Comparison of Chemical and Biological Methods of Filtering Cryptosporidia from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Investigated Samples (Zeolite)
2.2. Preparation of Studied Samples (Shrimp)
2.3. Filtration, Cleaning, Centrifugation (Zeolite)
2.4. Sampling (Shrimp)
2.5. DNA Extraction
2.6. PCR Amplification
3. Results
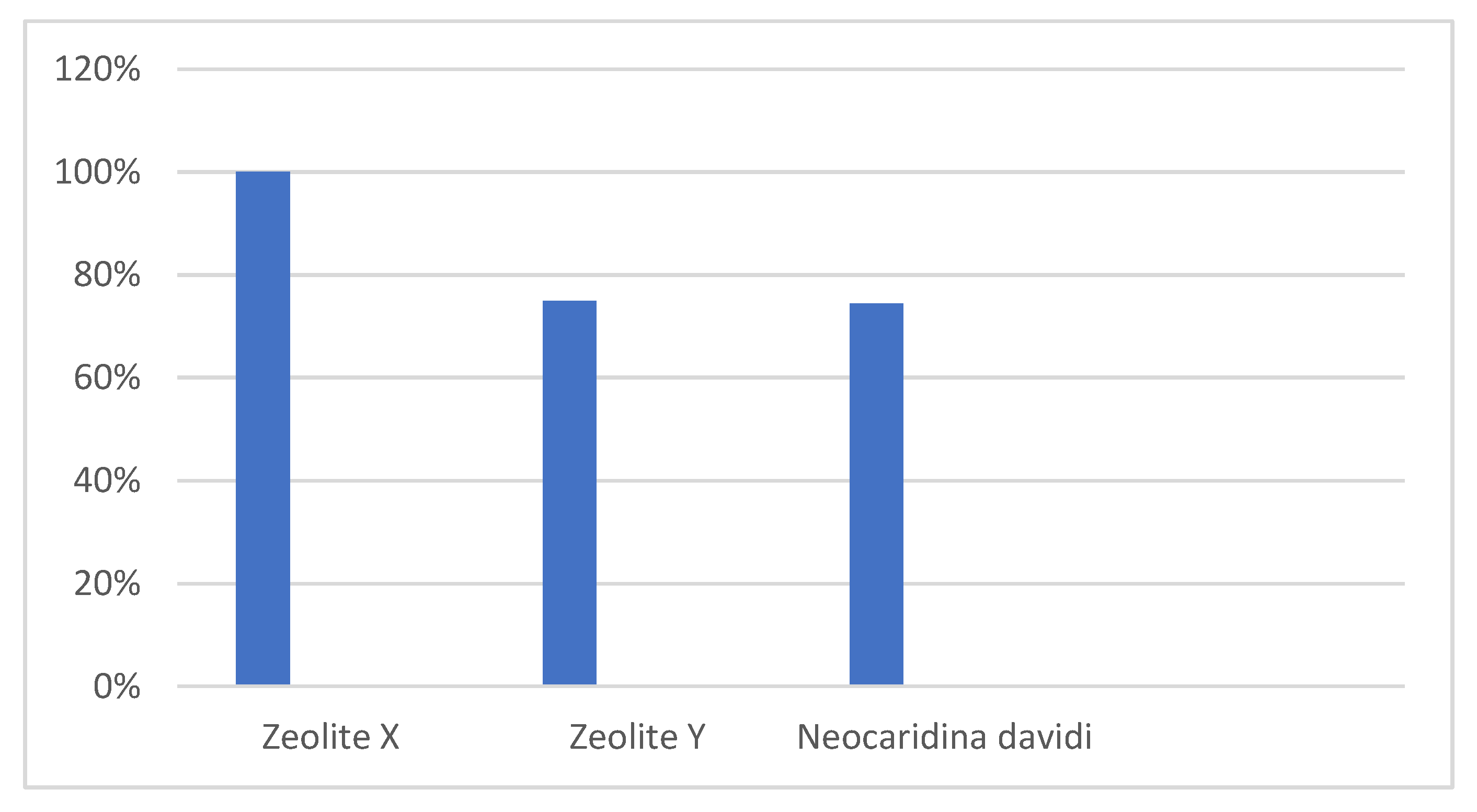
3.1. Zeolite Filtration
