Using One-Step Acid Leaching for the Recovering of Coal Gasification Fine Slag as Functional Adsorbents: Preparation and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Acid Leaching Process Optimization
2.3. Characterization
2.4. Adsorption Mechanisms Investigation
2.4.1. Adsorption Process
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Isotherm
2.4.4. Adsorption Thermodynamics
2.5. Analysis of Data
3. Results and Discussion
3.1. Acid Leaching Process Optimization
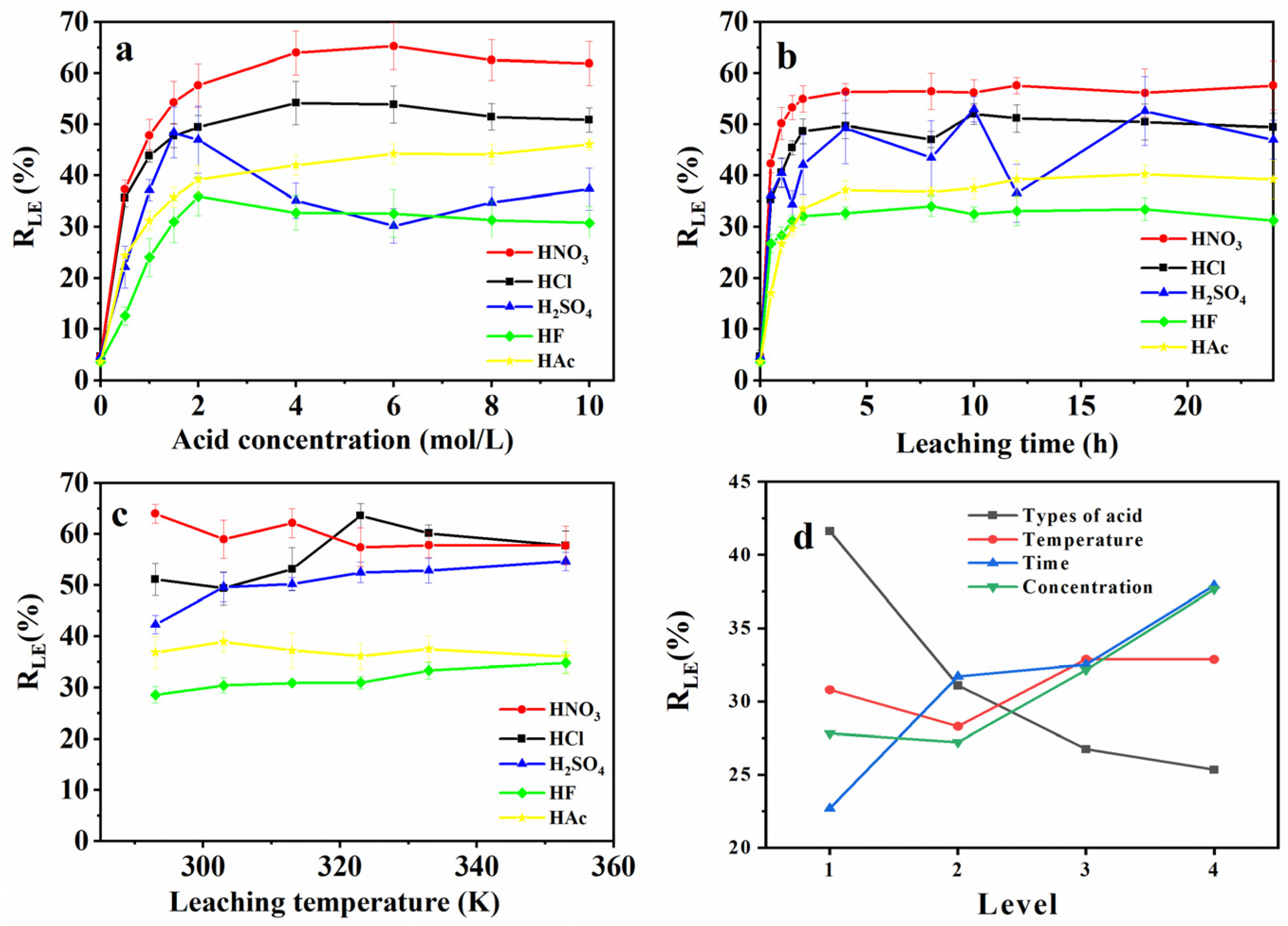
3.1.1. Acid Type Effect
3.1.2. Acid Concentration Effect
3.1.3. Leaching Time Effect
3.1.4. Leaching Temperature Effect
3.2. Optimization of Leaching Conditions
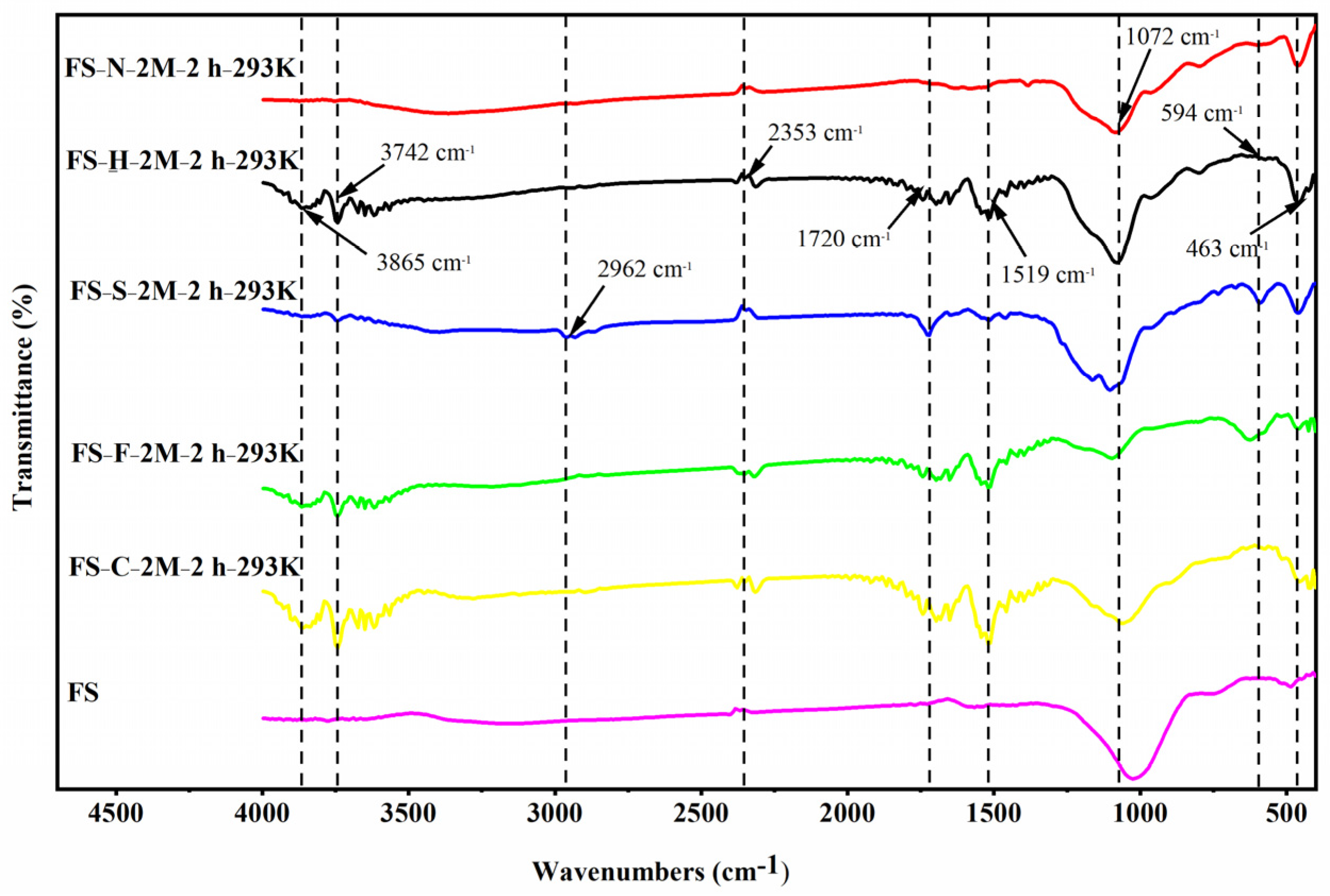
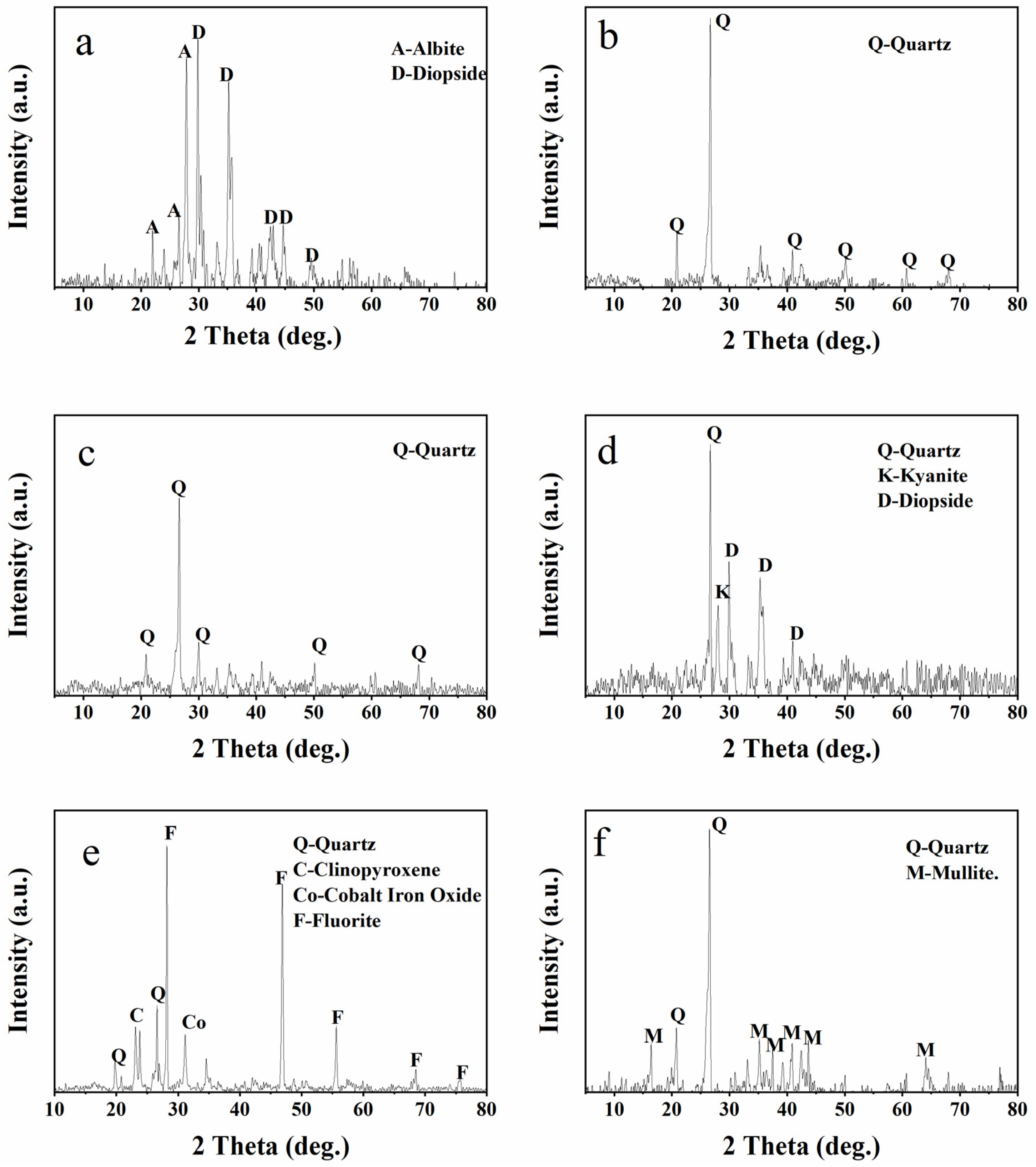
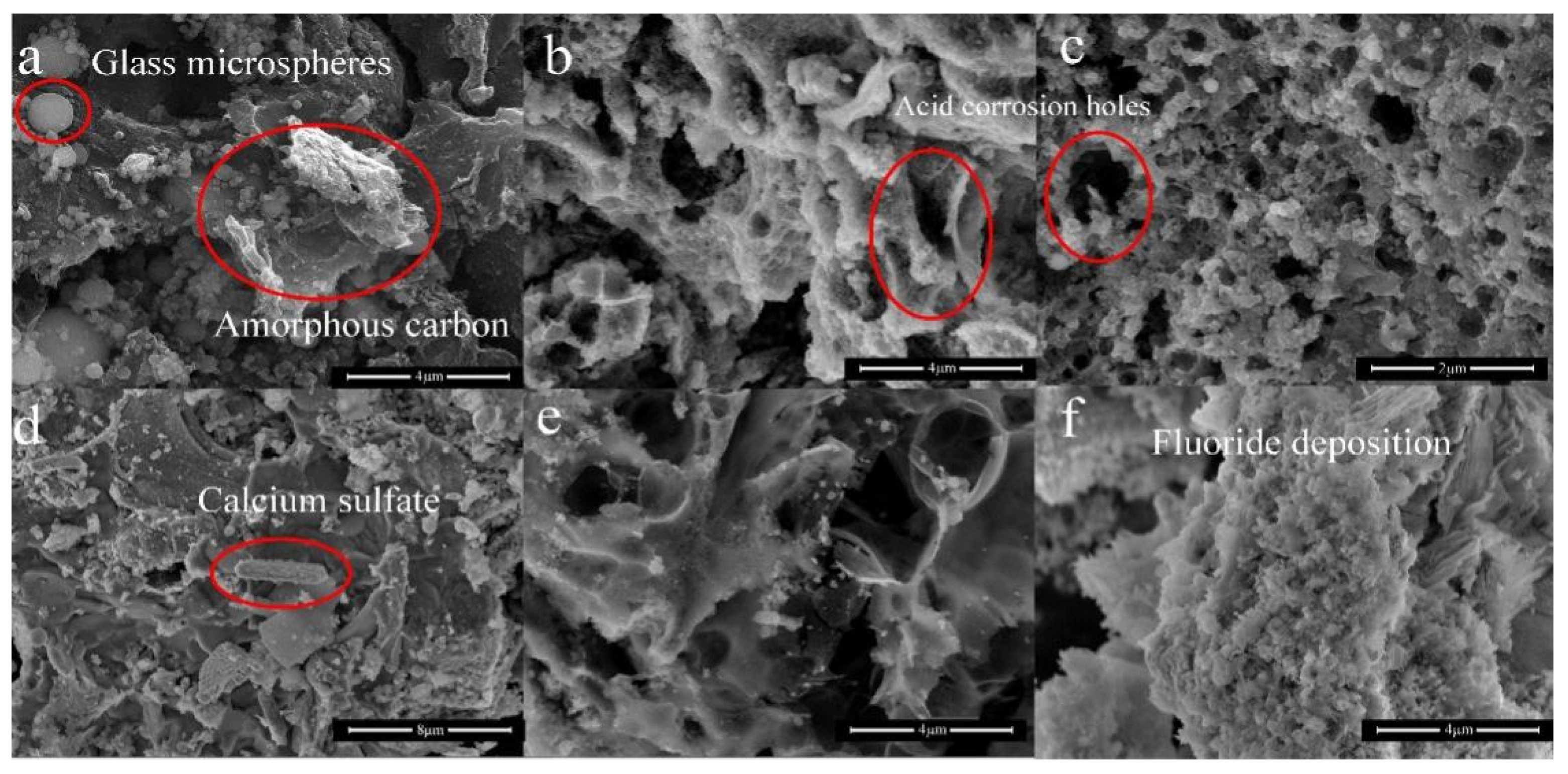
3.3. Characterization of Functional Adsorbents
3.4. Adsorption Mechanisms Investigation
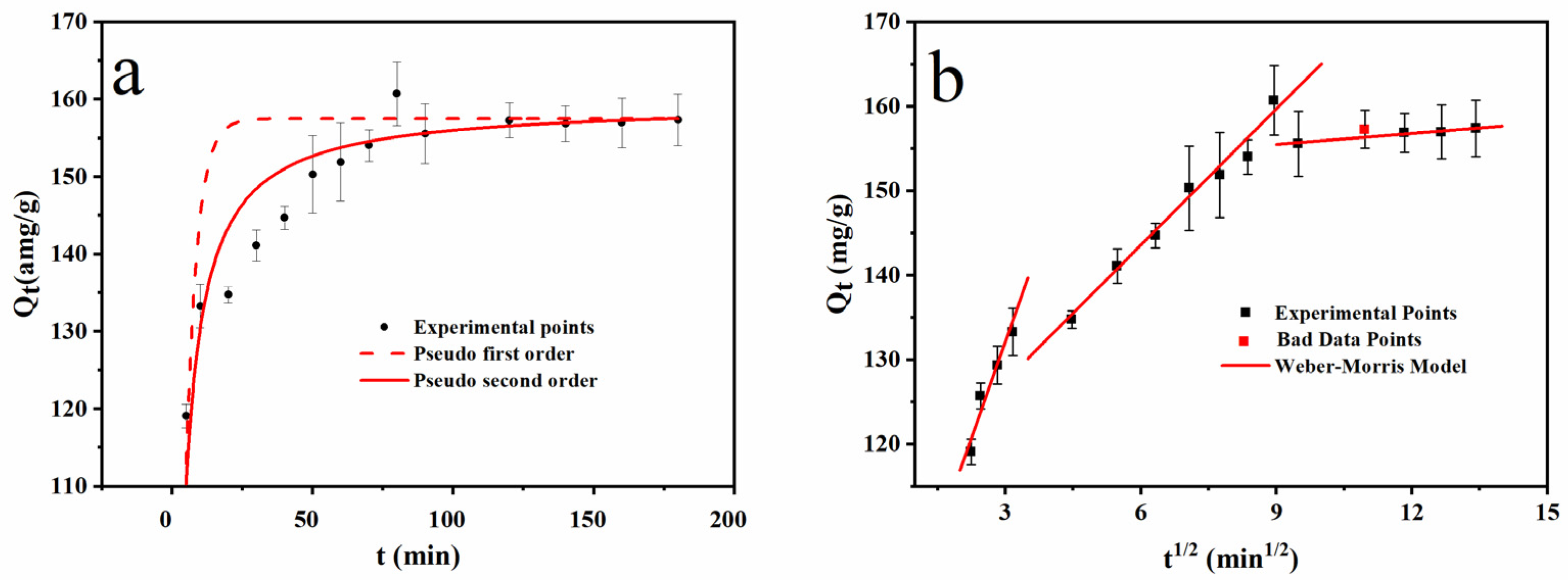
3.4.1. Adsorption Kinetics
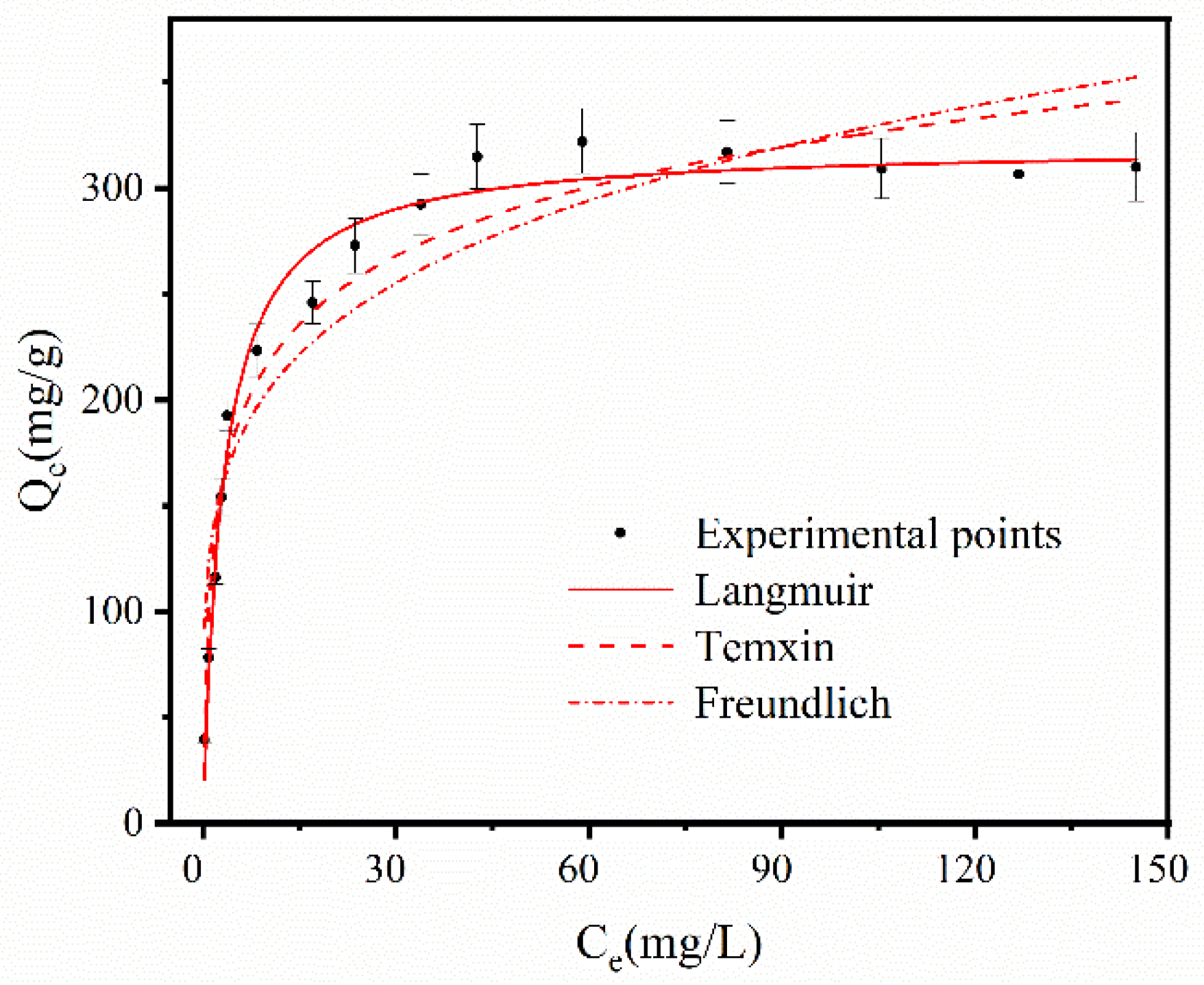
3.4.2. Adsorption Isotherm
3.4.3. Adsorption Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CGS | Coal gasification slag |
| FS | Coal gasification fine slag |
| CS | Coal gasification coarse slag |
| MB | Methylene blue |
| RLE | Leaching efficiency |
| CAC | Adsorption capacity |
| FS-N-2M-2 h-293K | The sample was obtained by 2.0 mol/L HNO3 at 293 K for 2 h. |
| FS-H-2M-2 h-293K | The sample was obtained by 2.0 mol/L HCl at 293 K for 2 h. |
| FS-S-2M-2 h-293K | The sample was obtained by 2.0 mol/L H2SO4 at 293 K for 2 h. |
| FS-F-2M-2 h-293K | The sample was obtained by 2.0 mol/L HF at 293 K for 2 h. |
| FS-C-2M-2 h-293K | The sample was obtained by 2.0 mol/L HAc at 293 K for 2 h. |
References
- Ding, L.; Dai, Z.H.; Wei, J.T.; Zhou, Z.J.; Yu, G.S. Catalytic effects of alkali carbonates on coal char gasification. J. Energy Inst. 2017, 90, 588–601. [Google Scholar] [CrossRef]
- Dai, G.F.; Zheng, S.J.; Wang, X.B.; Bai, Y.H.; Dong, Y.S.; Du, J.; Sun, X.W.; Tan, H.Z. Combustibility analysis of high-carbon fine slags from an entrained flow gasifier. J. Environ. Manag. 2020, 271, 9. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.D. High-value application of glass beads/porous carbon obtained from coal gasification fine slag as alternative for carbon black in natural rubber composite. J. Vinyl Addit. Technol. 2022, 28, 542–552. [Google Scholar] [CrossRef]
