Effects of High Intensity Interval Training versus Sprint Interval Training on Cardiac Autonomic Modulation in Healthy Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Participants
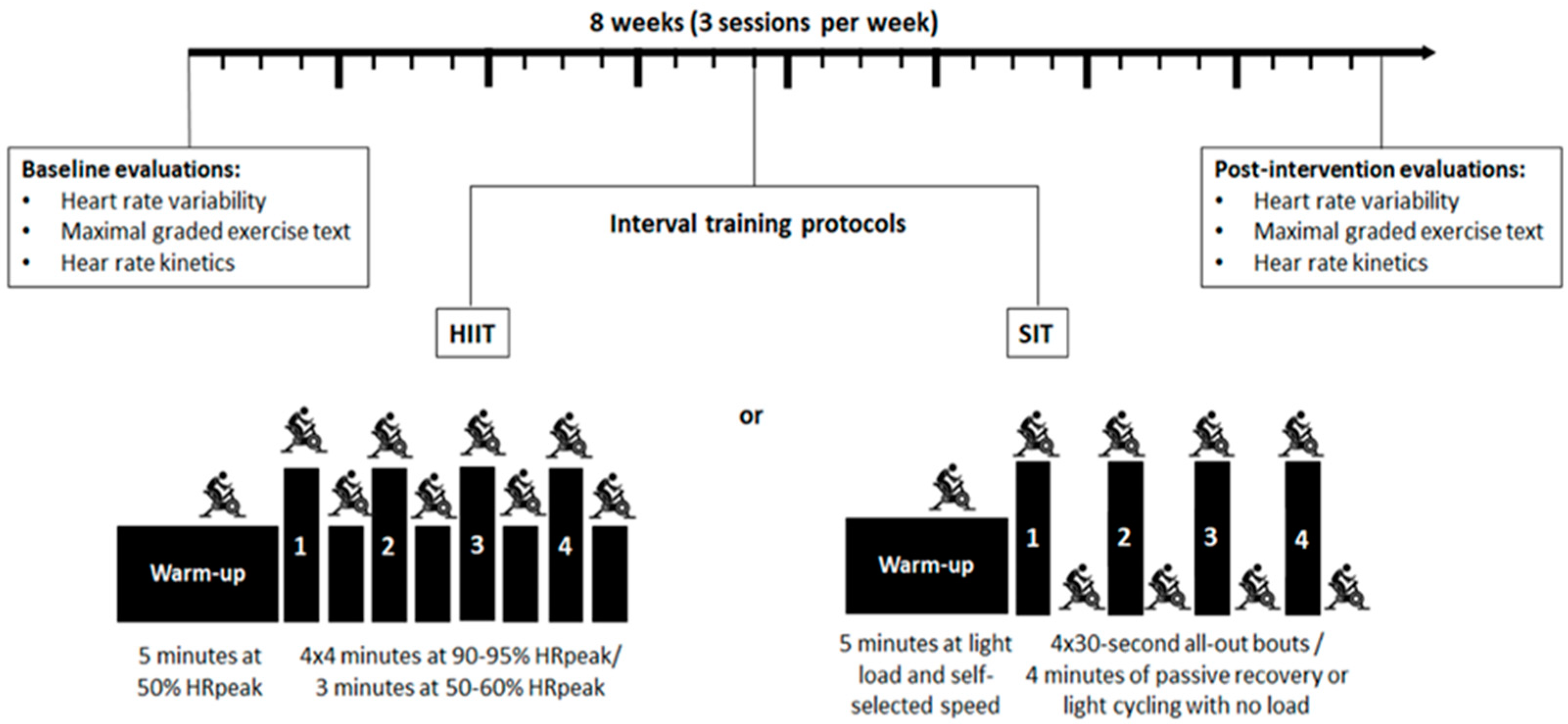
2.2. Study Design
2.3. Assessment of Cardiorespiratory Fitness
2.4. R-R Record and Heart Rate Variability
2.5. Evaluation of Heart Rate Kinetics
2.6. Delta Analysis
2.7. Intervention with Interval Training
2.8. Statistical Analysis
3. Results
3.1. Participants
3.2. Heart Rate Variability and VO2
3.3. Entropy and Symbolic Analysis
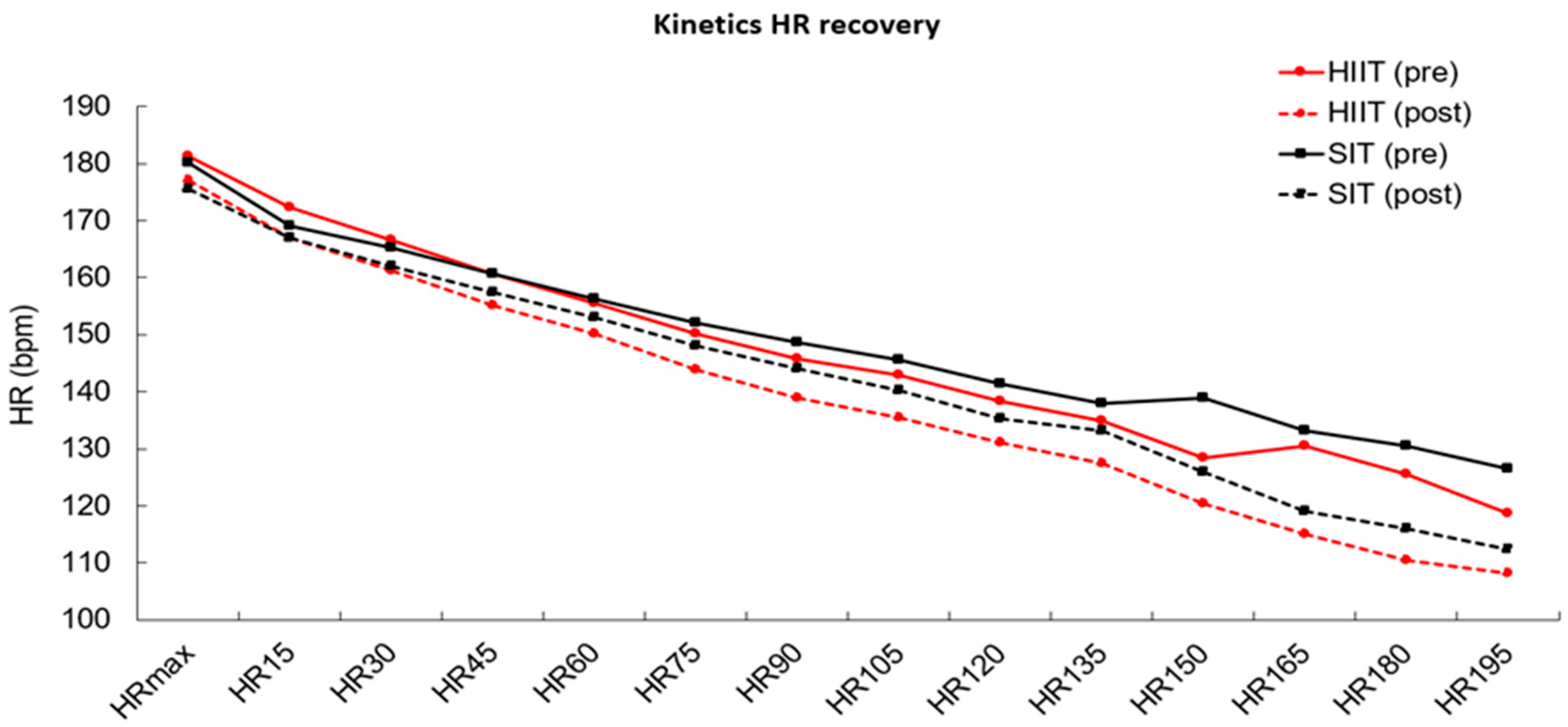
3.4. Heart Rate Kinetics
4. Discussion
Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Précoma, D.B.; De Oliveira, G.M.M.; Simão, A.F.; Dutra, O.P.; Coelho, O.R.; Izar, M.C.D.O.; Póvoa, R.M.D.S.; Giuliano, I.D.C.B.; Filho, A.C.D.A.; Machado, C.A.; et al. Atualização da Diretriz de Prevenção Cardiovascular da Sociedade Brasileira de Cardiologia—2019. Arq. Bras. Cardiol. 2019, 113, 787–891. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, T.; Milani, M.; Ferraz, A.S.; Da Silveira, A.D.; Herdy, A.H.; Hossri, C.; e Silva, C.G.S.; Gil Araujo, C.; Rocco, E.A.; Teixeira, J.A.C.; et al. Diretriz Brasileira de Reabilitação Cardiovascular—2020. Arq. Bras. Cardiol. 2020, 114, 943–987. [Google Scholar] [CrossRef] [PubMed]
- Vanderlei, L.; Pastre, C.M.; Hoshi, R.; Carvalho, T.; Godoy, M. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Rev. Bras. Cir. Cardiovasc. 2009, 24, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Veeranki, S.P.; Magnussen, C.G.; Xi, B. Recommended physical activity and all cause and cause specific mortality in US adults: Prospective cohort study. BMJ 2020, 370, m2031. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, A.E. Heart Rate Monitoring Applications and Limitations. Sport Med. 2003, 33, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.; Graham, K.S.; Oam, G.M.D. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals—A Review. Front. Physiol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.R.B.E.; Zamunér, A.R.; Gentil, P.; Alves, F.M.; Leal, A.G.F.; Soares, V.; Silva, M.S.; Vieira, M.F.; Simões, K.; Pedrino, G.R.; et al. Cardiac Autonomic Modulation and the Kinetics of Heart Rate Responses in the On- and Off-Transient during Exercise in Women with Metabolic Syndrome. Front. Physiol. 2017, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Catai, A.M.; Pastre, C.M.; Godoy, M.F.; Silva, E.D.; Takahashi, A.C.M.; Vanderlei, L.C.M. Heart rate variability: Are you using it properly? Standardisation checklist of procedures. Braz. J. Phys. Ther. 2020, 24, 91–102. [Google Scholar] [CrossRef]