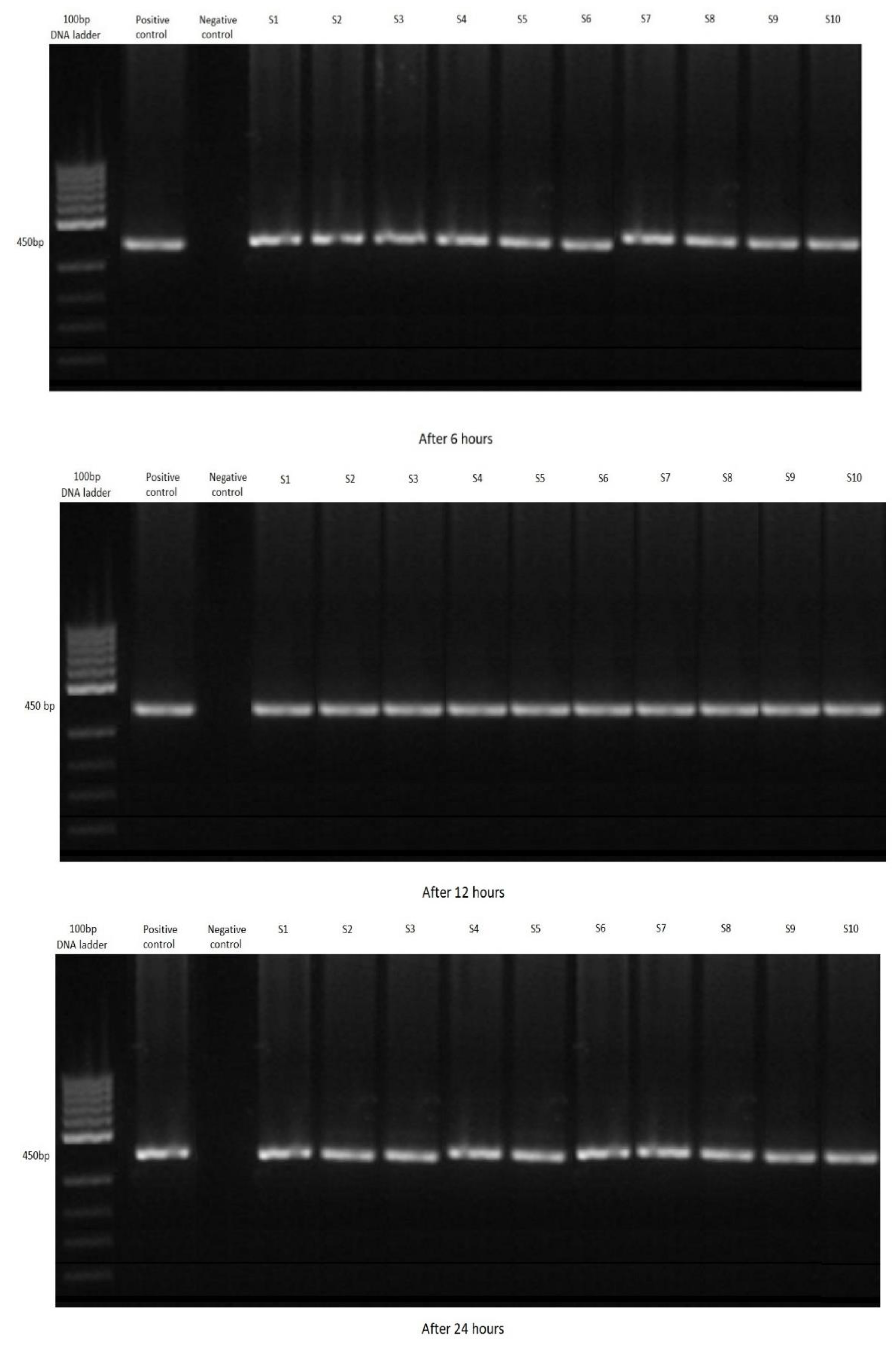
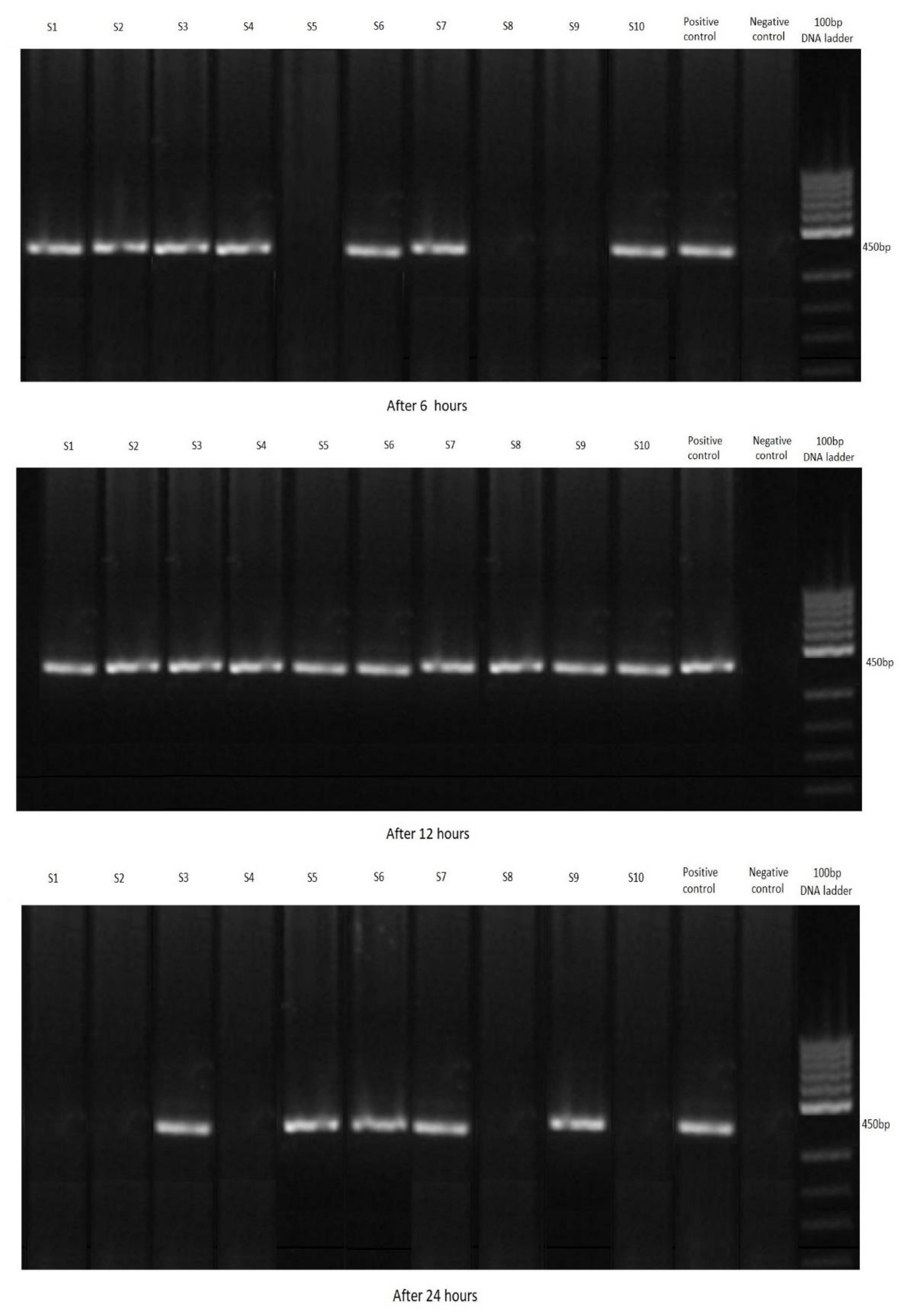
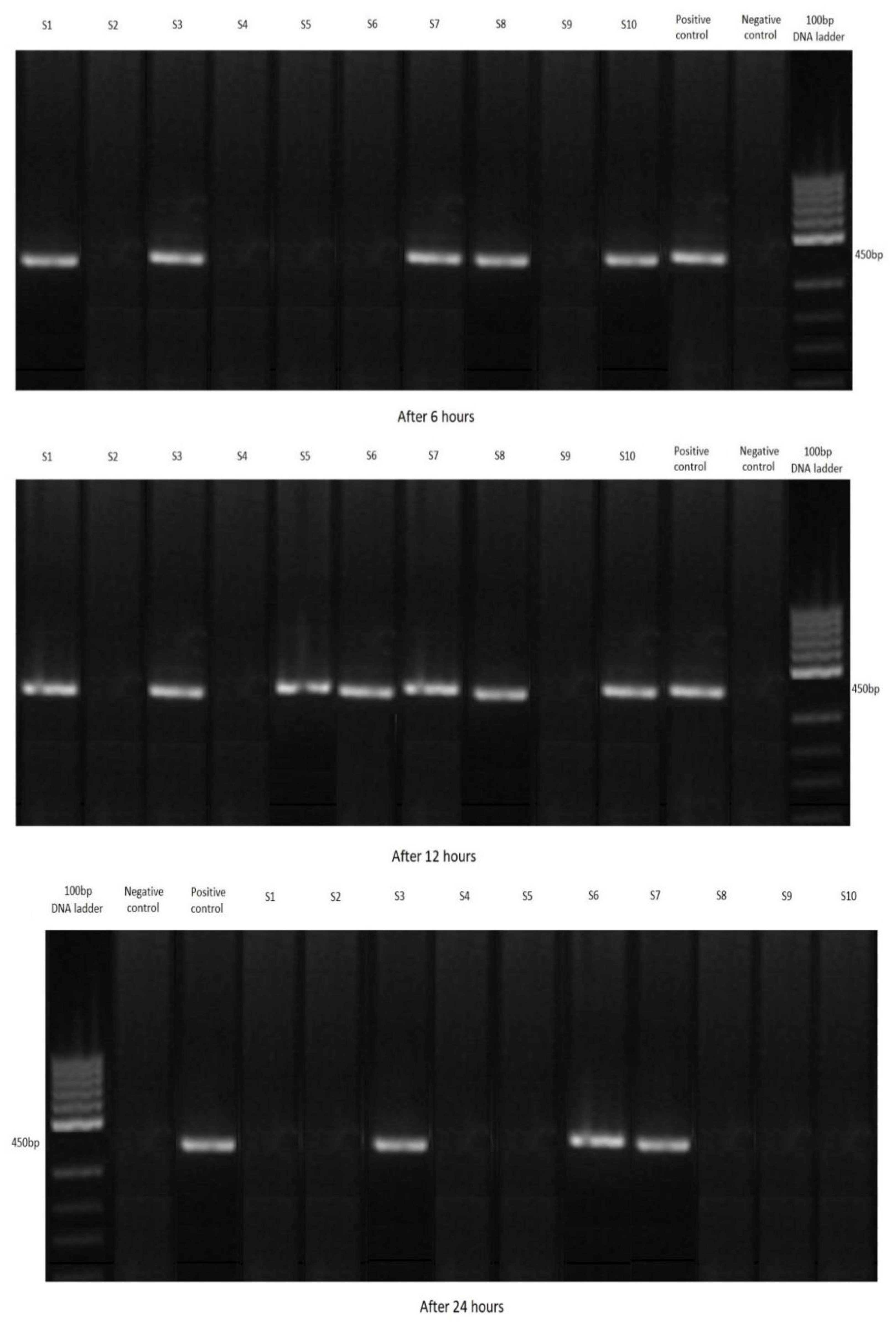
3.2. Filtration by Shrimp
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Leary, J.K.; Sleator, R.D.; Lucey, B. Cryptosporidium spp. diagnosis and research in the 21st century. Food Waterborne Parasitol. 2021, 24, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 11, 45–47. [Google Scholar] [CrossRef] [Green Version]
- Tankersley, K.B.; Dunning, N.P.; Carr, C.; Lentz, D.L.; Scarborough, V.L. Zeolite water purification at Tikal, an ancient Maya city in Guatemala. Sci. Rep. 2020, 10, 18021. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, L.; Zou, S.; Xiao, L.; Fan, J. Zeolite Cotton in Tube: A Simple Robust Household Water Treatment Filter for Heavy Metal Removal. Sci. Rep. 2020, 10, 4719. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar, C.; Bowman, R.; Schulze, D.; Dotson, T.; Fan, T.; Jenkins, A. Evaluation of Surfactant- Modified Zeolite for Control of Cryptosporidium and Giardia Species in Drinking Water. 2004. Available online: http://www.ees.nmt.edu/outside/alumni/papers/2004t_salazar_cm.pdf (accessed on 18 August 2022).
