnant women in improving IPTp-SP delivery and broadly the prevention of malaria in pregnancy. On average, women who never received IPTp-SP before have a 75% chance of receiving an IPTp-SP prescription at their second trimester ANC visits. The probability increases to 80% for IPTp-SP1 women, 78% for IPTp-SP2 women, and 94% for IPTp-SP3 women (results not shown). Although IPTp-SP history is an important reference for providers to prescribe SP in ANC settings, how IPTp-SP history predicts the uptake of IPTp-SP was rarely documented in previous literature. Although the SPA surveys do not document the interactions leading to whether or not to provide IPTp-SP, the higher likelihood among pregnant women to whom the IPTp-SP is optional suggests IPTp history-dependent disparity. This disparity might possibly be explained by the characteristics of care providers and pregnant women. For instance, pregnant women who had received IPTp-SP before could be more aware of the risk and the adverse consequence of malaria infection; thus, they could be more likely to be proactive and initiate the conversation regarding IPTp-SP with ANC providers. Conversely, pregnant women with no or low IPTp-SP history may be less aware of the importance of IPTp-SP and rely on ANC providers to complete the required IPTp-SP doses. Further data collection and research would be warranted to examine those hypotheses.
After the IPTp intervention has been adopted in Senegal for nearly a decade, converting guideline recommendations at the policy level to care delivery in the field remains challenging. These findings raised the question: what steps can further close the gaps to the IPTp policy targets? As Crawley et al. pointed out, collaboration with reproduction health programs is the key step in implementing a malaria prevention policy targeting pregnant women at risk. [
32] One area of actionable improvement is malaria IPTp training. For each sampled facility, only an average of 31% of care providers received malaria IPTp training in the past 24 months. At the facility level, moving from the bottom quintile in the share of IPTp trained staff to the top quintile is associated with a nearly four-time higher odds ratio (odds ratio is calculated using logit regression coefficients) (CI = [1.54, 9.80]) of providing IPTp-SP to pregnant women at the ANC visit. Emphasis on malaria IPTp training among ANC providers, particularly during the rainy season, would be an effective step to narrow the gap between IPTp policy-making and care delivery [
33,
34]. As Senegal moves from a minimum of two IPTp-SP doses to a three doses requirement, updating training programs and enforcing regular care provision assessments are necessary to ensure a smooth transition to the latest IPTp policy goal as well as future intervention upscaling [
14].
While improving training for the provider side is necessary, dissemination of IPTp education among pregnant women such that more women at risk are aware of the IPTp-SP as an integrated part of ANC will help accelerate the improvement of IPTp-SP provision [
35,
36,
37]. In addition, according to the updated WHO guidelines for malaria, other delivery methods (such as community health workers) could be explored to close the gap in ANC-based delivery of IPTp-SP [
38]. These community-based services might be particularly helpful in reaching women who live far from ANC facilities.
This study has several limitations. First, our results draw from a cross-sectional analysis using a convenience sample. They are thus prone to non-response bias if pregnant women who participate in the survey differ from those who do not. Moreover, with insufficient panel data to track individual pregnant women’s ANC and IPTp-SP history, we can only draw an association, rather than temporal causality, between the likelihood of receiving IPTp-SP at ANC visit and explanatory variables. Particularly, the time interval between receiving the last IPTp-SP and this ANC visit is unknown in the SPA data. As a result, it is possible that not all women with at least one IPTp-SP dose before need to be prescribed SP at this ANC visit. However, women with one prior IPTp-SP dose had SP prescription probability not statistically different from 100% starting from their 4-month pregnancy. Thus, the omitted variable of time to the last IPTp-SP should not be a major threat to our findings. Other omitted variables due to data limitations (such as the distance to the ANC facility) could also potentially threaten the findings.
Second, we use IPTp-SP treatment or prescription by care providers to approximate IPTp-SP administration. This approximation may be problematic if the patient compliance level is low, which would negatively bias our estimations, and is likely to have a differential effect on IPTp history [
39,
40]. Thus, our results shall be interpreted as the best-case scenario probabilities. Further research is needed on IPTp compliance and its interaction with IPTp provision.
Third, we recover the IPTp-SP history for each interviewed pregnant woman based on the interviewers’ observations and records from pregnant women’s ANC cards, assuming that the ANC cards were updated timely and accurately. Although we remove observations with IPTp records that occurred too early in pregnancy (<8 weeks), recall bias/measurement error could bias our results away from the null. On the other hand, if those erroneous observations are a truthful reflection of IPTp history, they raise a concern about over-provision. Future IPTp policy shall address this issue to avoid adverse outcomes such as SP resistance.