- Maddison, R.; Rawstorn, J.C.; Islam, S.M.S.; Ball, K.; Tighe, S.; Gant, N.; Whittaker, R.M.; Chow, C.K. mHealth Interventions for Exercise and Risk Factor Modification in Cardiovascular Disease. Exerc. Sport Sci. Rev. 2019, 47, 86–90. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sport. Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Zaffalon Júnior, J.K.; Viana, A.O.; Melo, G.E.L.; Angelis, K. The impact of sedentarism on heart rate variability (HRV) at rest and in response to mental stress in young women. Physiol. Rep. 2018, 6, e13873. [Google Scholar] [CrossRef]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H.C. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int. J. Environ. Res. Public Health 2018, 15, 1508. [Google Scholar] [CrossRef] [Green Version]
- Besnier, F.; Labrunée, M.; Richard, L.; Faggianelli, F.; Kerros, H.; Soukarié, L.; Bousquet, M.; Garcia, J.L.; Pathak, A.; Gales, C.; et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann. Phys. Rehabil. Med. 2019, 62, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Flores, S.; Medeiros, A.R.; Voltarelli, F.A.; Iglesias-Soler, E.; Doma, K.; Simões, H.G.; Rosa, T.S.; Boullosa, D.A. Combined effects of very short “all out” efforts during sprint and resistance training on physical and physiological adaptations after 2 weeks of training. Eur. J. Appl. Physiol. 2019, 119, 1337–1351. [Google Scholar] [CrossRef]
- Arboleda-Serna, V.H.; Feito, Y.; Patiño-Villada, F.A.; Vargas-Romero, A.V.; Arango-Vélez, E.F. Effects of highintensity interval training compared to moderate-intensity continuous training on maximal oxygen consumption and blood pressure in healthy men: A randomized controlled trial. Biomédica 2019, 39, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Naves, J.P.A.; Viana, R.B.; Rebelo, A.C.S.; Lira, C.A.B.; Pimentel, G.D.; Lobo, P.C.B.; Oliveira, J.C.M.; Ramirez-Campillo, R.; Gentil, P. Effects of High-Intensity Interval Training vs. Sprint Interval Training on Anthropometric Measures and Cardiorespiratory Fitness in Healthy Young Women. Front. Physiol. 