Activation of Bisulfite with Pyrophosphate-Complexed Mn(III) for Fast Oxidation of Organic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
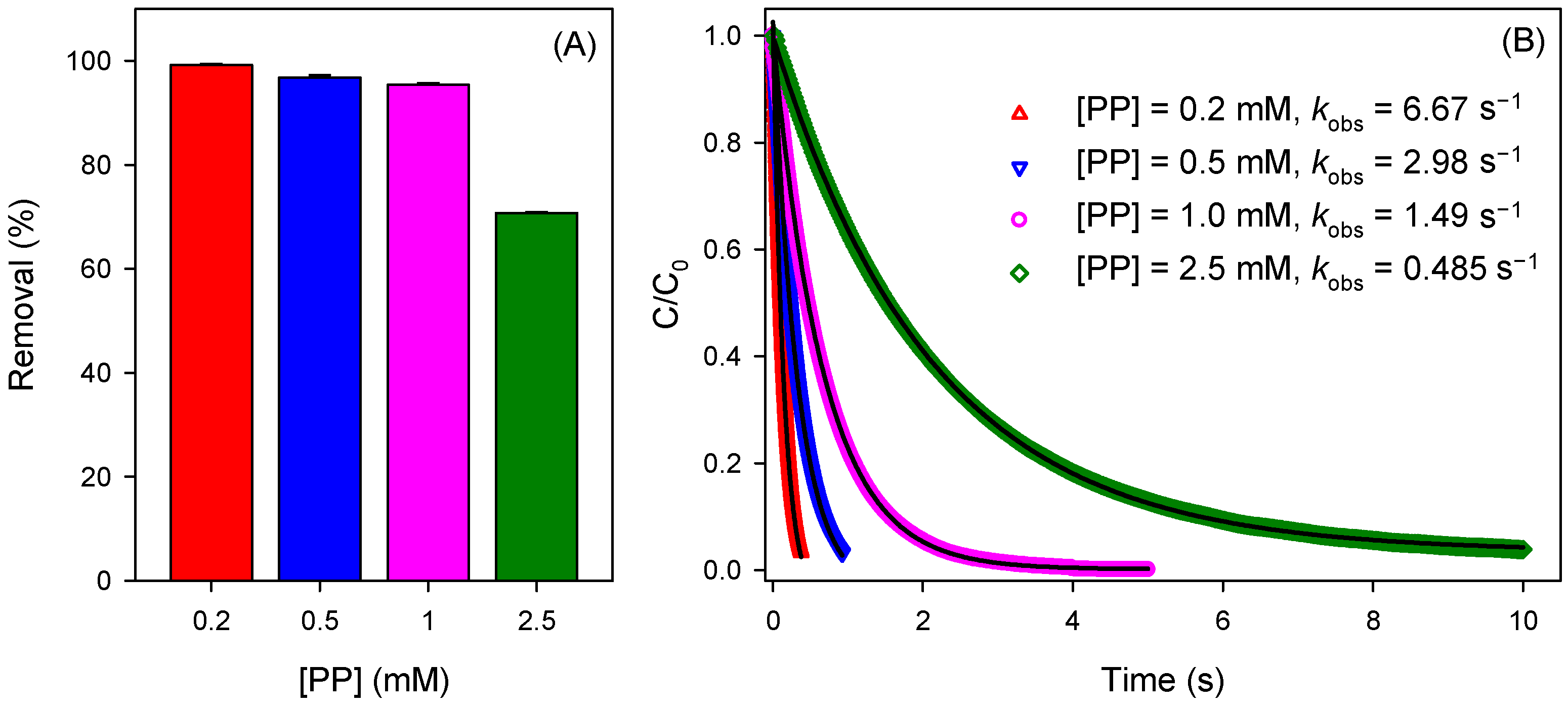
3.1. Pollutant Removal by the Mn(III)-Activated HSO3− Process
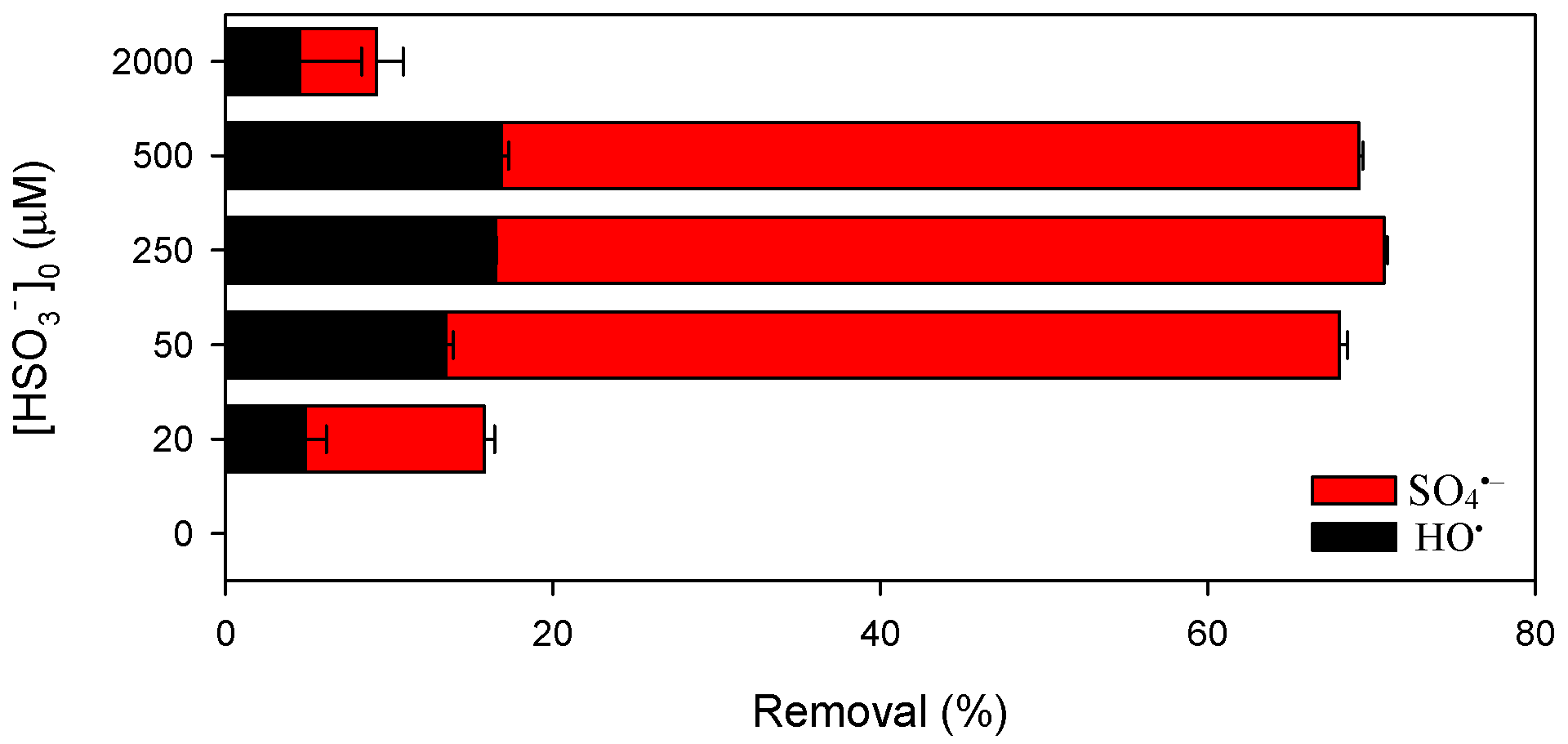
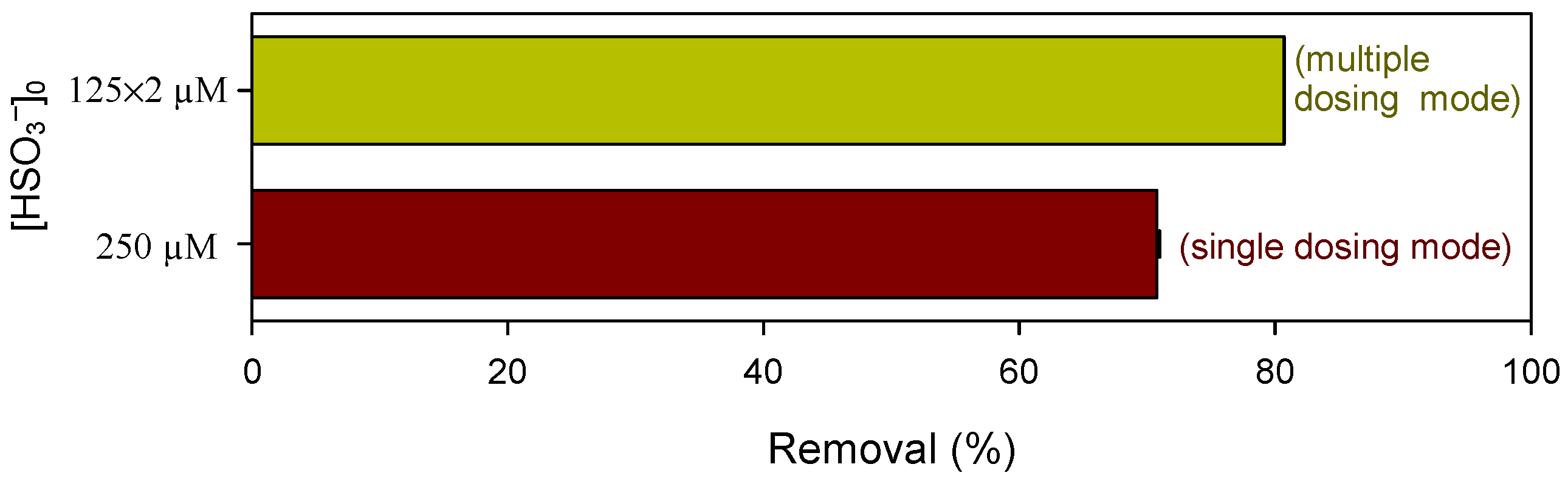
3.2. Influence of Pyrophosphate:Mn(III) Ratio of on Pollutant Abatement by Mn(III)/HSO3− Process
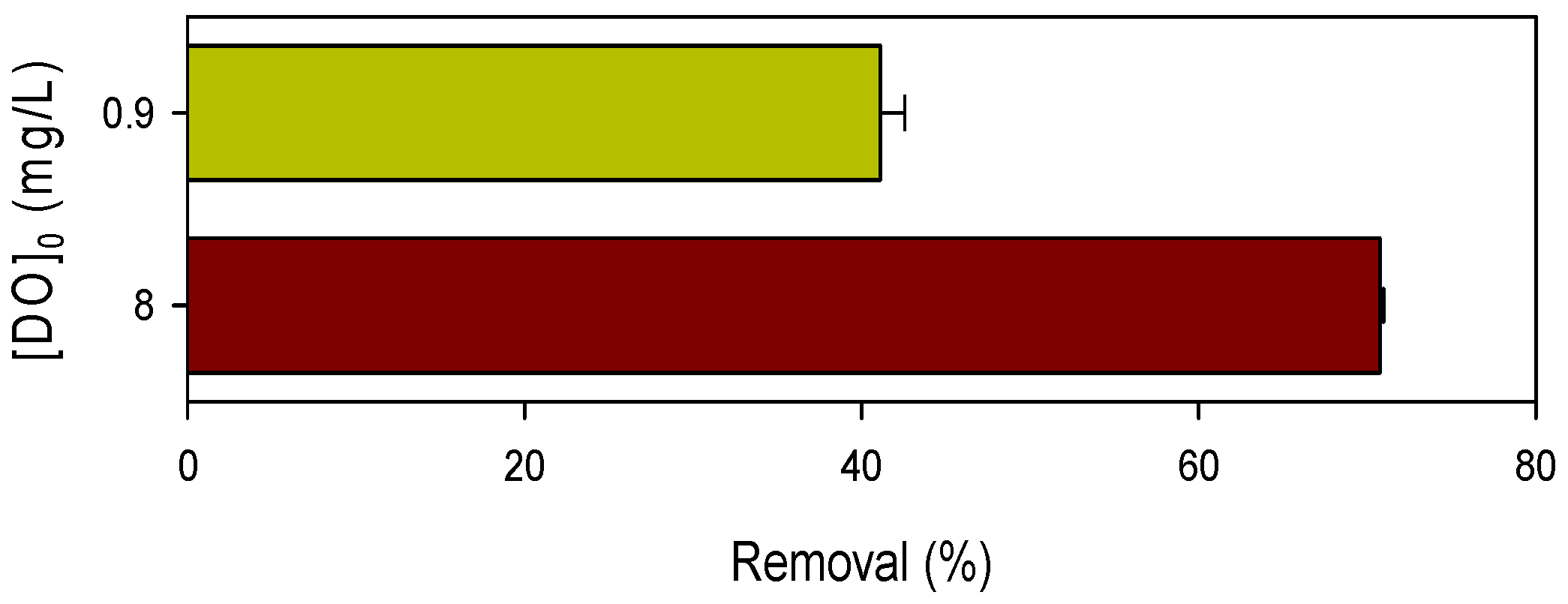
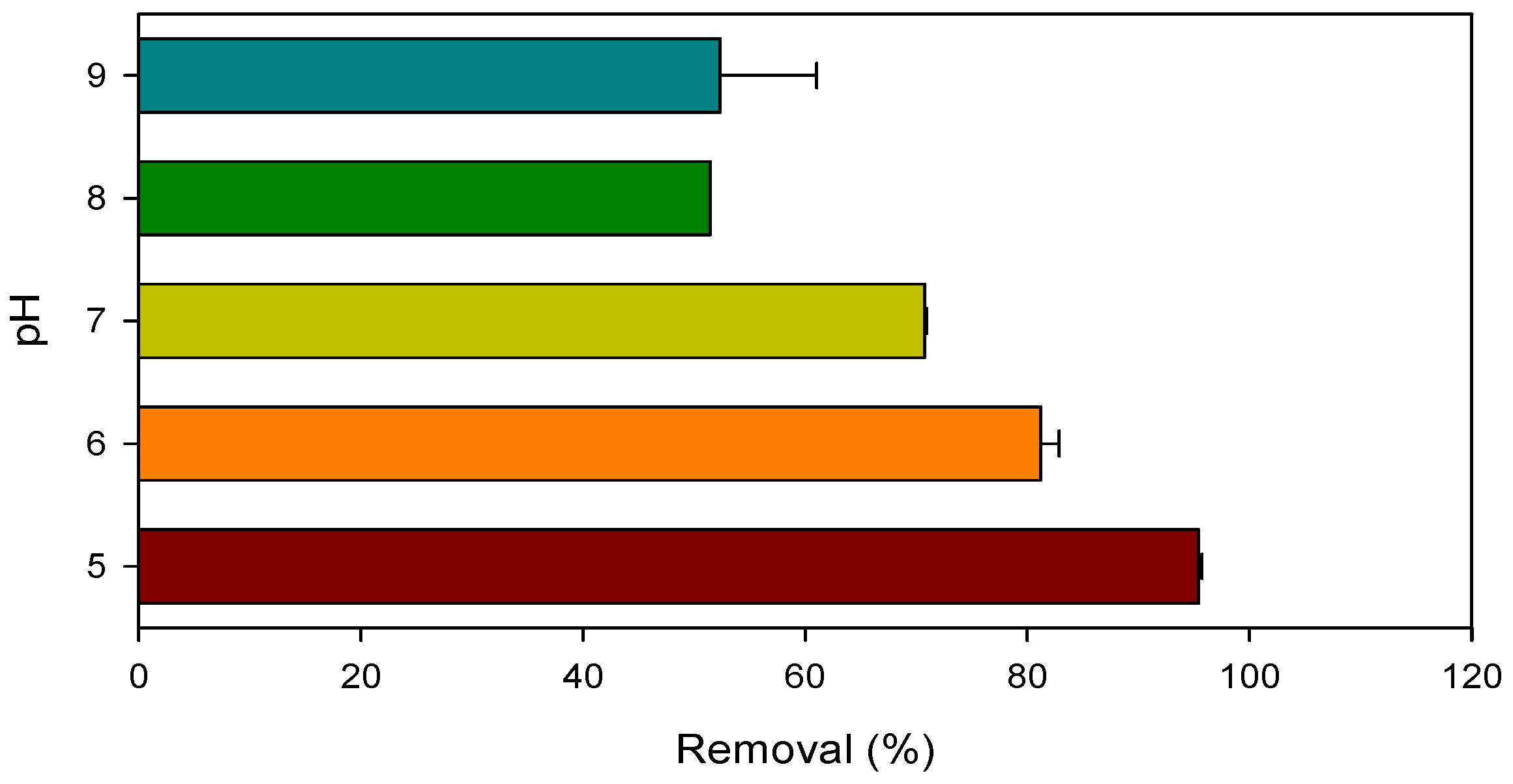
3.3. Influence of Dissolved Oxygen and pH on Pollutant Degradation by Mn(III)/HSO3− Process
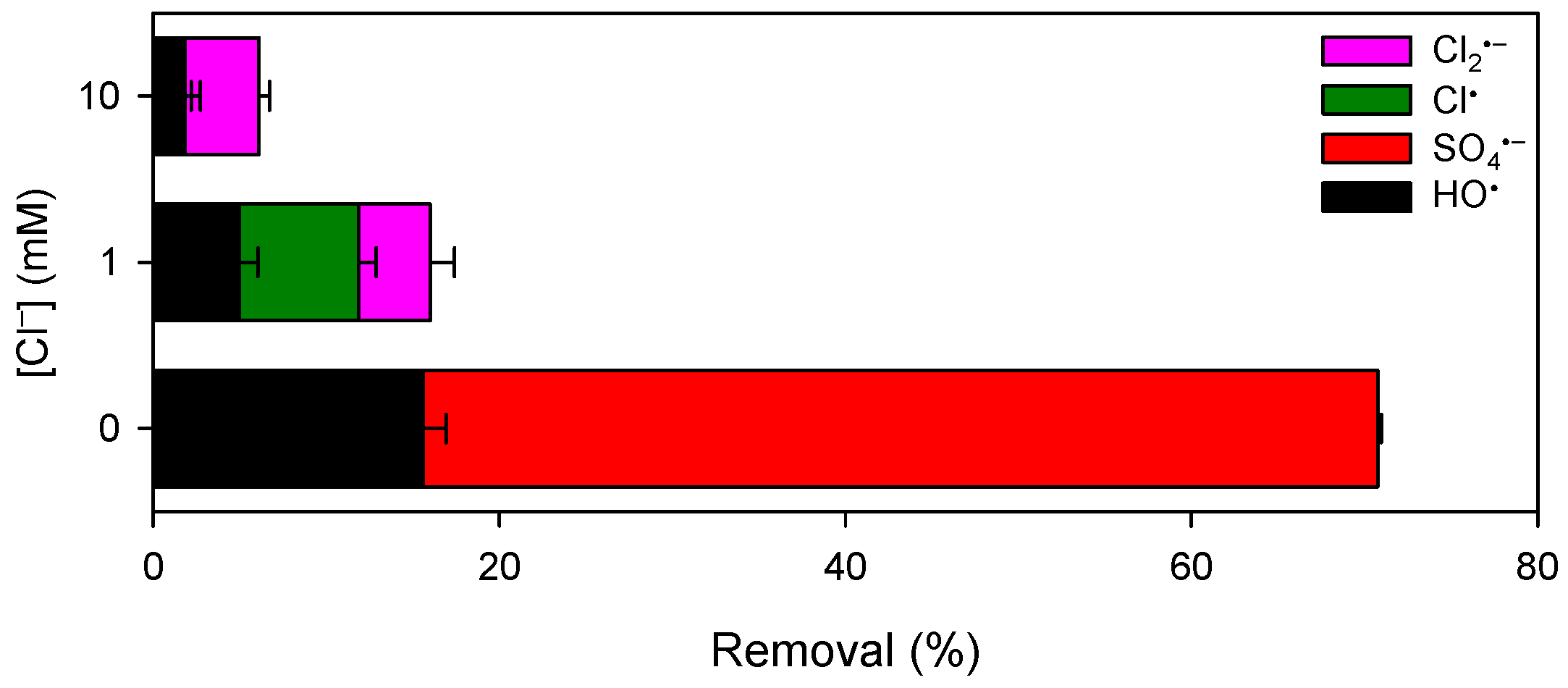
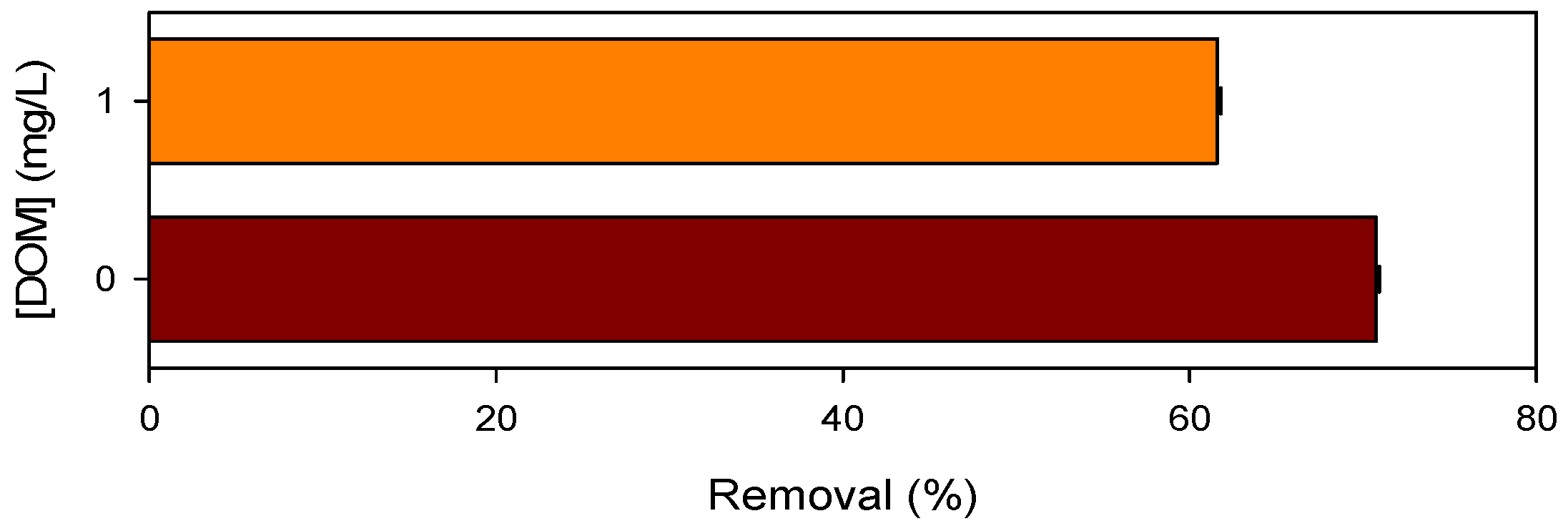
3.4. Influence of Chloride and DOM on Pollutant Degradation by Mn(III)/HSO3−
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkateswarlu, K. Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett. 2021, 19, 3887–3950. [Google Scholar] [CrossRef]
- Vallinayagam, S.; Rajendran, K.; Lakkaboyana, S.K.; Soontarapa, K.; Remya, R.R.; Sharma, V.K.; Kumar, V.; Venkateswarlu, K.; Koduru, J.R. Recent developments in magnetic nanoparticles and nano-composites for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106553. [Google Scholar] [CrossRef]
- Lakshmidevi, J.; Naidu, B.R.; Avula, S.K.; Majhi, A.; Chia, P.W.; Al-Harrasi, A.; Venkateswarlu, K. A waste valorization strategy for the synthesis of phenols from (hetero)arylboronic acids using pomegranate peel ash extract. Green Chem. Lett. Rev. 2022, 15, 426–435. [Google Scholar] [CrossRef]
- Appa, R.M.; Naidu, B.R.; Venkateswarlu, D.; Hanafiah, M.M.; Lakkaboyana, S.K.; Lakshmidevi, J.; Venkateswarlu, K. Water extract of pomegranate ash-I-2 as sustainable system for external oxidant/metal/catalyst-free oxidative iodination of (hetero)arenes. Green Chem. Lett. Rev. 2021, 14, 710–722. [Google Scholar] [CrossRef]