- Abbaszadegan, A.; Morteza, M.; Ouwens, P.; Ryu, R.; Hodon, A.; Absar, I. Removal and Inactivation of Cryptosporidium and Microbial Indicators by a Quaternary Ammonium Chloride (QAC)-Treated Zeolite in Pilot Filters. J. Environ. Sci. Health 2006, 41, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Moropeng, R.C.; Momba, M.N.B. Mechanism of silver incorporated in biosand zeolite clay granular filters for the removal of Cryptosporidium parvum and Giardia lamblia from surface water at point of use. Desalination Water Treat. 2020, 28, 286–295. [Google Scholar] [CrossRef]
- Sonakowska-Czajka, L.; Śróbka, J.; Ostróżka, A.; Rost-Roszkowska, M. Postembryonic development and differentiation of the midgut in the freshwater shrimp Neocaridina davidi (Crustacea, Malacostraca, Decapoda) larvae. J. Morphol. 2021, 282, 48–65. [Google Scholar] [CrossRef]
- Nur, F.A.H.; Christianus, A. Breeding and life cycle of Neocaridina denticulate sinensis (Kemp, 1918). Asian J. Anim. Vet. Adv. 2013, 8, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Pantaleão, J.A.F.; Gregati, R.A.; Da Costa, R.C.; López-Greco, L.S.; Negreiros-Fransozo, M.L. Post-hatching development of the ornamental “Red Cherry Shrimp” Neocaridina davidi (Bouvier, 1904) (Crustacea, Caridea, Atyidae) under laboratory conditions. Aquac. Res. 2017, 48, 553–569. [Google Scholar] [CrossRef]
- Cai, Y. The genus Neocaridina (Crustacea: Decapoda: Atyidae). Acta Zootaxonomica Sin. 1996, 21, 129–160. [Google Scholar]
- Nishino, M.; Niwa, N. Invasion of an alien freshwater shrimp Neocaridina dentriculata sinensis to Lake Biwa. Lake Biwa Res. Inst. News 2004, 80, 3. [Google Scholar]
- Englund, R.A.; Cai, Y. The occurrence and description of Neocaridina denticulata sinensis (Kemp, 1918) (Crustacea: Decapoda: Atyidae), a new introduction to the Hawaiian Islands. Bish. Mus. Occas. Pap. 1999, 58, 58–65. [Google Scholar]
- Klotz, W.; Miesen, F.W.; Hüllen, S.; Herder, F. Two Asian fresh water shrimp species found in a thermally polluted stream system in North Rhine-Westphalia, Germany. Aquat Invasions 2013, 8, 333–339. [Google Scholar] [CrossRef]
- Tropea, C.; Stumpf, L.; Lopez, G. Effect of temperature on the biochemical composition, growth and reproduction of the ornamental red cherry shrimp Neocaridina heteropoda (Decapoda, Caridea). PLoS ONE 2015, 10, e0119468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Włodarczyk, A.; Sonakowska, L.; Kamińska, K.; Marchewka, A.; Wilczek, G.; Wilczek, P.; Rost-Roszkowska, M.M. Effect of starvation and refeeding on mitochondrial potential in the midgut of Neocaridina davidi (Crustacea, Malacostraca). PLoS ONE 2017, 12, e0173563. [Google Scholar] [CrossRef] [Green Version]
- Siregar, P.; Suryanto, M.E.; Chen, K.H.; Huang, J.C.; Chen, H.M.; Kurnia, K.A.; Santoso, F.; Hussain, A.; Ngoc Hieu, B.T.; Saputra, F.; et al. Exploiting the Freshwater Shrimp Neocaridina denticulata as Aquatic Invertebrate Model to Evaluate Nontargeted Pesticide Induced Toxicity by Investigating Physiologic and Biochemical Parameters. Antioxidants 2021, 10, 391. [Google Scholar] [CrossRef]
- Ostróżka, A.; Tiffert, Z.; Wilczek, G.; Rost-Roszkowska, M. Can insecticide-free clean water regenerate the midgut epithelium of the freshwater shrimp after dimethoate treatment? Micron 2022, 155, 103162. [Google Scholar] [CrossRef]
- Klein, K.; Heß, S.; Nungeß, S.; Schulte-Oehlmann, U.; Oehlmann, J. Particle shape does not affect ingestion and egestion of microplastics by the freshwater shrimp Neocaridina palmata. Environ. Sci. Pollut. Res. Int. 2021, 28, 62246–62254. [Google Scholar] [CrossRef]