2018, 9, 1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naves, J.P.A.; Rebelo, A.C.S.; Silva, L.R.B.; Silva, M.S.; Ramirez-Campillo, R.; Ramírez-Vélez, R.; Gentil, P. Cardiorespiratory and perceptual responses of two interval training and a continuous training protocol in healthy young men. Eur. J. Sport Sci. 2019, 5, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sport Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Leicht, A.S.; Allen, G.D.; Hoey, A.J. Influence of intensive cycling training on heart rate variability during rest and exercise. Can. J. Appl. Physiol. 2003, 28, 898–909. [Google Scholar] [CrossRef]
- Viana, R.B.; de Lira, C.A.B.; Naves, J.P.A.; Coswig, V.S.; Del Vecchio, F.B.; Ramirez-Campillo, R.; Vieira, C.A.; Gentil, P. Can we draw general conclusions from interval training studies? Sport Med. 2018, 48, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.B.; Gentil, P.R.V.; Beltrame, T.; Basso Filho, M.A.; Alves, F.M.; Silva, M.S.; Pedrino, G.R.; Ramirez-Campillo, R.; Coswig, V.; Rebelo, A.C.S. Exponential model for analysis of heart rate responses and autonomic cardiac modulation during different intensities of physical exercise. R. Soc. Open Sci. 2019, 6, 190639. [Google Scholar] [CrossRef] [Green Version]
- Bellenger, C.R.; Fuller, J.T.; Thomson, R.L.; Davison, K.; Robertson, E.Y.; Buckley, J.D. Monitoring Athletic Training Status Through Autonomic Heart Rate Regulation: A Systematic Review and Meta-Analysis. Sport Med. 2016, 46, 1461–1486. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Gibala, M.J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2014, 39, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.S.; Dalleck, L.C.; Borrani, F.; Beetham, K.S.; Mielke, G.I.; Dias, K.A.; Wallen, M.P.; Keating, S.E.; Fassett, R.G.; Coombes, J.S. High-intensity interval training and cardiac autonomic control in individuals with metabolic syndrome: A randomized trial. Int. J. Cardiol. 2017, 245, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.; Van Den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Task Force American Heart. Guidelines Heart rate variability. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Raimundo, R.D.; Godleski, J.J. Variabilidade da frequência cardíaca na síndrome metabólica. J. Hum. Growth Dev. 2015, 25, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Porta, A.; Baselli, G.; Liberati, D.; Montano, N.; Cogliati, C.; Gnecchi-Ruscone, T.; Malliani, A.; Cerutti, S. Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biol. Cybern. 1998, 78, 71–78. [Google Scholar] [CrossRef]
- Guzzetti, S.; Borroni, E.; Garbelli, P.E.; Ceriani, E.; Della Bella, P.; Montano, N.; Cogliati, C.; Somers, V.K.; Mallani, A.; Porta, A. Symbolic dynamics of heart rate variability: A probe to investigate cardiac autonomic modulation. Circulation 2005, 112, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Porta, A.; Guzzetti, S.; Montano, N.; Furlan, R.; Pagani, M.; Malliani, A.; Cerutti, S. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans. Biomed. Eng. 2001, 48, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Wisløff, U.; Støylen, A.; Loennechen, J.P.; Bruvold, M.; Rognmo, Ø.; Haram, P.M.; Tjønna, A.E.; Helgerud, J.; Slørdahl, S.A.; Lee, S.J.; et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation 2007, 115, 3086–3094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibala, M.J.; Little, J.P.; Van Essen, M.; Wilkin, G.P.; Burgomaster, K.A.; Safdar, A.; Raha, S.; Tarnopolsky, M.A. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006, 575, 901–911. [Google Scholar] [CrossRef]
- Billman, G.E.; Kukielka, M. Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: Protection is not due to enhanced cardiac vagal regulation. J. Appl. Physiol. 2006, 100, 896–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac Parasympathetic’’ Reactivation Following Exercise: Implications for Training Prescription. Sport Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Tamburus, N.Y.; Paula, R.F.; Kunz, V.C.; César, M.C.; Moreno, M.A.; Silva, E. Interval training based on ventilatory anaerobic threshold increases cardiac vagal modulation and decreases high-sensitivity c-reative protein: Randomized clinical trial in coronary artery disease. Braz. J. Phys. Ther. 2015, 19, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Bhati, P.; Bansal, V.; Ali Moiz, J. Comparison of diferent volumes of high intensity interval training on cardiac autonomic function in sedentary young women. Int. J. Asolescent Med. Health 2017, 31, 1–13. [Google Scholar] [CrossRef]