- Ai, W.D.; Liu, S.; Zhang, J.P.; Miao, S.D.; Wei, C.D. Mechanical and nonisothermal crystallization properties of coal gasification fine slag glass bead-filled polypropylene composites. J. Appl. Polym. Sci. 2019, 136, 47803. [Google Scholar] [CrossRef]
- Ai, W.D.; Xue, B.; Wei, C.D.; Dou, K.Z.; Miao, S.D. Mechanical and thermal properties of coal gasification fine slag reinforced low density polyethylene composites. J. Appl. Polym. Sci. 2018, 135, 46203. [Google Scholar] [CrossRef]
- Ai, W.D.; Zhang, J.P.; Zhang, J.Y.; Miao, S.D.; Wei, C.D. Mechanical properties and morphology of coal gasification fine slag glass bead-filled acrylonitrile-butadiene-styrene (ABS) composites. J. Appl. Polym. Sci. 2020, 137, 48601. [Google Scholar] [CrossRef]
- Zhu, D.D.; Miao, S.D.; Xue, B.; Jiang, Y.S.; Wei, C.D. Effect of Coal Gasification Fine Slag on the Physicochemical Properties of Soil. Water Air Soil Pollut. 2019, 230, 11. [Google Scholar] [CrossRef]
- Zhu, D.D.; Xue, B.; Jiang, Y.S.; Wei, C.D. Using chemical experiments and plant uptake to prove the feasibility and stability of coal gasification fine slag as silicon fertilizer. Environ. Sci. Pollut. Res. 2019, 26, 5925–5933. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Ren, X.N.; Liu, X.Y.; Zhang, Z.Q. Influence of fine coal gasification slag on greenhouse gases emission and volatile fatty acids during pig manure composting. Bioresour. Technol. 2020, 316, 9. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Jiao, M.N.; Awasthi, S.K.; Qin, S.Y.; Zhou, Y.W.; Liu, H.M.; Li, J.; Zhang, Z.Q. Changes of fungal diversity in fine coal gasification slag amendment pig manure composting. Bioresour. Technol. 2021, 325, 9. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, Y.Y.; Zhao, H.Y.; Chen, H.X.; He, R. Structure characteristics and composition of hydration products of coal gasification slag mixed cement and lime. Constr. Build. Mater. 2019, 213, 265–274. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, Q.H.; Chen, S.L.; Yuan, L.; Li, Y.W. Comparison of corrosion resistance of high chromia brick, high zirconia brick, fused AZS brick and Si3N4 bonded SiC brick against coal slag. Ceram. Int. 2020, 46, 10851–10860. [Google Scholar] [CrossRef]