- Lakshmidevi, J.; Naidu, B.R.; Reddy, S.; Venkateswarlu, K. Oxidative iododeborylation reaction of (hetero)arylboronic acids in water extract of pomegranate ash: A novel and sustainable synthesis of iodo(hetero)arenes. Waste Biomass Valorization 2002, 13, 2207–2216. [Google Scholar] [CrossRef]
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total Environ. 2008, 397, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Leung, K.; Snyder, S.A.; Alvarez, P. Which Micropollutants in Water Environments Deserve More Attention Globally? Environ. Sci. Technol. 2022, 56, 13–29. [Google Scholar] [CrossRef]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Lee, J.; Gunten, U.V.; Kim, J. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Qiao, L.; Shi, Y.; Cheng, Q.; Liu, B.; Liu, J. The removal efficiencies and mechanism of aniline degradation by peroxydisulfate activated with magnetic Fe-Mn oxides composite. J. Water Reuse Desalination 2021, 11, 212–223. [Google Scholar] [CrossRef]
- Schnabel, T.; Jautzus, N.; Mehling, S.; Springer, C.; Londong, J. Photocatalytic degradation of hydrocarbons and methylene blue using floatable titanium dioxide catalysts in contaminated water. J. Water Reuse Desalination 2021, 11, 224–235. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B-Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]
- He, X.; La Cruz, A.A.D.; Dionysiou, D.D. Destruction of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals and sulfate radicals using UV-254 nm activation of hydrogen peroxide, persulfate and peroxymonosulfate. J. Photochem. Photobiol. A 2013, 251, 160–166. [Google Scholar] [CrossRef]
