Physiological and Ankle Functions Are Discriminating Factors for the Risk of Falls in Women in Treatment of Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
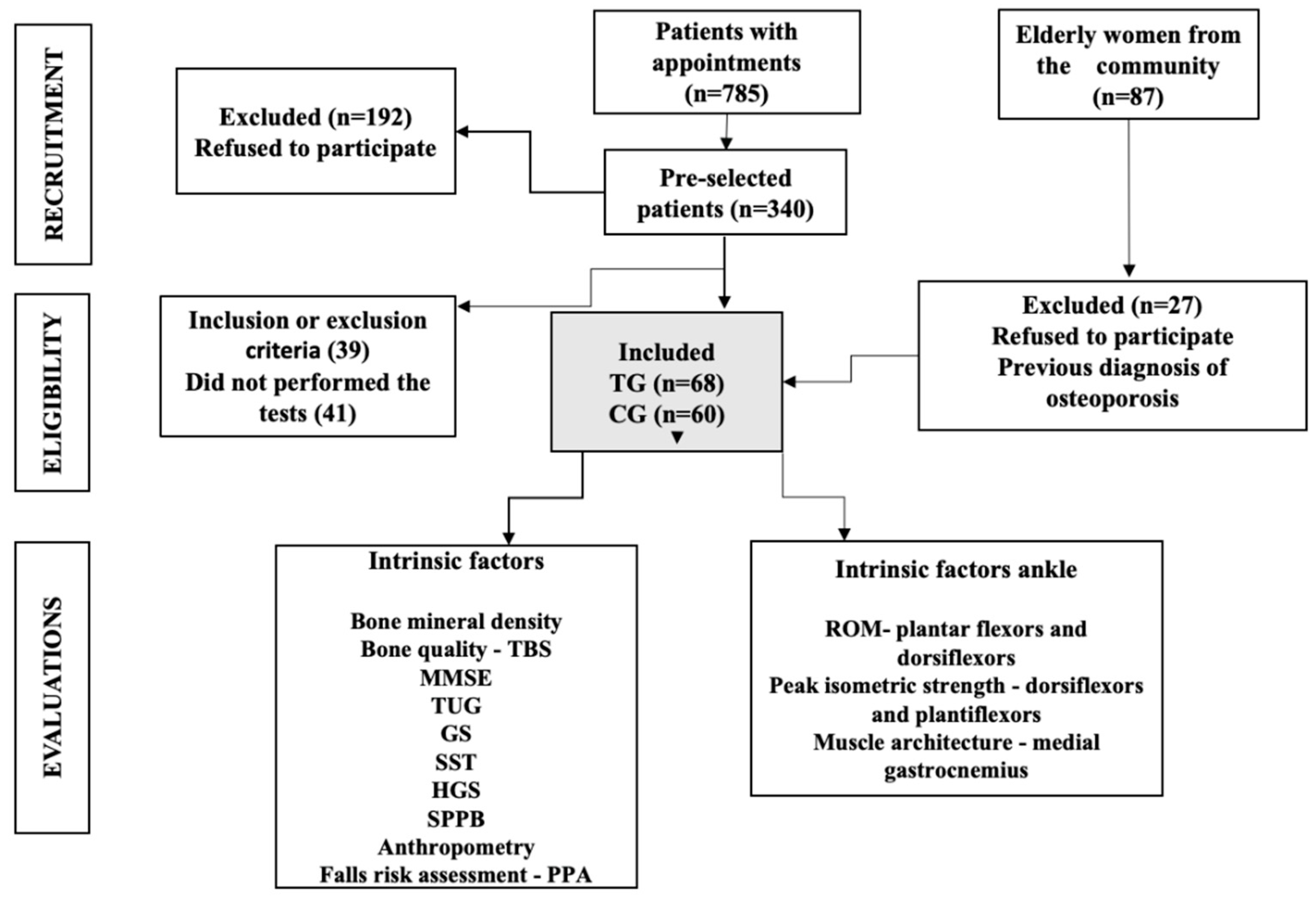
2.2. Participants
2.3. Study Procedures
2.4. Fall History
2.5. Sample Characterization
2.6. Anthropometric Assessment and Cognitive Screening
2.7. Bone Mineral Density
2.8. Cognitive Screening
2.9. Physical Performance
2.10. Strength
2.11. Ankle Musculoskeletal Function
2.12. Muscle Architecture
2.13. Falls Risk Assessment
2.14. Statistical Analysis
3. Results
3.1. Comparison between Groups
3.2. Intrinsic Factors for Falls
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bok, S.-K.; Lee, T.H.; Lee, S.S. The Effects of changes of ankle strength and range of motion according to aging on balance. Ann. Rehabil. Med. 2013, 37, 10–16. [Google Scholar] [CrossRef] [PubMed]
- LaRoche, D.P.; Cremin, K.A.; Greenleaf, B.; Croce, R.V. Rapid torque development in older female fallers and nonfallers: A comparison across lower-extremity muscles. J. Electromyogr. Kinesiol. 2010, 20, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Reddy, S.; Berkman, N.; Cullen, K.; Middleton, J.C.; Nicholson, W.K.; Kahwati, L.C. Screening to Prevent Osteoporotic Fractures: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 2532–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, R.B.; Burke, N.; Von Keyserlingk, C.; Leslie, W.D.; Morin, S.N.; Adachi, J.D.; Papaioannou, A.; Bessette, L.; Brown, J.P.; Pericleous, L.; et al. The current economic burden of illness of osteoporosis in Canada. Osteoporos. Int. 2016, 27, 3023–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanis, J.A.; Svedbom, A.; Harvey, N.; McCloskey, E.V. The Osteoporosis Treatment Gap. J. Bone Miner. Res. 2014, 29, 1926–1928. [Google Scholar] [CrossRef] [PubMed]
- Kothawala, P.; Badamgarav, E.; Ryu, S.; Miller, R.M.; Halbert, R.J. Systematic Review and Meta-analysis of Real-World Adherence to Drug Therapy for Osteoporosis. Mayo Clinic Proc. 2007, 82, 1493–1501. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Fullman, R.L.; Marshall, L.; Mackey, D.C.; Fink, H.A.; Cauley, J.A.; Cummings, S.R.; Orwoll, E.S.; Ensrud, K.E.; Osteoporotic Fractures in Men (MrOS) Research Group. Physical performance and risk of hip fractures in older men. J. Bone Miner. Res. 2008, 23, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Baccaro, L.F.; Conde, D.M.; Costa-Paiva, L.; Pinto-Neto, A.M. The epidemiology and management of postmenopausal osteoporosis: A viewpoint from Brazil. Clin. Interv. Aging 2015, 10, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, R.C. Fractures and Repeated Falls. J. Clin. Densitom. 2017, 20, 425–431. [Google Scholar] [CrossRef]
