Effects of the COVID-19 Pandemic on Physical Function of Community-Dwelling People with Disabilities in Japan
Abstract
1. Introduction
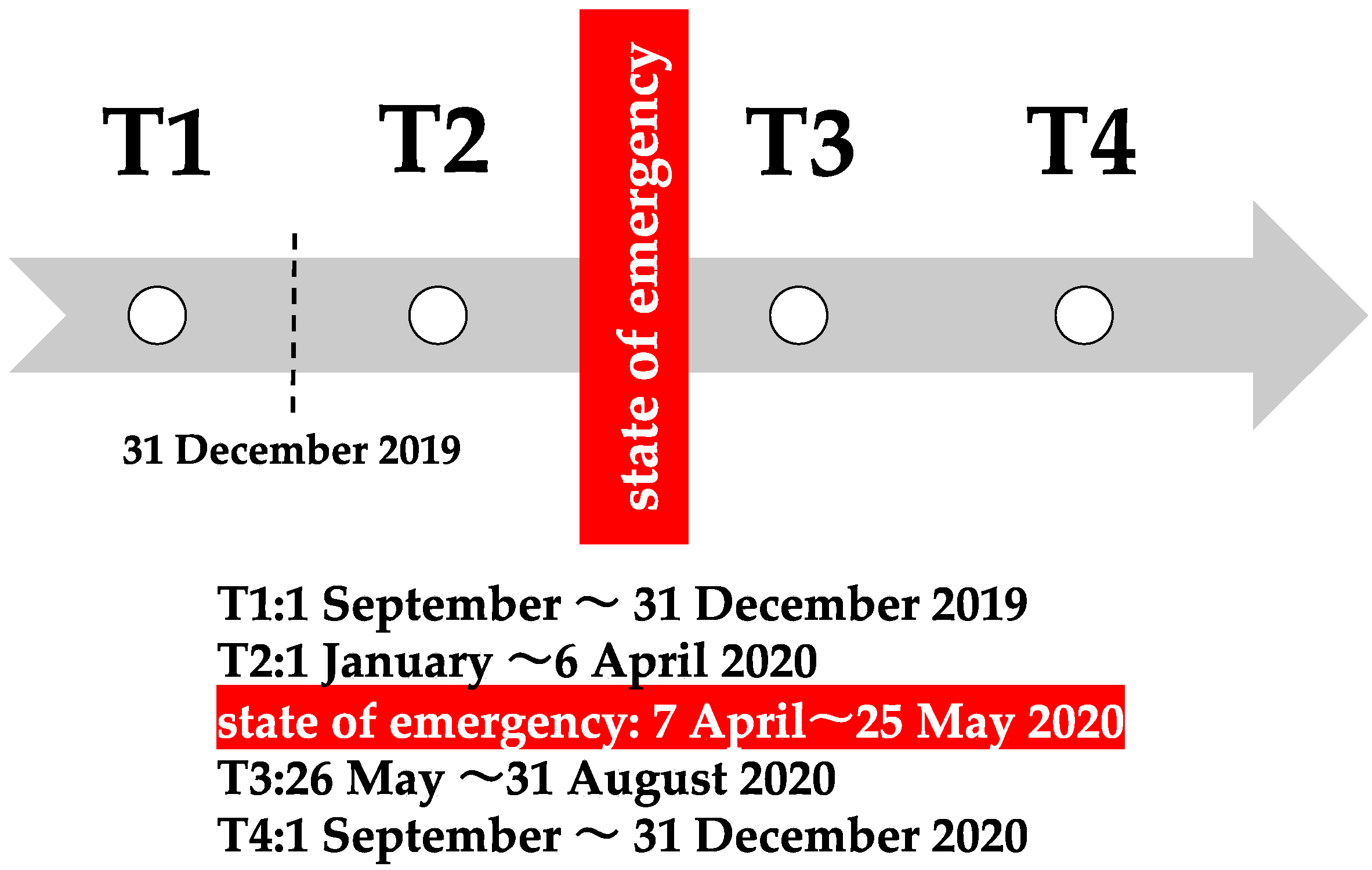
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Daycare and Long-Term Care Insurance (LTCI)
2.3. Physical Data
2.4. Statistical Analyses
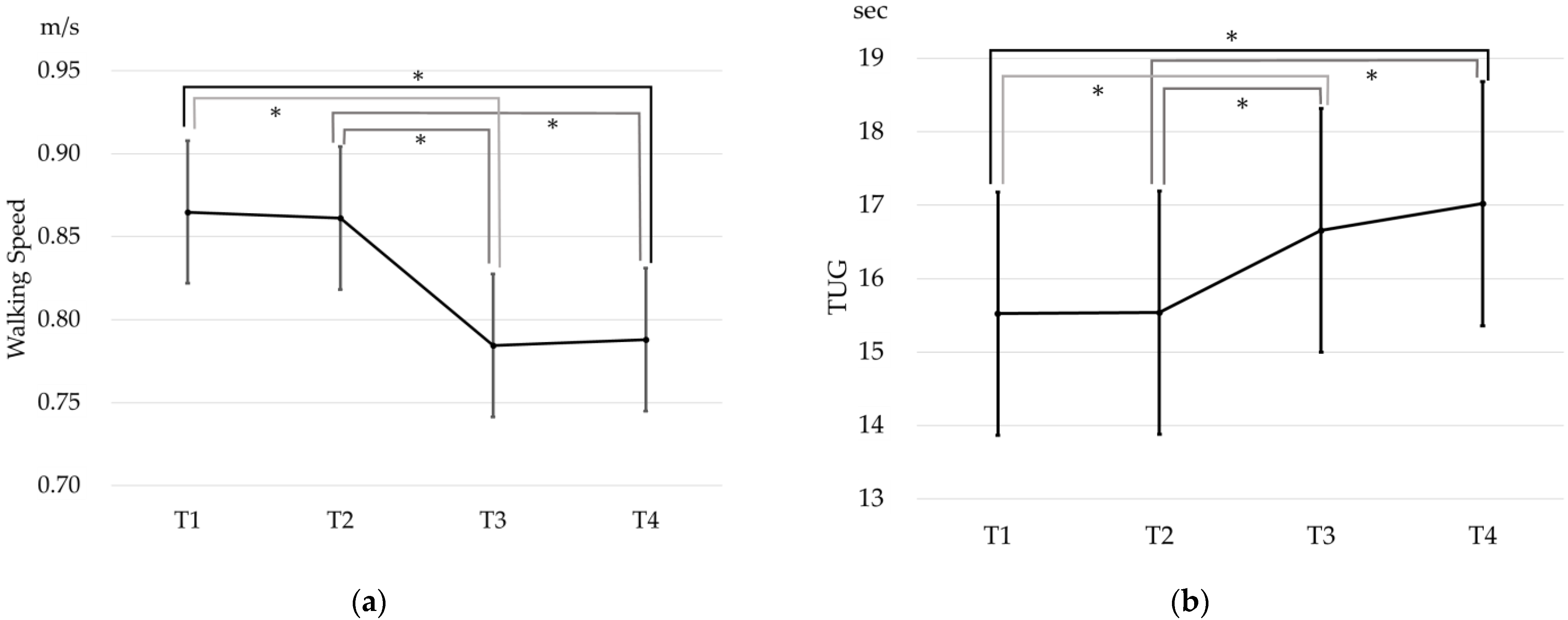
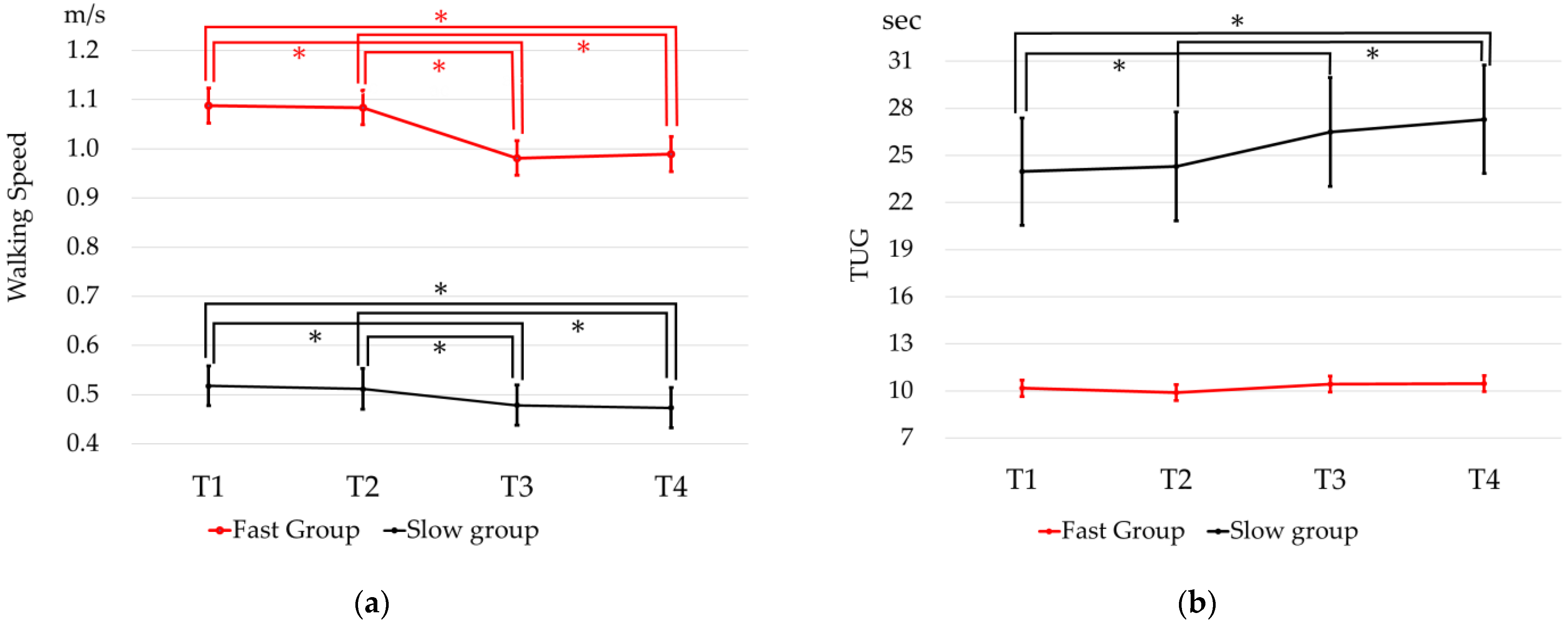
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parmet, W.E.; Sinha, M.S. COVID-19—The Law and Limits of Quarantine. N. Engl. J. Med. 2020, 382, e28. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, H.; Wilder-Smith, A.; Osman, S.; Farooq, Z.; Rocklöv, J. Only strict quarantine measures can curb the coronavirus disease (COVID-19) outbreak in Italy, 2020. Euro. Surveill. 2020, 25, 2000280. [Google Scholar] [CrossRef] [PubMed]
- Looi, M.-K. COVID-19: Japan declares state of emergency as Tokyo cases soar. BMJ 2020, 369, m1447. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Bulzing, R.A.; Meyer, J.; Vancampfort, D.; Firth, J.; Stubbs, B.; Grabovac, I.; Willeit, P.; Tavares, V.D.O.; Calegaro, V.C.; et al. Associations of moderate to vigorous physical activity and sedentary behavior with depressive and anxiety symptoms in self-isolating people during the COVID-19 pandemic: A cross-sectional survey in Brazil. Psychiatry Res. 2020, 292, 113339. [Google Scholar] [CrossRef]
- Kirwan, R.; McCullough, D.; Butler, T.; Perez de Heredia, F.P.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. GeroScience 2020, 42, 1547–1578. [Google Scholar] [CrossRef]
- da Rocha, A.Q.; Lobo, P.C.B.; Pimentel, G.D. Muscle Function Loss and Gain of Body Weight during the COVID-19 Pandemic in Elderly Women: Effects of One Year of Lockdown. J. Nutr. Health Aging 2021, 25, 1028–1029. [Google Scholar] [CrossRef]
- Amanzio, M.; Canessa, N.; Bartoli, M.; Cipriani, G.E.; Palermo, S.; Cappa, S.F. Lockdown Effects on Healthy Cognitive Aging During the COVID-19 Pandemic: A Longitudinal Study. Front. Psychol. 2021, 12, 685180. [Google Scholar] [CrossRef]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kikuchi, T.; Kusumi, H.; Arai, H. Effect of the COVID-19 Epidemic on Physical Activity in Community-Dwelling Older Adults in Japan: A Cross-Sectional Online Survey. J. Nutr. Health Aging 2020, 24, 948–950. [Google Scholar] [CrossRef]