- Mykles, D.L.; Hui, J.H. Neocaridina denticulata: A Decapod Crustacean Model for Functional Genomics. Integr. Comp. Biol. 2015, 55, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Sing, A.; Limor, J.; Graczyk, T.K.; Gradus, S.; Lal, A.A. Molecular characterisation of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 2001, 67, 1091–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iber, B.T.; Kasan, N.A. Recent advances in Shrimp aquaculture wastewater management. Heliyon 2021, 7, 8283. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010, 124, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Omarova, A.; Tussupova, K.; Berndtsson, R.; Kalishev, M.; Sharapatova, K. Protozoan Parasites in Drinking Water: A System Approach for Improved Water, Sanitation and Hygiene in Developing Countries. Int. J. Environ. Res. Public Health 2018, 15, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, Y.; Dong, S.; Wang, H.; Li, P.; Zhang, H.; Chu, W. The occurrence and control of waterborne viruses in drinking water treatment: A review. Chemosphere 2021, 281, 130728. [Google Scholar] [CrossRef]
- Shirley, D.A.; Moonah, S.N.; Kotloff, K.L. Burden of disease from cryptosporidiosis. Curr. Opin. Infect Dis. 2012, 25, 555–563. [Google Scholar] [CrossRef]
- Iqbal, J.; Khalid, N.; Hira, P.R. Cryptosporidiosis in Kuwaiti children: Association of clinical characteristics with Cryptosporidium species and subtypes. J. Med. Microbiol. 2011, 60, 647–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, P.R.; Nichols, G. Epidemiology and clinical features of Cryptosporidium infection in immunocompromised patients. Clin. Microbiol. Rev. 2002, 15, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatalova, E.; Guman, T.; Bednarova, V.; Simova, V.T.; Logoida, M.; Halanova, M. Occurrence of cryptosporidium parvum IIaA17G1R1 in hospitalized hemato-oncological patients in Slovakia. Parasitol. Res. 2022, 121, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Liu, Y.; Yan, C.; Zhou, Y.; Zhang, R.; Sun, Y.; Zhang, J. Transcriptomic analysis of Neocaridina denticulate sinensis hepatopancreas indicates immune changes after copper exposure. Fish. Shellfish Immunol. 2022, 121, 23–30. [Google Scholar] [CrossRef]
- Méndez-Hermida, F.; Gómez-Couso, H.; Ares-Mazás, E. Artemia is capable of spreading oocysts of Cryptosporidium and cysts of Giardia. J. Eukaryot. Microbiol. 2006, 53, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hermida, F.; Gómez-Couso, H.; Ares-Mazás, E. Possible involvement of Artemia as live diet in the transmission of cryptosporidiosis in cultured fish. Parasitol. Res. 2007, 101, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Kalinová, J.; Valenčáková, A.; Hatalová, E.; Danišová, O.; Luptáková, L.; Špalková, M. Use of Artemia franciscana as a biofilter for catching Cryptosporidium parvum oocysts. Bulg. J. Vet. Med. 2017, 20, 158–161. [Google Scholar]
- Kociánová, J. The Fate of Cryptosporidial Oocysts in the Environment, in Contact with Different Groups of Invertebrates. Bachelor Thesis, University of South Bohemia in České Budějovice, Faculty of Science, České Budějovice, Czech republic, 2009. Volume 65. [Google Scholar]
- Križanová, M. Interaction between Bivalves (Sinanodonta Woodiana) and Cryptosporidium (Cryptosporidium parvum), JN Neumann Bishop’s High School, Church Elementary School and Elementary Art School in České Budějovice, High School Professional Thesis. 2007. Available online: https://adoc.pub/interakce-mezi-mli-sinanodonta-woodiana-a-kryptosporidiemi-c.html (accessed on 17 August 2022).
