Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemicals
2.3. Sample Preparation
2.4. HPLC-MS/MS Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Sample Description
3.2. Quantification of Plasma 8-Isoprostane
3.3. Sample Preparation
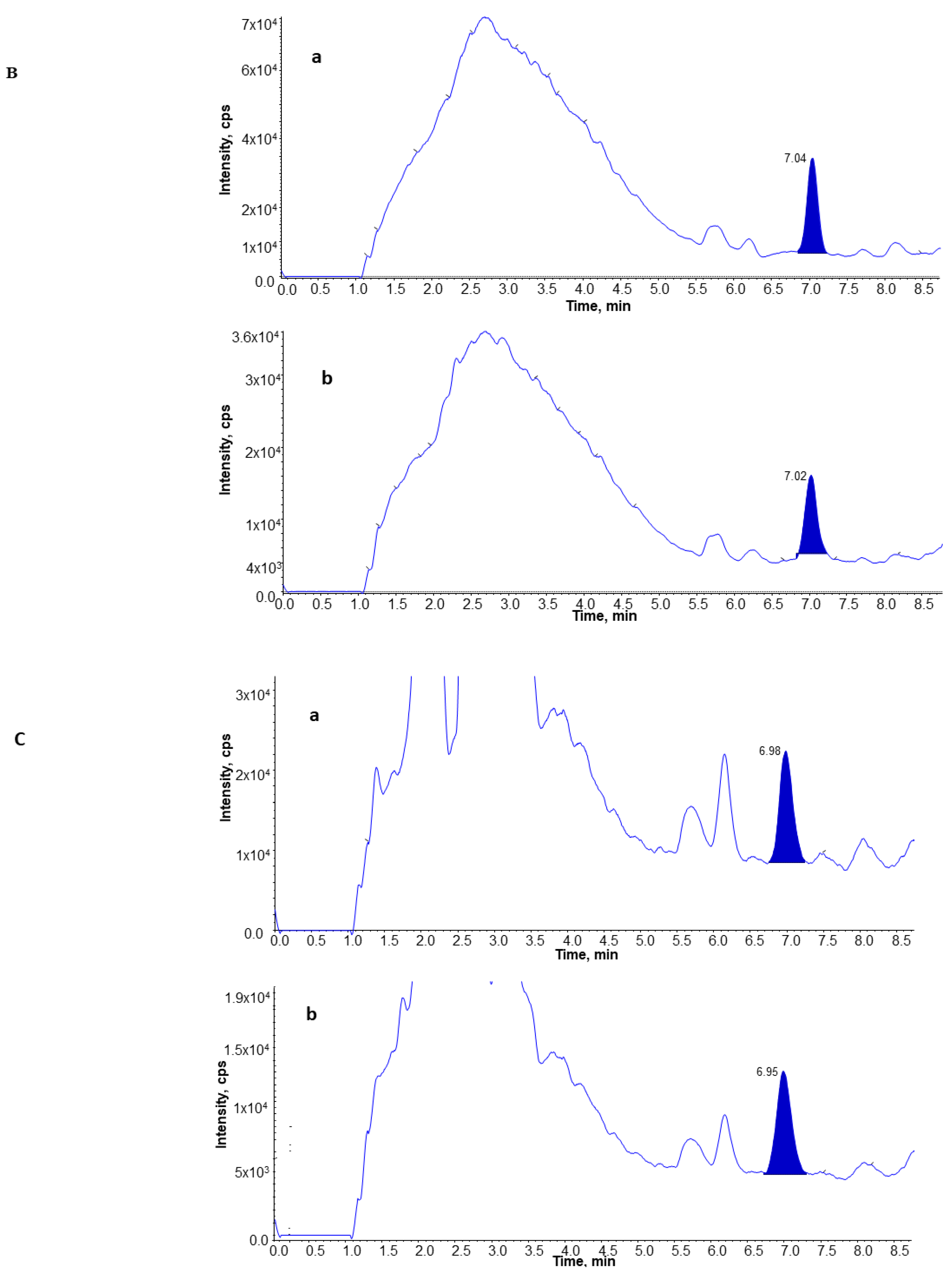
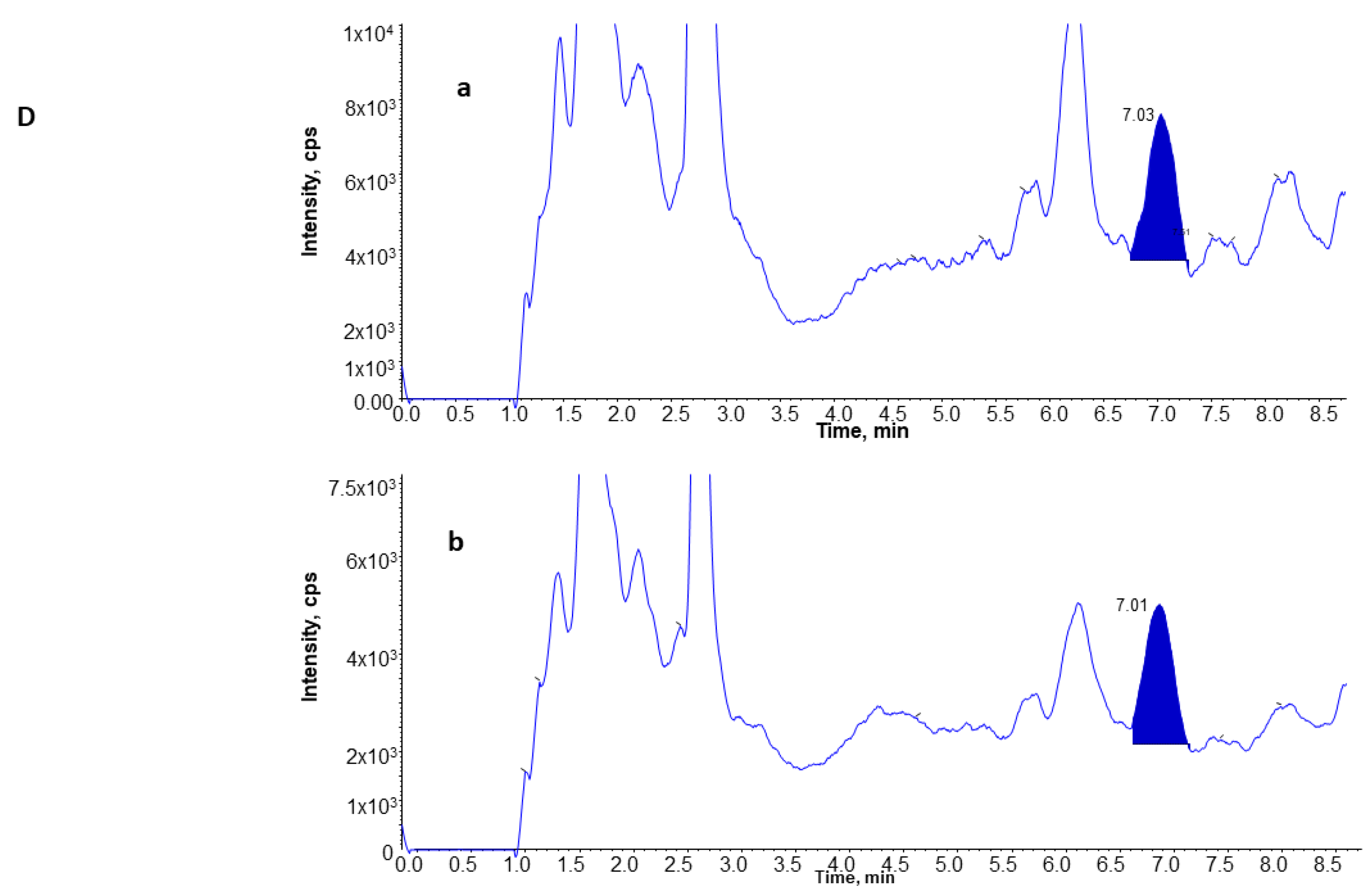
3.4. UPLC and MS Analysis of 8-Isoprostane