- Shakespeare, T.; Ndagire, F.; Seketi, Q.E. Triple jeopardy: Disabled people and the COVID-19 pandemic. Lancet 2021, 397, 1331–1333. [Google Scholar] [CrossRef]
- Palmer, K.; Vetrano, D.L.; Padua, L.; Romano, V.; Rivoiro, C.; Scelfo, B.; Marengoni, A.; Bernabei, R.; Onder, G. Frailty Syndromes in Persons with Cerebrovascular Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Brennan, L.; Gaunt, D.M.; Ben-Shlomo, Y.; Henderson, E. Frailty in Parkinson’s Disease: A Systematic Review. J. Park. Dis. 2019, 9, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Aboelmagd, T.; Dainty, J.R.; MacGregor, A.; Smith, T.O. Trajectory of physical activity after hip fracture: An analysis of community-dwelling individuals from the English Longitudinal Study of Ageing. Injury 2018, 49, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Marengoni, A.; Zucchelli, A.; Vetrano, D.L.; Aloisi, G.; Brandi, V.; Ciutan, M.; Panait, C.L.; Bernabei, R.; Onder, G.; Palmer, K. Heart failure, frailty, and pre-frailty: A systematic review and meta-analysis of observational studies. Int. J. Cardiol. 2020, 316, 161–171. [Google Scholar] [CrossRef]
- Luis-Martínez, R.; Di Marco, R.; Weis, L.; Cianci, V.; Pistonesi, F.; Baba, A.; Carecchio, M.; Biundo, R.; Tedesco, C.; Masiero, S.; et al. Impact of social and mobility restrictions in Parkinson’s disease during COVID-19 lockdown. BMC Neurol. 2021, 21, 332. [Google Scholar] [CrossRef]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Manonelles, P.; Abellán-Aynés, O.; Rubio-Arias, J.Á. The impact of COVID-19 home confinement on neuromuscular performance, functional capacity, and psychological state in Spanish people with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 53, 103047. [Google Scholar] [CrossRef]