- Ling, S.K.; Wang, S.; Peng, Y. Oxidative degradation of dyes in water using Co2+/H2O2 and Co2+/peroxymonosulfate. J. Hazard. Mater. 2010, 178, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Y.; Wang, W.H.; Song, L.; Lu, L.Q.; Wang, Z.Y.; Jiang, X.F.; Dong, C.N.; Qiu, R.Y. Acceleration comparison between Fe2+/H2O2 and Co2+/oxone for decolouration of azo dyes in homogeneous systems. Chem. Eng. J. 2013, 234, 475–483. [Google Scholar]
- Ahn, Y.Y.; Bae, H.; Kim, H.I.; Kim, S.H.; Kim, J.H.; Lee, S.G.; Lee, J. Surface-loaded metal nanoparticles for peroxymonosulfate activation: Efficiency and mechanism reconnaissance. Appl. Catal. B-Environ. 2019, 241, 561–569. [Google Scholar] [CrossRef]
- Duan, X.G.; Ao, Z.M.; Zhang, H.Y.; Saunders, M.; Sun, H.Q. Nanodiamonds in sp2/sp3 configuration for radical to nonradical oxidation: Core-shell layer dependence. Appl. Catal. B-Environ. 2018, 222, 176–181. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2016, 310, 307–315. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.J.; Orbell, D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Zhu, S.S.; Li, X.J.; Kang, J.; Duan, X.G.; Wang, S.B. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.Y.; Li, J.; Lu, X.T.; Yuan, L.P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941. [Google Scholar] [CrossRef]
- Yin, H.X.; Li, J.; Yan, H.D.; Cai, H.Y.; Wan, Y.J.; Yao, G.; Guo, Y.; Lai, B. Activation of peroxymonosulfate by CuCo2O4 nano-particles towards long-lasting removal of atrazine. J. Water Reuse Desalination 2021, 11, 542–559. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xiao, M. Enhanced Decolorization of Orange II Solutions by the Fe(ll)-Sulfite System under Xenon Lamp Irradiation. Ind. Eng. Chem. Res. 2013, 52, 10089–10094. [Google Scholar] [CrossRef]