- Moreira, N.B.; Rodacki, A.L.F.; Pereira, G.; Bento, P.C.B. Does functional capacity, fall risk awareness and physical activity level predict falls in older adults in different age groups? Arch. Gerontol. Geriatr. 2018, 77, 57–63. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.; Huang, H.; Zeng, L.; Yang, W.; Pan, J.; Liu, J. Identification of risk factors for falls in postmenopausal women: A systematic review and meta-analysis. Osteoporos. Int. 2020, 31, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Radominski, S.C.; Bernardo, W.; Paula, A.P.; Albergaria, B.H.; Moreira, C.; Fernandes, C.E.; Castro, C.H.M.; Zerbini, C.A.F.; Domiciano, D.S.; Mendonça, L.M.C.; et al. Brazilian guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Rev. Bras. Reumatol. 2017, 57, 452–466. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, R.A.; Veras, R.P. Mini-Exame do Estado Mental: Características psicométricas em idosos ambulatoriais. Mini-Mental State Examination: Psychometric characteristics in elderly outpatients. Rev. De Saúde Pública 2006, 40, 712–719. [Google Scholar] [CrossRef]
- Barron, R.L.; Oster, G.; Grauer, A.; Crittenden, D.B.; Weycker, D. Determinants of imminent fracture risk in postmenopausal women with osteoporosis. Osteoporos. Int. 2020, 31, 2103–2111. [Google Scholar] [CrossRef]
- Report of a WHO Expert Committee. Physical status: The use and interpretation of anthropometry. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- McCloskey, E.V.; Oden, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A meta-analysis oftrabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Burns, J.; 1000 Norms Project Consortium. Normative reference values for strength and flexibility of 1000 children and adults. Neurology 2016, 88, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch. Phys. Med. Rehabil. 1997, 78, 26–32. [Google Scholar] [CrossRef]
- Kuyumcu, M.E.; Halil, M.; Kara, Ö.; Çuni, B.; Çağlayan, G.; Güven, S.; Yeşil, Y.; Arık, G.; Yavuz, B.B.; Cankurtaran, M.; et al. Ultrasonographic evaluation of the calf muscle mass and architecture in elderly patients with and without sarcopenia. Arch. Gerontol. Geriatr. 2016, 65, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Maganaris, C.N.; Reeves, N.D.; Capodaglio, P. Effect of aging on human muscle architecture. J. Appl. Physiol. (1985). 2003, 95, 2229–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojciechowski, A.S.; Biesek, S.; Filho, J.M.; Rabito, E.I.; Amaral, M.P.D.; Gomes, A.R.S. Effects of physical training with the Nintendo Wii Fit Plus ® and protein supplementation on musculoskeletal function and the risk of falls in pre-frail older women: Protocol for a randomized controlled clinical trial (the WiiProtein study). Maturitas 2018, 111, 53–60. [Google Scholar] [CrossRef]
- Lord, S.R.; Menz, H.B.; Tiedemann, A. A physiological profile approach to falls risk assessment and prevention. Phys. Ther. 2003, 83, 237–252. [Google Scholar] [CrossRef]