- Taylor, J.K.; Ndiaye, H.; Daniels, M.; Ahmed, F.; Triage-HF Plus Investigators. Lockdown, slow down: Impact of the COVID-19 pandemic on physical activity—An observational study. Open Heart 2021, 8, e001600. [Google Scholar] [CrossRef]
- Bertacchini, L.; Paneroni, M.; Comini, L.; Scalvini, S.; Vitacca, M. Recovering of oxygenation, physical function and disability in patients with COVID-19. Monaldi Arch. Chest Dis. 2021, 91, 4. [Google Scholar] [CrossRef]
- Vieira, A.G.D.S.; Pinto, A.C.P.N.; Garcia, B.M.S.P.; Eid, R.A.C.; Mól, C.G.; Nawa, R.K. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: A systematic review. J. Physiother. 2022, 68, 90–98. [Google Scholar] [CrossRef]
- Yamada, M.; Arai, H. Long-Term Care System in Japan. Ann. Geriatr. Med. Res. 2020, 24, 174–180. [Google Scholar] [CrossRef]
- Collen, F.M.; Wade, D.T.; Bradshaw, C.M. Mobility after stroke: Reliability of measures of impairment and disability. Int. Disabil. Stud. 1990, 12, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Hollman, J.H.; Beckman, B.A.; Brandt, R.A.; Merriwether, E.N.; Williams, R.T.; Nordrum, J.T. Minimum detectable change in gait velocity during acute rehabilitation following hip fracture. J. Geriatr. Phys. Ther. 2008, 31, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Scivoletto, G.; Tamburella, F.; Laurenza, L.; Foti, C.; Ditunno, J.F.; Molinari, M. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord. 2011, 49, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Chan, W.L.S.; Pin, T.W. Reliability, validity and minimal detectable change of 2-min walk test and 10-m walk test in frail older adults receiving day care and residential care. Aging Clin. Exp. Res. 2019, 32, 597–604. [Google Scholar] [CrossRef]
- Ng, S.; Au, K.; Chan, E.; Chan, D.; Keung, G.; Lee, J.; Kwong, P.; Tam, E.; Fong, S. Effect of acceleration and deceleration distance on the walking speed of people with chronic stroke. J. Rehabil. Med. 2016, 48, 666–670. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Paillaud, E.; Zelek, L.; Laurent, M.; Lévy, V.; Landre, T.; Sebbane, G. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J. Geriatr. Oncol. 2015, 6, 484–496. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Mathias, S.; Nayak, U.S.; Isaacs, B. Balance in elderly patients: The “get-up and go” test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar] [PubMed]