- Long, C.; Peng, X.; Liu, J.; Li, J.; Feng, W. Decolorization of Orange II in Aqueous Solution by an Fe(II)/sulfite System: Replacement of Persulfate. Ind. Eng. Chem. Res. 2012, 51, 13632–13638. [Google Scholar]
- Sun, B.; Guan, X.; Fang, J.; Tratnyek, P.G. Activation of Manganese Oxidants with Bisulfite for Enhanced Oxidation of Organic Contaminants: The Involvement of Mn(III). Environ. Sci. Technol. 2015, 49, 12414–12421. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xiao, Z.; Dong, H.; Ma, S.; Wei, G.; Cao, T.; Guan, X. Bisulfite triggers fast oxidation of organic pollutants by colloidal MnO2. J. Hazard. Mater. 2019, 363, 412–420. [Google Scholar] [CrossRef]
- Sun, S.; Pang, S.Y.; Jiang, J.; Ma, J.; Huang, Z.; Zhang, J.; Liu, Y.; Xu, C.; Liu, Q.; Yuan, Y. The combination of ferrate(VI) and sulfite as a novel advanced oxidation process for enhanced degradation of organic contaminants. Chem. Eng. J. 2017, 333, 11–19. [Google Scholar] [CrossRef]
- Wang, J.W.; Teng, Y.G.; Zhang, C.X.; Liao, X.P.; Zhai, Y.Z.; Zuo, R. Activation of manganese dioxide with bisulfite for enhanced abiotic degradation of typical organophosphorus pesticides: Kinetics and transformation pathway. Chemosphere 2019, 226, 858–864. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Wu, F.; Mailhot, G.; Zhou, D.N.; Hanna, K. Rapid catalytic oxidation of arsenite to arsenate in an iron(III)/sulfite system under visible light. Appl. Catal. B-Environ. 2016, 186, 56–61. [Google Scholar] [CrossRef]