- Siddique, N.; Fallon, N.; Casey, M.C.; Walsh, J.B. Statistical analysis of fat and muscle mass in osteoporosis in elderly population using total body DXA scans. Ir. J. Med. Sci. 2020, 189, 1105–1113. [Google Scholar] [CrossRef]
- Peraza-Delgado, A.; Sánchez-Gómez, M.B.; Gómez-Salgado, J.; Romero-Martín, M.; Novo-Muñoz, M.; Duarte-Clíments, G. Non-Pharmacological Interventions towards Preventing the Triad Osteoporosis-Falls Risk-Hip Fracture, in Population Older than Scoping Review. J. Clin. Med. 2020, 9, 2329. [Google Scholar] [CrossRef]
- Body, J.-J.; Bergmann, P.; Boonen, S.; Boutsen, Y.; Bruyere, O.; Devogelaer, J.-P.; Goemaere, S.; Hollevoet, N.; Kaufman, J.-M.; Milisen, K.; et al. Non-pharmacological management of osteoporosis: A consensus of the Belgian Bone Club. Osteoporos. Int. 2011, 22, 2769–2788. [Google Scholar] [CrossRef] [Green Version]
- Babiuch, A.S.; Oestervemb, K.; Lipińska, A.; Stańczak, M.L.; Cholewa, M.; Makulec, K.; Nowakowska, K.; Derengowska, M.H. Differences in the level of physical fitness and mobility among older women with osteoporosis and healthy women—Cross-sectional study. Sci. Rep. 2021, 11, 14179. [Google Scholar] [CrossRef]
- Menz, H.B.; Morris, M.E.; Lord, S.R. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1546–1552. [Google Scholar] [CrossRef]
- Spink, M.J.; Fotoohabadi, M.R.; Wee, E.; Hill, K.D.; Lord, S.R.; Menz, H.B. Foot and ankle strength, range of motion, posture, and deformity are associated with balance and functional ability in older adults. Arch. Phys. Med. Rehabil. 2011, 92, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kemoun, G.; Thoumie, P.; Boisson, D.; Guieu, J.D. Ankle dorsiflexion delay can predict falls in the elderly. J. Rehabil. Med. 2002, 34, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menz, H.B.; Auhl, M.; Spink, M.J. Foot problems as a risk factor for falls in community-dwelling older people: A systematic review and meta-analysis. Maturitas 2018, 118, 7–14. [Google Scholar] [CrossRef]
- Pollock, R.D.; Carter, S.; Velloso, C.P.; Duggal, N.A.; Lord, J.M.; Lazarus, N.R.; Harridge, S.D.R. An investigation into the relationship between age and physiological function in highly active older adults. J. Physiol. 2015, 593, 657–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, S.R.; Delbaere, K.; Gandevia, S.C. Use of a physiological profile to document motor impairment in ageing and in clinical groups. J. Physiol. 2016, 594, 4513–4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, S.R.; Sambrook, P.N.; Gilbert, C.; Kelly, P.J.; Nguyen, T.; Webster, I.W.; Eisman, J.A. Postural stability, falls and fractures in the elderly: Results from the Dubbo Osteoporosis Epidemiology Study. Med. J. Aust. 1994, 160, 684–685. [Google Scholar] [CrossRef]
| Variables | TG (n = 68) Mean ± SD | CG (n = 60) Mean ± SD | p |
|---|---|---|---|
| Age (years) | 71.98 ± 3.47 | 71.13 ± 2.41 | 0.113 |
| Race | |||
| White | 88.23% (n = 60) | 98.33% (n = 59) | 0.577 |