- Xin, J.; Liu, L.; Jiang, Q.; Yang, P.; Qu, H.S.; Xie, G. Early-age hydration characteristics of modified coal gasification slag-cement-aeolian sand paste backfill. Constr. Build. Mater. 2022, 322, 125936. [Google Scholar] [CrossRef]
- Guo, F.H.; Guo, Y.; Guo, Z.K.; Miao, Z.K.; Zhao, X.; Zhang, Y.X.; Li, J.; Wu, J.J. Recycling Residual Carbon from Gasification Fine Slag and Its Application for Preparing Slurry Fuels. ACS Sustain. Chem. Eng. 2020, 8, 8830–8839. [Google Scholar] [CrossRef]
- Guo, F.H.; Zhao, X.; Guo, Y.; Zhang, Y.X.; Wu, J.J. Fractal analysis and pore structure of gasification fine slag and its flotation residual carbon. Colloid Surf. A-Physicochem. Eng. Asp. 2020, 585, 124148. [Google Scholar] [CrossRef]
- Guo, Q.H.; Huang, Y.C.; Gong, Y.; Zhuang, X.D.; Richter, A.; Yu, G.S. Recovered Carbon from Coal Gasification Fine Slag as Electrocatalyst for Oxygen Reduction Reaction and Zinc-Air Battery. Energy Technol. 2021, 9, 8. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, F.H.; Zhou, L.; Guo, Z.K.; Miao, Z.K.; Liu, H.; Zhang, X.X.; Wu, J.J.; Zhang, Y.X. Investigation on co-combustion of coal gasification fine slag residual carbon and sawdust char blends: Physiochemical properties, combustion characteristic and kinetic behavior. Fuel 2021, 292, 10. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.T.; Ai, W.D.; Wei, C.D. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.H.; Zhang, Y.X.; Wu, J.J. Water distribution and adsorption behaviors of two typical coal gasification fine slags from Ningxia Region. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 625, 7. [Google Scholar] [CrossRef]
- Ji, W.X.; Feng, N.; Zhao, P.D.; Zhang, S.Y.; Zhang, S.S.; Lan, L.P.; Huang, H.L.; Li, K.N.; Sun, Y.G.; Li, Y.Y.; et al. Synthesis of Single-Phase Zeolite A by Coal Gasification Fine Slag from Ningdong and Its Application as a High-Efficiency Adsorbent for Cu2+ and Pb2+ in Simulated Waste Water. Chem. Eng. 2020, 4, 65. [Google Scholar] [CrossRef]
- Ji, W.X.; Zhang, S.Y.; Zhao, P.D.; Zhang, S.S.; Feng, N.; Lan, L.P.; Zhang, X.G.; Sun, Y.G.; Li, Y.Y.; Ma, Y.L. Green Synthesis Method and Application of NaP Zeolite Prepared by Coal Gasification Coarse Slag from Ningdong, China. Appl. Sci. 2020, 10, 2694. [Google Scholar] [CrossRef]