- Yuan, Y.N.; Yang, S.J.; Zhou, D.N.; Wu, F. A simple Cr(VI)-S(IV)-O-2 system for rapid and simultaneous reduction of Cr(VI) and oxidative degradation of organic pollutants. J. Hazard. Mater. 2016, 307, 294–301. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Zhang, C.B.; Yu, Y.T.; Zhang, L.; Wu, F. A novel photochemical system of ferrous sulfite complex: Kinetics and mechanisms of rapid decolorization of Acid Orange 7 in aqueous solutions. Water Res. 2014, 57, 87–95. [Google Scholar] [CrossRef]
- Jia, Y.; Xi, B.; Jiang, Y.; Guo, H.; Yang, Y.; Lian, X.; Han, S. Distribution, formation and human-induced evolution of geogenic contaminated groundwater in China: A review. Sci. Total Environ. 2018, 643, 967–993. [Google Scholar] [CrossRef]
- Cui, H.; Liu, X.; Tan, W.; Feng, X.; Liu, F.; Huada, D.R. Influence of Mn(III) Availability on the Phase Transformation From Layered Buserite to Tunnel-structured Todorokite. Clays Clay Miner. 2008, 56, 397–403. [Google Scholar] [CrossRef]
- Ukrainczyk, L.; McBride, M.B. Oxidation of phenol in acidic aqueous suspensions of manganese oxides. Clays Clay Miner. 1992, 40, 157–166. [Google Scholar] [CrossRef]
- Remucal, C.K. A critical review of the reactivity of manganese oxides with organic contaminants. Environ. Sci.-Proc. Impacts 2014, 16, 1247–1266. [Google Scholar] [CrossRef]
- Trouwborst, R.E.; Clement, B.G.; Tebo, B.M.; Glazer, B.T.; Rd, L.G. Soluble Mn(III) in suboxic zones. Science 2006, 313, 1955–1957. [Google Scholar] [CrossRef] [Green Version]
- Madison, A.S.; Tebo, B.M.; Alfonso, M.S.; Bjørn, S.; Luther, G.W. Abundant porewater Mn(III) is a major component of the sedimentary redox system. Science 2013, 341, 875–878. [Google Scholar] [CrossRef]