- Rousková, L. The Role of Barnacles as Filters of Cryptosporidial Oocysts in the Water Column. Bachelor Thesis, University of South Bohemia in České Budějovice, Faculty of Science, České Budějovice, Czech Republic, 2008. Volume 30. [Google Scholar]
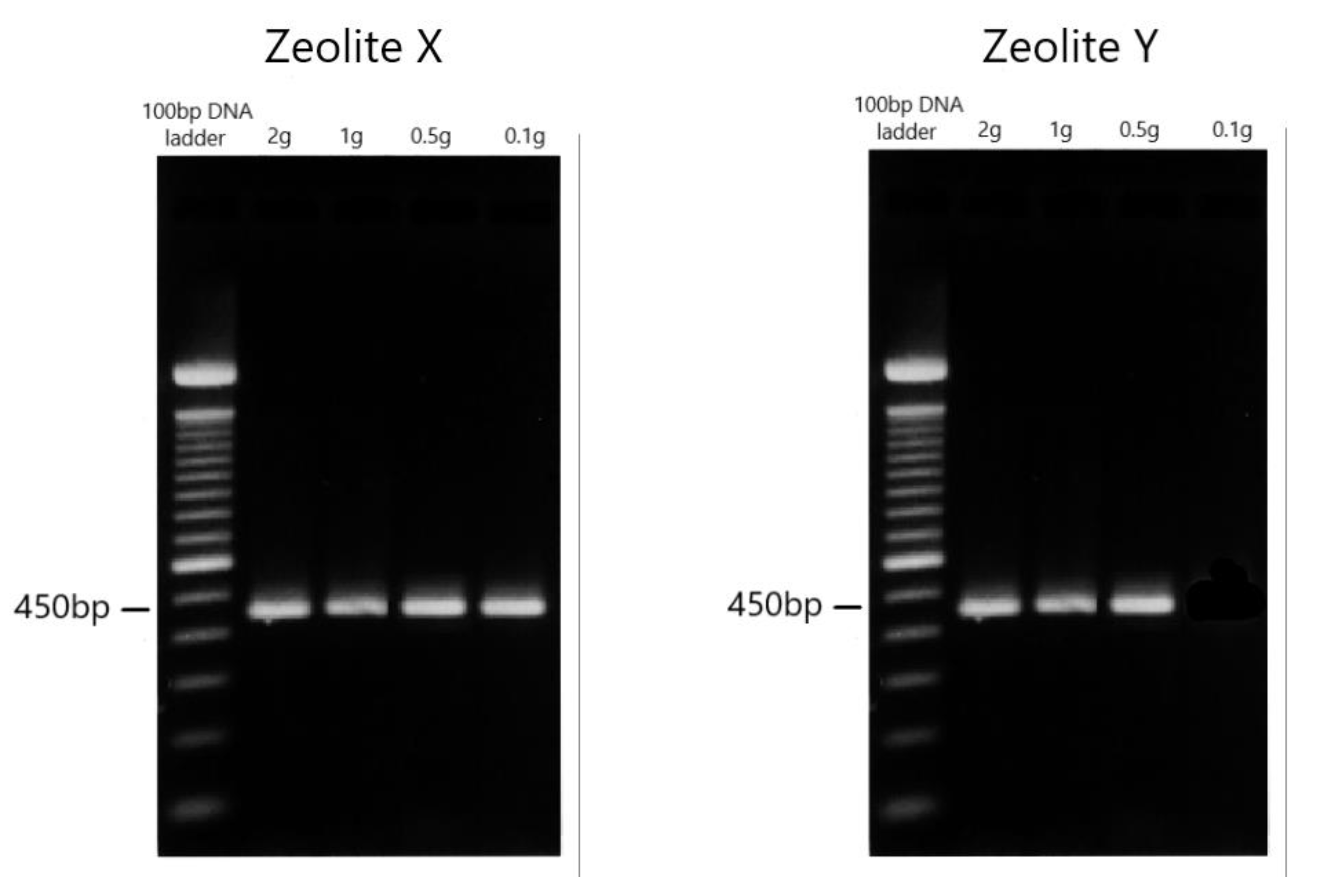
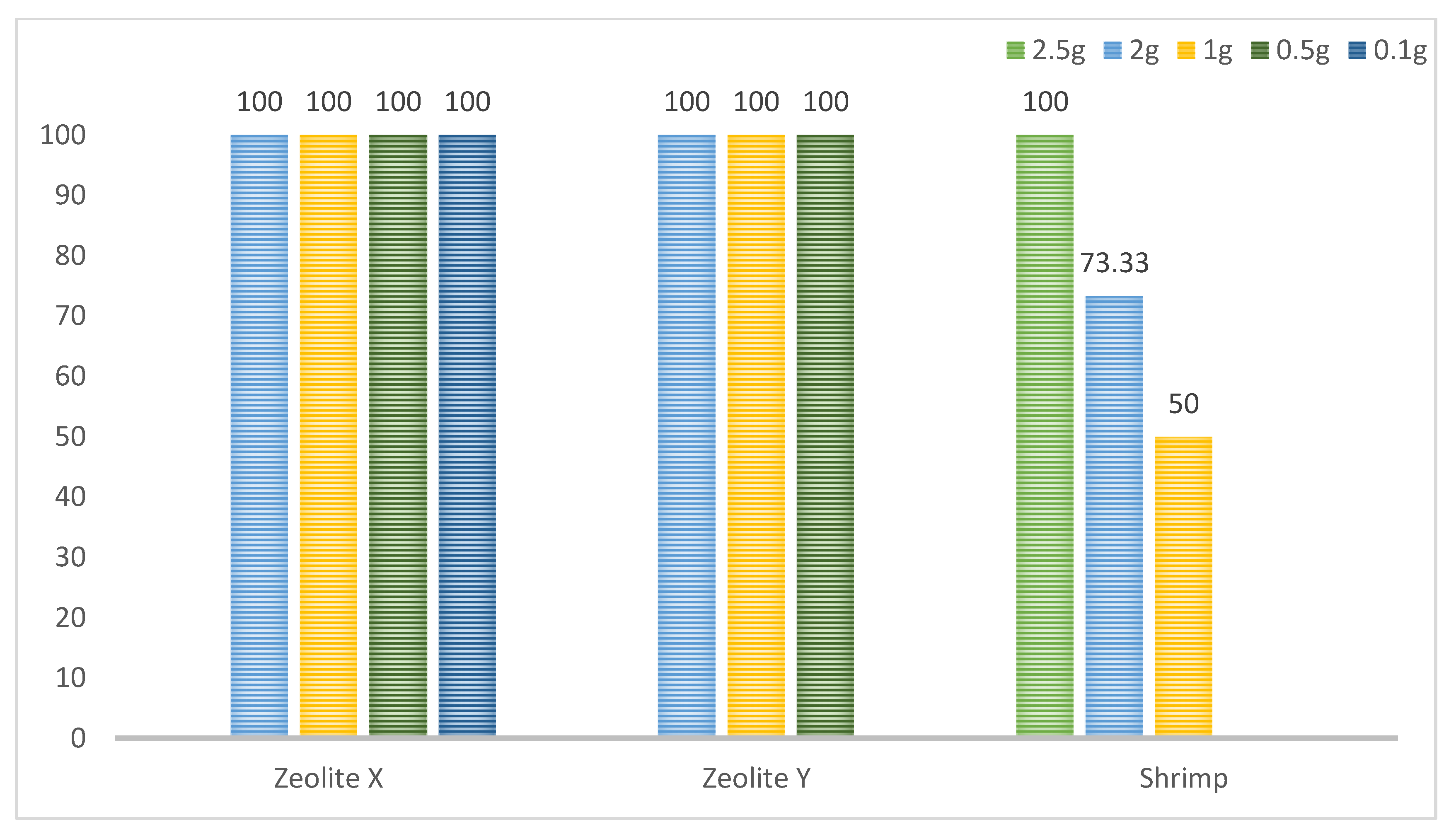
| Zeolite | Filtration Time | 2 g | 1 g | 0.5 g | 0.1 g |
|---|---|---|---|---|---|
| X: 0.2–0.6 mm | 15 min | Posit | Posit | Posit | Posit |
| Y: 0–0.3 mm | 30 min | Posit | Posit | Posit | - |
| C. parvum-Positive Shrimp | ||||
|---|---|---|---|---|
| Marking | Total | After 6 h | After 12 h | After 24 h |
| Group A (2.5 g) | 30 | 10 | 10 | 10 |
| Group B (2 g) | 22 | 7 | 10 | 5 |
| Group C (1 g) | 15 | 5 | 7 | 3 |
| Control group (K) | 0 | 0 | 0 | 0 |
| Total | 67 | 22 | 27 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sučik, M.; Valenčáková, A. Comparison of Chemical and Biological Methods of Filtering Cryptosporidia from Water. Int. J. Environ. Res. Public Health 2022, 19, 12675. https://doi.org/10.3390/ijerph191912675
Sučik M, Valenčáková A. Comparison of Chemical and Biological Methods of Filtering Cryptosporidia from Water. International Journal of Environmental Research and Public Health. 2022; 19(19):12675. https://doi.org/10.3390/ijerph191912675
Chicago/Turabian StyleSučik, Monika, and Alexandra Valenčáková. 2022. "Comparison of Chemical and Biological Methods of Filtering Cryptosporidia from Water" International Journal of Environmental Research and Public Health 19, no. 19: 12675. https://doi.org/10.3390/ijerph191912675