| Black | 11.70% (n = 8) | 1.66% (n = 1) | 0.600 |
| Height (m) | 1.54 ± 0.06 | 1.58 ± 0.08 | 0.002 |
| Weight (Kg) | 64.13 ± 11.13 | 68.85 ± 10.54 | 0.015 |
| BMI (kg/m2) | 26.52 ± 3.71 | 21.77 ± 3.35 | <0.000 |
| Underweight | - | 16.70% (n = 10) | <0.000 |
| Normal | 41.17% (n = 28) | 56.70% (n = 34) | <0.000 |
| Pre-obese | 38.23% (n = 26) | 23.40% (n = 14) | <0.000 |
| Obese | 20.59% (n = 14) | - | <0.000 |
| Comorbidities (N) | 1.46 ± 1.30 | 0.76 ± 0.96 | <0.000 |
| Hypertension | 46.37% (n = 32) | 53.33% (n = 32) | 0.246 |
| Hyperglycemia | 79.71% (n = 55) | 76.66% (n = 46) | 0.855 |
| Dyslipidemia | 60.86% (n = 42) | 43.33% (n = 26) | 0.085 |
| DM2 | 79.71% (n = 55) | 76.65% (n = 46) | 0.855 |
| Medications (N) | 4.10 ± 2.96 | 2.30 ± 2.56 | 0.000 |
| Osteoporosis treatment | |||
| Alendronate | 50.0% (n = 34) | - | |
| Risedronate | 2.94% (n = 2) | - | |
| Pamidronate | 23.53% (n = 16) | - | |
| Zoledronic acid | 2.94 % (n = 2) | - | |
| No treatment # | 20.59% (n = 14) | - | |
| Treatment time (years) | 1.94 ± 4.38 | - | |
| BMD (g/m2) | |||
| Spine | 0.768 ± 0.13 | 0.901 ± 0.19 | <0.000 |
| Femoral neck | 0.647 ± 0.90 | 0.715 ± 0.27 | 0.057 |
| Total hip | 0.748 ± 0.11 | 0.693 ± 0.15 | 0.023 |
| BMD classification | |||
| Normal | 2.90% (n = 2) | 21.70% (n = 13) | <0.000 |
| Osteopenia | 20.30% (n = 14) | 58.30% (n = 35) | <0.000 |
| Osteoporosis | 76.80% (n = 57) | 20.00% (n = 12) | <0.000 |
| Low | 97.10% (n = 67) | 78.30% (n = 47) | <0.000 |
| TBS score | 1.23 ± 0.10 | 1.30 ± 0.14 | 0.001 |
| Normal | 30.00% (n = 14) | 56.60% (n = 34) | <0.000 |
| Partially degraded | 38.60% (n = 27) | 23.30% (n = 14) | <0.000 |
| Degraded | 41.40% (n = 29) | 20.00% (n = 12) | <0.000 |
| Past fracture % (N) | 15.30% (n = 11) | 13.50% (n = 8) | 0.455 |
| 1 | 13.40% (n = 9) | 11.40% (n = 7) | 0.142 |
| ≥2 | 1.10% (n = 1) | 0.90% (n = 1) | 0.142 |
| Falls classification | |||
| Non-faller | 78.5% (n = 53) | 81% (n = 49) | 0.866 |
| Faller | 16.5% (n = 11) | 11%(n = 7) | 0.866 |
| Recurrent faller | 5% (n = 4) | 6% (n = 4) | 0.866 |
| MMSE | 26.50 ± 3.11 | 27.96 ± 1.62 | 0.001 |
| Variables | TG (n = 69) | CG(n = 60) | p | ||
|---|---|---|---|---|---|
| Mean ± SD | Cohen D | Mean ± SD | Cohen D | ||
| n (%) | n (%) | ||||
| Physical performance | |||||
| TUG (s) | 10.06 ± 2.70 | 2.2 | 11.43 ± 3.04 | 0.48 | 0.008 |
| Reduced (<9.2 s) | 61.4% (n = 43) | 75.9% (n = 44) | 0.090 | ||
| GS (s) | 0.90 ± 0.17 | 4.11 | 0.81 ± 0.14 | 0.58 | 0.004 |
| Low (≤0.8 m/s) | 74.3% (n = 52) | 46.6% (n = 27) | 0.001 | ||
| SPPB (score) | 9.30 ± 2.40 | 4.87 | 7.88 ± 1.36 | 0.72 | 0.000 |
| 0 (worst performance) | |||||
| 12 (best performance) | |||||
| Strength | |||||
| SST (s) | 12.30 ± 2.74 | 0.3 | 12.79 ± 3.10 | 0.17 | 0.343 |
| Low (%) | 45.7% (n = 32) | 41.4% (n = 53) | 0.182 | ||
| HGS (kgf) | 21.24 ± 3.78 | 4.62 | 22.46 ± 4.24 | 0.31 | 0.087 |
| Low (≤18 kgf) | 11.4% (n = 8) | 8.6% (n = 11) | 0.174 | ||