- Johnson, K.L.; Mccann, C.M.; Wilkinson, J.L.; Jones, M.; Tebo, B.M.; West, M.; Elgy, C.; Clarke, C.E.; Gowdy, C.; Hudson-Edwards, K.A. Dissolved Mn(III) in water treatment works: Prevalence and significance. Water Res. 2018, 140, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, R.E.; Ward, M.H.; Hinze, W. Spectrophotometric determination of sulfite with 4,4′-dithio-dipyridine and 5,5′-dithiobis (2-nitrobenzoic acid). Anal. Chem. 1970, 42, 698–702. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Chen, J.; Rao, D.; Dong, H.; Sun, B.; Shao, B.; Cao, G.; Guan, X. The role of active manganese species and free radicals in permanganate/bisulfite process. J. Hazard. Mater. 2020, 388, 121735. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wei, G.; Yin, D.; Guan, X. Mechanistic insight into the generation of reactive oxygen species in sulfite activation with Fe(III) for contaminants degradation. J. Hazard. Mater. 2019, 384, 121497. [Google Scholar] [CrossRef] [PubMed]
- Klewicki, J.K.; Morgan, J.J. Kinetic behavior of Mn(III) complexes of pyrophosphate, EDTA, and citrate. Environ. Sci. Technol. 1998, 32, 2916–2922. [Google Scholar] [CrossRef]
- Jiang, J.; Pang, S.Y.; Ma, J. Role of ligands in permanganate oxidation of organics. Environ. Sci. Technol. 2010, 44, 4270–4275. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, B.; Qiao, J.; Guan, X. Influence of pyrophosphate on the generation of soluble mn(iii) from reactions involving Mn oxides and Mn(VII). Environ. Sci. Technol. 2019, 53, 10227–10235. [Google Scholar] [CrossRef]
- Sun, B.; Dong, H.; He, D.; Rao, D.; Guan, X. Modeling the kinetics of contaminants oxidation and the generation of Manganese(III) in the permanganate/bisulfite process. Environ. Sci. Technol. 2016, 50, 1473–1482. [Google Scholar] [CrossRef]