- Banks, J.L.; Marotta, C.A. Outcomes validity and reliability of the modified rankin scale: Implications for Stroke Clinical Trials: A literature review and synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.; Morrow, V.R.; Yonemura, N.M.; Hopkins, P.N.; Marcus, R.L.; Rondina, M.T.; Lin, Y.K.; Drummond, M.J. Skeletal muscle ceramides and relationship with insulin sensitivity after 2 weeks of simulated sedentary behaviour and recovery in healthy older adults. J. Physiol. 2018, 596, 5217–5236. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Tison, G.H.; Avram, R.; Kuhar, P.; Abreau, M.S.; Marcus, G.M.; Pletcher, M.J.; Olgin, J.E. Worldwide Effect of COVID-19 on Physical Activity: A Descriptive Study. Ann. Intern. Med. 2020, 173, 767–770. [Google Scholar] [CrossRef]
- Yamada, M.; Kimura, Y.; Ishiyama, D.; Otobe, Y.; Suzuki, M.; Koyama, S.; Kikuchi, T.; Kusumi, H.; Arai, H. The Influence of the COVID-19 Pandemic on Physical Activity and New Incidence of Frailty among Initially Non-Frail Older Adults in Japan: A Follow-Up Online Survey. J. Nutr. Health Aging 2021, 25, 751–756. [Google Scholar] [CrossRef]
- Barry, E.; Galvin, R.; Keogh, C.; Horgan, F.; Fahey, T. Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta- analysis. BMC Geriatr. 2014, 14, 14. [Google Scholar] [CrossRef]
- Castell, M.V.; Sánchez, M.; Julián, R.; Queipo, R.; Martín, S.; Otero, A. Frailty prevalence and slow walking speed in persons age 65 and older: Implications for primary care. BMC Fam. Pract. 2013, 14, 86. [Google Scholar] [CrossRef]
- Navarrete-Villanueva, D.; Gómez-Cabello, A.; Marín-Puyalto, J.; Moreno, L.A.; Vicente-Rodríguez, G.; Casajús, J.A. Frailty and Physical Fitness in Elderly People: A Systematic Review and Meta-analysis. Sports Med. 2021, 51, 143–160. [Google Scholar] [CrossRef]
| Mean | SD | n | % | T1–T2 Mean Walking Speed (m/s) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | ||||||||