- Li, C.C.; Qiao, X.C.; Yu, J.G. Large surface area MCM-41 prepared from acid leaching residue of coal gasification slag. Mater. Lett. 2016, 167, 246–249. [Google Scholar] [CrossRef]
- Wu, Y.H.; Ma, Y.L.; Sun, Y.G.; Xue, K.; Ma, Q.L.; Ma, T.; Ji, W.X. Graded synthesis of highly ordered MCM-41 and carbon/zeolite composite from coal gasification fine residue for crystal violet removal. J. Clean. Prod. 2020, 277, 16. [Google Scholar] [CrossRef]
- Liu, L.J.; Ji, W.X.; Li, K.N.; Yu, W.H.; Lan, L.P.; Ye, J.J.; Gao, X.; Li, Y.Y.; Ma, Y.L.; Sun, Y.G. Solid Phase ZSM-5 Synthesis from Coal Gasification Coarse Slag. Silicon 2022. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, R.M.; Qiu, G.F.; Jia, W.K.; Guo, Y.; Guo, F.H.; Wu, J.J. Synthesis of Porous Material from Coal Gasification Fine Slag Residual Carbon and Its Application in Removal of Methylene Blue. Molecules 2021, 26, 6116. [Google Scholar] [CrossRef]
- Duan, L.Y.; Hu, X.D.; Sun, D.S.; Liu, Y.Z.; Guo, Q.J.; Zhang, T.K.; Zhang, B.T. Rapid removal of low concentrations of mercury from wastewater using coal gasification slag. Korean J. Chem. Eng. 2020, 37, 1166–1173. [Google Scholar] [CrossRef]
- Zhang, J.P.; Liu, Y.; Zhang, J.Y.; Zuo, J.; Zhang, J.Y.; Qiu, F.G.; Wei, C.D.; Miao, S.D. Preparation of mesoporous coal gasification slag and applications in polypropylene resin reinforcement and deodorization. Powder Technol. 2021, 386, 437–448. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zuo, J.; Jiang, Y.S.; Zhu, D.D.; Zhang, J.P.; Wei, C.D. Kinetic analysis on the mesoporous formation of coal gasification slag by acid leaching and its thermal stability. Solid State Sci. 2020, 100, 11. [Google Scholar] [CrossRef]
- Gao, S.T.; Zhang, Y.C.; Li, H.X.; He, J.; Xu, H.; Wu, C.L. The microwave absorption properties of residual carbon from coal gasification fine slag. Fuel 2021, 290, 8. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zuo, J.; Ai, W.D.; Zhang, J.Y.; Zhu, D.D.; Miao, S.D.; Wei, C.D. Preparation of mesoporous coal-gasification fine slag adsorbent via amine modification and applications in CO2 capture. Appl. Surf. Sci. 2021, 537, 11. [Google Scholar] [CrossRef]