- Sun, B.; Zheng, Y.Z.; Shang, C.; Yin, R. Concentration-dependent chloride effect on radical distribution and micropollutant degradation in the sulfate radical-based AOPs. J. Hazard. Mater. 2022, 430, 128450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, H.; Sun, B.; Zhang, J.; Wang, W. The altered effects of chloride on the treatment efficiency of SO4−-based AOPs by other background water constituents. Chem. Eng. J. 2022, 441, 135914. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Sun, J.; Meng, X.; Luo, J.; Zhou, D.; Crittenden, J.C. Impact of chloride ions on UV/H2O2 and UV/persulfate advanced oxidation processes. Environ. Sci. Technol. 2018, 52, 7380–7389. [Google Scholar] [CrossRef] [PubMed]
- How, S.; Ling, L.; Dionysiou, D.D.; Wan, Y.; Huang, J.; Guo, K.; Li, X.; Fang, J. Chlorate formation mechanism in the presence of sulfate radical, chloride, bromide and natural organic matter. Environ. Sci. Technol. 2018, 52, 6317–6325. [Google Scholar]
- Sun, B.; Wang, Y.; Xiang, Y.; Shang, C. Influence of pre-ozonation of DOM on micropollutant abatement by UV-based advanced oxidation processes. J. Hazard. Mater. 2020, 391, 122201. [Google Scholar] [CrossRef]
- Huie, R.E.; Clifton, C.L. Temperature dependence of the rate constants for reactions of the sulfate radical, SO4•–, with anions. J. Phys. Chem. 1990, 94, 8561–8567. [Google Scholar] [CrossRef]
- Barker, J.R. Hydrogen Peroxide Photolysis in Acidic Aqueous Solutions Containing Chloride Ions. II. Quantum Yield of HO•(Aq) Radicals. J. Phys. Chem. A 2003, 107, 1325–1332. [Google Scholar]
- Margerum, D.W. Kinetics of Reversible Chlorine Hydrolysis: Temperature Dependence and General-Acid/Base-Assisted Mechanisms. Inorg. Chem. 1994, 33, 1050–1055. [Google Scholar]
- Kläning, U.K.; Wolff, T. Laser Flash Photolysis of HCIO, CIO–, HBrO, and BrO– in Aqueous Solution. Reactions of Cl− and Br-Atoms. Ber. Bunsen-Ges. Phys. Chem. 1985, 89, 243–245. [Google Scholar] [CrossRef]