| Ankle ROM (°) | 0.06 | 0 | |||
| Dorsiflexors | 22.16 ± 6.10 | 21.98 ± 4.09 | 0.8 | 0.989 | |
| Low (<26 ± 6.3°) | 100% | 100% | 1 | ||
| Plantiflexors | 46.46 ± 7.17 | 4.13 | 41.23 ± 5.86 | 0.55 | 0.001 |
| Low (<57 ± 7.2°) | 100% | 100% | 1 | ||
| Muscle architecture | |||||
| Medial gastrocnemius | |||||
| MT | 1.40 ± 0.23 | 11.23 | 1.37 ± 0.26 | 0.12 | 0.452 |
| FL | 4.43 ± 0.88 | 4.61 | 4.36 ±0.97 | 0.08 | 0.686 |
| PA | 19.09 ± 4.70 | 4.24 | 20.45 ± 4.36 | 0.3 | 0.092 |
| PIS—ankle | |||||
| Dorsiflexion | 7.78 ± 2.96 | 3.47 | 8.23 ± 1.21 | 0.2 | 0.275 |
| Plantarflexion | 10.41 ± 4.19 | 0.69 | 11.87± 2.45 | 0.42 | 0.019 |
| TG (n = 69) Mean ± SD n (%) | D-Cohen | CG (n = 60) Mean ± SD n (%) | p | Cohen-D | |
|---|---|---|---|---|---|
| Visual contrast | 16.92 ± 2.15 | 2.22 | 17.21 ± 1.57 | 0.391 | 0.15 |
| Excellent/good | 8.6% (n = 6) | 1.6% (n = 1) | 0.047 | ||
| Fair/bad | 91.30% (n = 63) | 93.3% (n = 56) | 0.046 | ||
| Proprioception | 2.51 ± 1.54 | 9.53 | 1.80 ± 0.90 | 0.002 | 0.56 |
| Good/fair | 84.05% (n = 58) | 95.00% (n = 57) | 0.018 | ||
| Bad | 11.94% (n = 11) | 5.00% (n = 3) | 0.018 | ||
| Strength LM | 23.82 ± 5.82 | 5.16 | 27.60 ± 6.17 | 0.001 | 0.67 |
| Excellent/good | 69.56% (n = 48) | 93.33% (n = 56) | 0.002 | ||
| Fair/bad | 30.43% (n = 21) | 6.66% (n = 4) | 0.003 | ||
| Reaction time | 391.04 ± 111.59 | 4.48 | 394.86 ± 109.40 | 1 | 0.03 |
| Excellent/good | 17.39%(n = 12) | 18.33% (n = 11) | 1 | ||
| Fair/bad | 82.60%(n = 57) | 81.66% (n = 49) | 1 | ||
| Postural sway | 322.60 ± 147.99 | 0.55 | 319.33 ± 157.60 | 0.564 | 0.02 |
| Excellent/good | 100.00% (n = 69) | 98.33% (n = 59) | 0.166 | ||
| Falls risk | 2.22 ± 1.17 | 2.98 | 1.56 ± 0.94 | 0.050 | 0.62 |
| Very low | 5.7% (n = 4) | 26.66% (n = 16) | 0.003 | ||
| Low | 73.91% (n = 51) | 65.00% (n = 39) | 0.002 | ||
| Moderate/very high | 14.49% (n = 10) | 5.00% (n = 3) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correa, R.G.P.; Silveira Gomes, A.R.; Borba, V.Z.C. Physiological and Ankle Functions Are Discriminating Factors for the Risk of Falls in Women in Treatment of Osteoporosis. Int. J. Environ. Res. Public Health 2022, 19, 12643. https://doi.org/10.3390/ijerph191912643
Correa RGP, Silveira Gomes AR, Borba VZC. Physiological and Ankle Functions Are Discriminating Factors for the Risk of Falls in Women in Treatment of Osteoporosis. International Journal of Environmental Research and Public Health. 2022; 19(19):12643. https://doi.org/10.3390/ijerph191912643
Chicago/Turabian StyleCorrea, Renata Gonçalves Pinheiro, Anna Raquel Silveira Gomes, and Victoria Zeghbi Cochenski Borba. 2022. "Physiological and Ankle Functions Are Discriminating Factors for the Risk of Falls in Women in Treatment of Osteoporosis" International Journal of Environmental Research and Public Health 19, no. 19: 12643. https://doi.org/10.3390/ijerph191912643