- Miao, Z.K.; Guo, Z.K.; Qiu, G.F.; Jia, W.K.; Jiao, K.R.; Zhang, Y.X.; Wu, J.J. Insight into the role of slag particles in coal gasification fine slag on hierarchical porous composites preparation and CO2 capture. Fuel 2022, 310, 9. [Google Scholar] [CrossRef]
- Abbas, N.; Rubab, N.; Sadiq, N.; Manzoor, S.; Khan, M.I.; Garcia, J.F.; Aragao, I.B.; Tariq, M.; Akhtar, Z.; Yasmin, G. Aluminum-Doped Cobalt Ferrite as an Efficient Photocatalyst for the Abatement of Methylene Blue. Water 2020, 12, 2285. [Google Scholar] [CrossRef]
- Alayli, A.; Nadaroglu, H.; Turgut, E. Nanobiocatalyst beds with Fenton process for removal of methylene blue. Appl. Water Sci. 2021, 11, 8. [Google Scholar] [CrossRef]
- Tenenhaus, A.; Tenenhaus, M. Regularized Generalized Canonical Correlation Analysis. Psychometrika 2011, 76, 257–284. [Google Scholar] [CrossRef] [Green Version]
- Holgado-Tello, F.P.; Chacon-Moscoso, S.; Barbero-Garcia, I.; Vila-Abad, E. Polychoric versus Pearson correlations in exploratory and confirmatory factor analysis of ordinal variables. Qual. Quant. 2010, 44, 153–166. [Google Scholar] [CrossRef]
- Dosmukhamedov, N.K.; Zholdasbay, E.E.; Daruesh, G.S.; Argyn, A.A.; Kurmanseitov, M.B. Study of the mechanism of pre-burned ash leaching by hydrochloric acid. Kompleks Ispol. Miner. Syra 2021, 4, 72–80. [Google Scholar] [CrossRef]
- Schuster, J.; Ebin, B. Investigation of indium and other valuable metals leaching from unground waste LCD screens by organic and inorganic acid leaching. Sep. Purif. Technol. 2021, 279, 11. [Google Scholar] [CrossRef]
- Porada, S.; Rozwadowski, A.; Zubek, K. Studies of catalytic coal gasification with steam. Pol. J. Chem. Technol. 2016, 18, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Bilgin, O.; Hacifazlioglu, H.; Sert, D. Leaching of coal by trioxoboric acid for coal cleaning. Physicochem. Probl. Mineral Pro. 2021, 57, 129–136. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Sedajova, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds. C-J. Carbon Res. 2020, 6, 61. [Google Scholar] [CrossRef]