- Schaun, G.Z.; Del Vecchio, F.B. Acute impact of high-intensity interval exercise on heart rate variability: Comparison between full-body and stationary bicycle protocols. J. Strength Cond. Res. 2018, 32, 223–229. [Google Scholar] [CrossRef]
- Buchheit, M.; Papelier, Y.; Laursen, P.B. Noninvasive assessment of cardiac parasympathetic function: Postexercise heart rate recovery or heart rate variability? Am. Physiol. Soc. 2007, 293, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Michael, S.; Jay, O.; Graham, K.S.; Davis, G.M. Higher exercise intensity delays postexercise recovery of impedance-derived cardiac sympathetic activity. Appl. Physiol. Nutr. Metab. 2017, 42, 834–840. [Google Scholar] [CrossRef]
- Kaikkonen, P.; Hynynen, E.; Mann, T.; Rusko, H.; Nummela, A. Can HRV be used to assess training load in constant load exercises? Eur. J. Appl. Physiol. 2010, 108, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Martinmäki, K.; Häkkinen, K.; Mikkola, J.; Rusko, H. Effect of low-dose endurance training on heart rate variability at rest and during an incremental maximal exercise test. Eur. J. Appl. Physiol. 2008, 104, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Jay, O.; Graham, K.S.; Davis, G.M. Longer exercise duration delays post-exercise recovery of cardiac parasympathetic but not sympathetic indices. Eur. J. Appl. Physiol. 2017, 117, 1897–1906. [Google Scholar] [CrossRef]

| HIIT (n = 22) | SIT (n = 21) | |
|---|---|---|
| Age (years) | 31.1 ± 6.5 | 28.8 ± 6.0 |
| Height (m) | 164.04 ± 4.75 | 164.95 ± 4.91 |
| Body mass (kg) | 66.70 ± 10.16 | 67.86 ± 8.32 |
| Body mass index (kg/m²) | 24.60 ± 3.46 | 24.98 ± 3.19 |
| Variables | HIIT (n = 22) | SIT (n = 21) | Group | Time | Group × Time | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| O2peak (mL/kg/min) | 37.3 ± 7.6 | 42.1 ± 6.0 | 32.0 ± 7.7 | 36.5 ± 7.0 | 0.001 * | 0.004 * | 0.924 |
| HRV índices | |||||||
| RMSSD (ms2) | 29.7 ± 13.7 | 36.0 ± 20.8 | 24.7 ± 9.2 | 41.0 ± 18.6 | 0.994 | 0.002 * | 0.159 |
| SDNN (ms2) | 34.3 ± 10.8 | 37.2 ± 13.5 | 29.4 ± 8.4 | 42.1 ± 13.5 | 0.990 | 0.003 * | 0.059 * |
| SD1 (ms) | 21.7 ± 10.1 | 25.5 ± 14.7 | 17.5 ± 6.5 | 29.1 ± 13.2 | 0.907 | 0.003 * | 0.122 |
| SD2 (ms) | 43.2 ± 13.8 | 45.4 ± 14.6 | 37.5 ± 10.8 | 51.1 ± 16.2 | 0.998 | 0.011 * | 0.062 |