| Age (years) | 72.39 | 10.21 | 0.89 | 0.34 | |||||
| Sex | male | 130 | 53.9% | 0.89 | 0.34 | 0.191 a | |||
| female | 111 | 46.1% | 0.83 | 0.35 | |||||
| Disease | stroke | 157 | 65.1% | 0.85 | 0.37 | 0.303 b | |||
| musculoskeletal disease | 58 | 24.1% | 0.92 | 0.26 | |||||
| others | 26 | 10.8% | 0.82 | 0.30 | |||||
| mRS | 1 | 58 | 94 | 39.0% | 0.85 | 0.35 | 0.739 a | ||
| 2 | 36 | ||||||||
| 3 | 130 | 147 | 61.0% | 0.87 | 0.34 | ||||
| 4 | 17 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamimoto, T.; Kawakami, M.; Morita, T.; Miyazaki, Y.; Hijikata, N.; Akimoto, T.; Tsujikawa, M.; Honaga, K.; Suzuki, K.; Kondo, K.; et al. Effects of the COVID-19 Pandemic on Physical Function of Community-Dwelling People with Disabilities in Japan. Int. J. Environ. Res. Public Health 2022, 19, 12599. https://doi.org/10.3390/ijerph191912599
Kamimoto T, Kawakami M, Morita T, Miyazaki Y, Hijikata N, Akimoto T, Tsujikawa M, Honaga K, Suzuki K, Kondo K, et al. Effects of the COVID-19 Pandemic on Physical Function of Community-Dwelling People with Disabilities in Japan. International Journal of Environmental Research and Public Health. 2022; 19(19):12599. https://doi.org/10.3390/ijerph191912599
Chicago/Turabian StyleKamimoto, Takayuki, Michiyuki Kawakami, Towa Morita, Yuta Miyazaki, Nanako Hijikata, Tomonori Akimoto, Masahiro Tsujikawa, Kaoru Honaga, Kanjiro Suzuki, Kunitsugu Kondo, and et al. 2022. "Effects of the COVID-19 Pandemic on Physical Function of Community-Dwelling People with Disabilities in Japan" International Journal of Environmental Research and Public Health 19, no. 19: 12599. https://doi.org/10.3390/ijerph191912599
APA StyleKamimoto, T., Kawakami, M., Morita, T., Miyazaki, Y., Hijikata, N., Akimoto, T., Tsujikawa, M., Honaga, K., Suzuki, K., Kondo, K., & Tsuji, T. (2022). Effects of the COVID-19 Pandemic on Physical Function of Community-Dwelling People with Disabilities in Japan. International Journal of Environmental Research and Public Health, 19(19), 12599. https://doi.org/10.3390/ijerph191912599