- Ettler, V.; Vrtiskova, R.; Mihaljevic, M.; Sebek, O.; Grygar, T.; Drahota, P. Cadmium, lead and zinc leaching from smelter fly ash in simple organic acids-Simulators of rhizospheric soil solutions. J. Hazard. Mater. 2009, 170, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.X.; Cheng, G.J.; Xue, X.X.; Yang, H. Kinetics and mechanism of hydrochloric acid leaching of rare earths from Bayan Obo slag and recovery of rare earth oxalate and high purity oxides. Hydrometallurgy 2022, 208, 16. [Google Scholar] [CrossRef]
- Du, M.J.; Huang, J.J.; Liu, Z.Y.; Zhou, X.; Guo, S.; Wang, Z.Q.; Fang, Y.T. Reaction characteristics and evolution of constituents and structure of a gasification slag during acid treatment. Fuel 2018, 224, 178–185. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Minisy, I.M.; Salahuddin, N.A.; Ayad, M.M. Adsorption of methylene blue onto chitosan? montmorillonite/polyaniline nanocomposite. Appl. Clay Sci. 2021, 203, 10. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Evrendilek, G.A.; Zerrouq, F.; Lairini, S. Recent Advances in Adsorption Kinetic Models: Their Application to Dye Types. Arab. J. Chem. 2021, 14, 24. [Google Scholar] [CrossRef]
- Lombardo, S.; Thielemans, W. Thermodynamics of adsorption on nanocellulose surfaces. Cellulose 2019, 26, 249–279. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 22. [Google Scholar] [CrossRef]
- Tong, D.S.; Wu, C.W.; Adebajo, M.O.; Jin, G.C.; Yu, W.H.; Ji, S.F.; Zhou, C.H. Adsorption of methylene blue from aqueous solution onto porous cellulose-derived carbon/montmorillonite nanocomposites. Appl. Clay Sci. 2018, 161, 256–264. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.P.; Zhou, S.Q. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Zhao, D.L.; Ding, Y.; Chen, S.H.; Bai, T.T.; Ma, Y.L. Adsorption of Methylene Blue on Carbon Nanotubes from Aqueous Solutions. Asian J. Chem. 2013, 25, 5756–5758. [Google Scholar] [CrossRef]
- Otcovska, T.P.; Muzikova, B.; Padevet, P. Influence of earth composition on adsorption capacity of methylene blue dye. In Proceedings of the 11th Conference on Nano and Macro Mechanics (NMM), Prague, Czech Republic, 17 September 2020; pp. 63–68. [Google Scholar]
- De Lima, A.F.; Fagnani, H.M.C.; Santos, W.L.F.; de Barros, M. Methylene blue adsorption in hydrocarbons textile waste. Materia 2020, 25, 16. [Google Scholar] [CrossRef]
- Truong, T.T.C.; Vo, N.T.T.; Nguyen, K.D.; Bui, H.M. Preparation of cellulose-based hydrogel derived from tea residue for the adsorption of methylene blue. Cell Chem. Technol. 2019, 53, 573–582. [Google Scholar] [CrossRef]