- Matthew, B.M.; Anastasio, C. A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 1-bromide solutions. Atmos. Chem. Phys. 2006, 6, 2423–2437. [Google Scholar] [CrossRef] [Green Version]
- Bulman, D.M.; Mezyk, S.P.; Remucal, C.K. The impact of pH and irradiation wavelength on the production of reactive oxidants during chlorine photolysis. Environ. Sci. Technol. 2019, 53, 4450–4459. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Meng, T.; Wang, Z.; Zhang, R.; Yao, H.; Yang, Y.; Zhao, L. Degradation of Organic Micropollutants in UV/NH2Cl Advanced Oxidation Process. Environ. Sci. Technol. 2019, 53, 9024–9033. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Qi, X.; Zhang, J.; Sun, B. Activation of Bisulfite with Pyrophosphate-Complexed Mn(III) for Fast Oxidation of Organic Pollutants. Int. J. Environ. Res. Public Health 2022, 19, 9437. https://doi.org/10.3390/ijerph19159437
Guo Q, Qi X, Zhang J, Sun B. Activation of Bisulfite with Pyrophosphate-Complexed Mn(III) for Fast Oxidation of Organic Pollutants. International Journal of Environmental Research and Public Health. 2022; 19(15):9437. https://doi.org/10.3390/ijerph19159437
Chicago/Turabian StyleGuo, Qianli, Xianhu Qi, Jian Zhang, and Bo Sun. 2022. "Activation of Bisulfite with Pyrophosphate-Complexed Mn(III) for Fast Oxidation of Organic Pollutants" International Journal of Environmental Research and Public Health 19, no. 15: 9437. https://doi.org/10.3390/ijerph19159437
APA StyleGuo, Q., Qi, X., Zhang, J., & Sun, B. (2022). Activation of Bisulfite with Pyrophosphate-Complexed Mn(III) for Fast Oxidation of Organic Pollutants. International Journal of Environmental Research and Public Health, 19(15), 9437. https://doi.org/10.3390/ijerph19159437