| SD2/SD1 | 2.3 ± 1.2 | 2.1 ± 0.7 | 2.3 ± 0.7 | 2.0 ± 0.6 | 0.692 | 0.133 | 0.826 |
| Entropy | |||||||
| ES | 3.10 ± 0.8 | 3.81 ± 0.4 | 3.53 ± 0.75 | 3.64 ± 0.54 | 0.657 | 0.004 * | 0.011 * |
| ECN | 0.70 ± 0.10 | 0.77 ± 0.11 | 0.71 ± 0.11 | 0.75 ± 0.09 | 0.864 | 0.031 * | 0.516 |
| EC | 0.92 ± 0.28 | 1.22 ± 0.19 | 1.06 ± 0.29 | 1.10 ± 0.22 | 0.887 | 0.003 * | 0.023 * |
| Simbolic Analisys | |||||||
| 0V% | 35.04 ± 20.10 | 18.42 ± 13.09 | 25.79 ± 19.49 | 26.37 ± 15.73 | 0.675 | 0.022 * | 0.051 * |
| 1V% | 41.30 ± 9.38 | 44.57 ± 6.15 | 44.48 ± 8.58 | 45.03 ± 6.29 | 0.222 | 0.200 | 0.551 |
| 2LV% | 7.81 ± 7.15 | 13.92 ± 6.51 | 14.18± 9.22 | 12.04 ± 7.92 | 0.146 | 0.193 | 0.026 * |
| 2UV% | 15.82 ± 8.89 | 23.05 ± 13.58 | 15.51 ± 8.81 | 16.53 ± 9.82 | 0.205 | 0.060 | 0.254 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, J.; Gentil, P.; Naves, J.P.; Souza Filho, L.F.; Silva, L.; Zamunér, A.R.; de Lira, C.A.; Rebelo, A. Effects of High Intensity Interval Training versus Sprint Interval Training on Cardiac Autonomic Modulation in Healthy Women. Int. J. Environ. Res. Public Health 2022, 19, 12863. https://doi.org/10.3390/ijerph191912863
Oliveira J, Gentil P, Naves JP, Souza Filho LF, Silva L, Zamunér AR, de Lira CA, Rebelo A. Effects of High Intensity Interval Training versus Sprint Interval Training on Cardiac Autonomic Modulation in Healthy Women. International Journal of Environmental Research and Public Health. 2022; 19(19):12863. https://doi.org/10.3390/ijerph191912863
Chicago/Turabian StyleOliveira, Jordana, Paulo Gentil, João Pedro Naves, Luiz Fernando Souza Filho, Lucas Silva, Antonio Roberto Zamunér, Claudio Andre de Lira, and Ana Rebelo. 2022. "Effects of High Intensity Interval Training versus Sprint Interval Training on Cardiac Autonomic Modulation in Healthy Women" International Journal of Environmental Research and Public Health 19, no. 19: 12863. https://doi.org/10.3390/ijerph191912863
APA StyleOliveira, J., Gentil, P., Naves, J. P., Souza Filho, L. F., Silva, L., Zamunér, A. R., de Lira, C. A., & Rebelo, A. (2022). Effects of High Intensity Interval Training versus Sprint Interval Training on Cardiac Autonomic Modulation in Healthy Women. International Journal of Environmental Research and Public Health, 19(19), 12863. https://doi.org/10.3390/ijerph191912863