- Song, X.S.; Zhou, J.; Fan, J.S.; Zhang, Q.Q.; Wang, S.F. Preparation and adsorption properties of magnetic graphene oxide composites for the removal of methylene blue from water. Mater. Res. Express 2022, 9, 15. [Google Scholar] [CrossRef]


| Si | Al | Fe | Ca | Na | Mg | K | S | Ti | Others | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FS | 21.20 | 11.15 | 13.90 | 13.44 | 1.39 | 1.22 | 1.06 | 0.82 | 0.70 | 1.47 | 33.68 |
| Pore Diameter (nm) | Total Pore Volume (cc/g) | Surface Area (m2/g) | |
|---|---|---|---|
| FS | 19.09 | 0.21 | 151.67 |
| FS-N-2M-2 h-293K | 3.82 | 0.26 | 340.40 |
| FS-H-2M-2 h-293K | 3.83 | 0.48 | 247.21 |
| FS-S-2M-2 h-293K | 3.82 | 0.15 | 162.09 |
| FS-C-2M-2 h-293K | 3.83 | 0.26 | 159.01 |
| FS-F-2M-2 h-293K | 3.83 | 0.12 | 98.83 |
| Kinetics Model | Parameters | ||||
|---|---|---|---|---|---|
| Pseudo-first-order | Qe | K1 | R2 | χ2 | |
| 156.10 | 0.26784 | 0.523 | 46.665 | ||
| Pseudo-second-order | Qe | K2 | R2 | χ2 | |
| 159.47 | 0.00282 | 0.875 | 12.193 | ||
| Weber-Morris model | Part I | Ci | Ki1 | R2 | χ2 |
| 86.57022 | 15.18055 | 0.90365 | 2.65117 | ||
| Part II | c | Ki2 | R2 | χ2 | |
| 111.009 | 5.381 | 0.979 | 9.32794 | ||
| Part III | c | Ki3 | R2 | χ2 | |
| 151.328 | 0.453 | 0.975 | 0.0452 | ||
|
Temperature (K) | Langmuir Isotherm Model | ||||
|---|---|---|---|---|---|
| KL | Qmax | R2 | ALL | Equation | |
| 293 | 0.321 | 320.134 | 0.982 | 0.135 | Qe = 102.808Ce/(1 + 0.321Ce) |
| 303 | 0.244 | 308.616 | 0.916 | 0.170 | Qe = 75.173Ce/(1 + 0.244Ce) |
| 313 | 0.167 | 287.880 | 0.955 | 0.230 | Qe = 48.145Ce/(1 + 0.167Ce) |
| 323 | 0.146 | 273.099 | 0.939 | 0.256 | Qe = 39.782Ce/(1 + 0.146Ce) |
| Temperature (K) | Freundlich Isotherm Model | ||||
| KF | n | R2 | Equation | ||
| 293 | 127.085 | 4.881 | 0.879 | Qe = 127.085Ce0.205 | |
| 303 | 107.450 | 4.332 | 0.838 | Qe = 107.45Ce0.231 | |
| 313 | 100.253 | 4.654 | 0.757 | Qe = 100.253Ce0.215 | |
| 323 | 92.136 | 4.591 | 0.748 | Qe = 92.136Ce0.218 | |
| Temperature (K) | Temkin Isotherm Model | ||||
| KT | b | R2 | Equation | ||
| 293 | 52.087 | 10.268 | 0.952 | Qe = 237.24ln (52.087Ce) | |
| 303 | 47.291 | 4.179 | 0.901 | Qe = 781.843ln (47.291Ce) | |
| 313 | 50.494 | 3.025 | 0.854 | Qe = 860.313ln (50.494Ce) | |
| 323 | 52.595 | 2.567 | 0.839 | Qe = 1046.271ln (52.595Ce) | |
| ΔG(kJ/mol) | ΔS | ΔH | R2 | |||
|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 323 K | (J/(mol·K)) | (kJ/mol) | |
| −4.21 | −3.936 | −3.905 | −3.825 | −82.398 | −37.526 | 0.9366 |
| Adsorbent | Qm (mg/g) | Equilibrum Time (min) | pH | T (K) | References |
|---|---|---|---|---|---|
| Carbon nanotubes | 65.36 | 120 | 9.4 | 293 | [51] |
| Clay | 88 | - | - | Room temperature | [52] |
| hydrocarbon textile waste | 72 | 90 | 10 | Room temperature | [53] |
| tea cellulose hydrogel | 41.67 | 300 | 10 | 298 | [54] |
| magnetic graphene oxide | 205.34 | 120 | 10 | 318 | [55] |
| FS-N-2M-2 h-293K | 162.94 | 120 | 9 | 293 | This paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; He, S.; Shen, T.; Sun, J.; Sun, C.; Pan, H.; Yu, D.; Lu, W.; Li, R.; Zhang, E.; et al. Using One-Step Acid Leaching for the Recovering of Coal Gasification Fine Slag as Functional Adsorbents: Preparation and Performance. Int. J. Environ. Res. Public Health 2022, 19, 12851. https://doi.org/10.3390/ijerph191912851
Li T, He S, Shen T, Sun J, Sun C, Pan H, Yu D, Lu W, Li R, Zhang E, et al. Using One-Step Acid Leaching for the Recovering of Coal Gasification Fine Slag as Functional Adsorbents: Preparation and Performance. International Journal of Environmental Research and Public Health. 2022; 19(19):12851. https://doi.org/10.3390/ijerph191912851
Chicago/Turabian StyleLi, Tianpeng, Shaocang He, Tingting Shen, Jing Sun, Chenxu Sun, Haoqi Pan, Dehai Yu, Wenxue Lu, Runyao Li, Enshan Zhang, and et al. 2022. "Using One-Step Acid Leaching for the Recovering of Coal Gasification Fine Slag as Functional Adsorbents: Preparation and Performance" International Journal of Environmental Research and Public Health 19, no. 19: 12851. https://doi.org/10.3390/ijerph191912851
APA StyleLi, T., He, S., Shen, T., Sun, J., Sun, C., Pan, H., Yu, D., Lu, W., Li, R., Zhang, E., Lu, X., Fan, Y., & Gao, G. (2022). Using One-Step Acid Leaching for the Recovering of Coal Gasification Fine Slag as Functional Adsorbents: Preparation and Performance. International Journal of Environmental Research and Public Health, 19(19), 12851. https://doi.org/10.3390/ijerph191912851