3.5. Levels of 8-Isoprostane in Plasma from Subjects with Lung Cancer, Benign Lung Nodules, and from Controls
3.6. Comparison of 8-Isoprostane Levels between Subjects with Lung Cancer and Benign Lung Nodules, and Controls
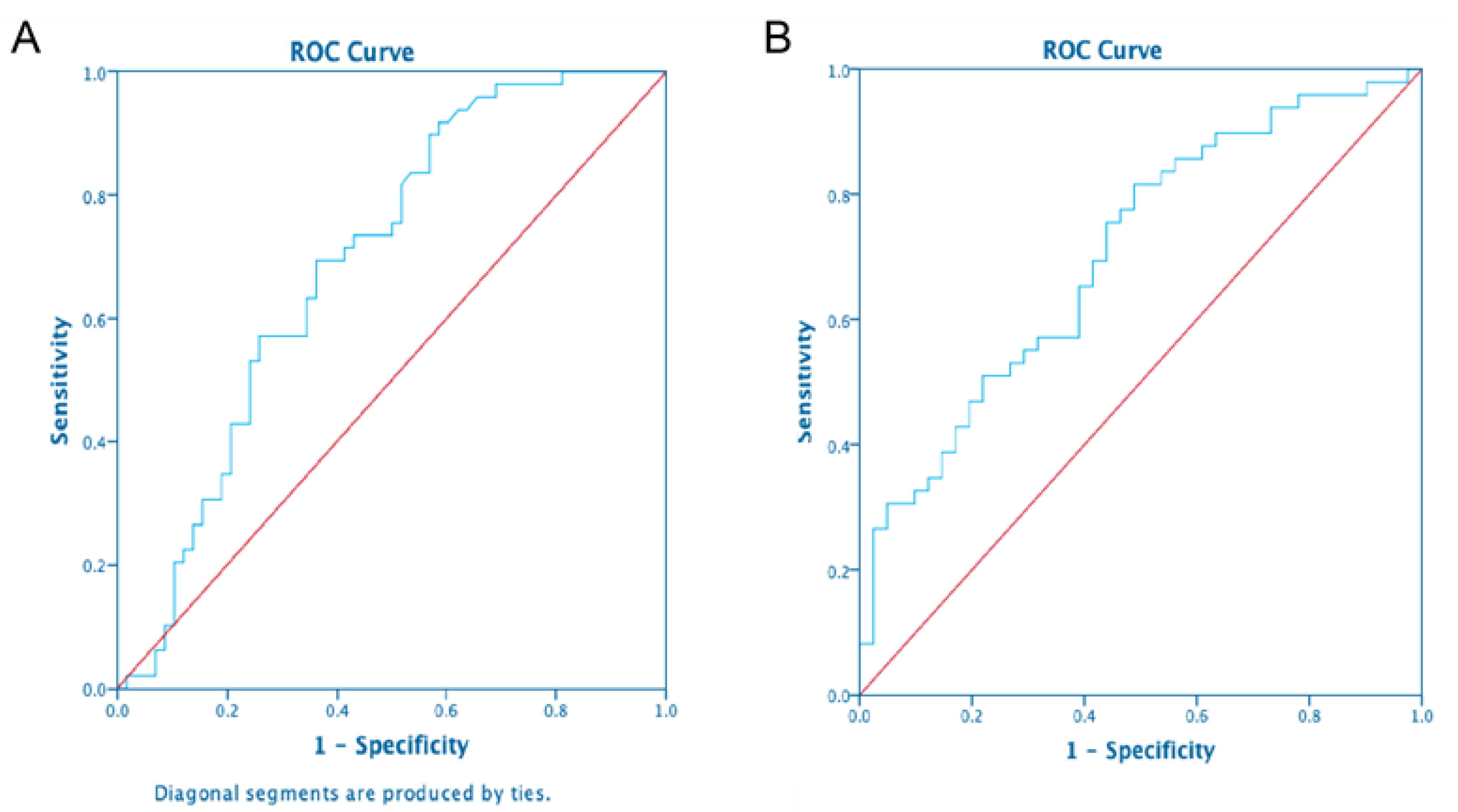
3.7. Predictors of 8-Isoprostane Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkaczyk, J.; Vízek, M. Oxidative stress in the lung tissue--sources of reactive oxygen species and antioxidant defence. Prague Med. Rep. 2007, 108, 105–114. [Google Scholar] [PubMed]
- Comhair, S.A.A.; Erzurum, S.C. Antioxidant responses to oxidant-mediated lung diseases. Am. J. Physiol. Cell. Mol. Physiol. 2002, 283, L246–L255. [Google Scholar] [CrossRef] [PubMed]
- Elfsmark, L.; Ågren, L.; Akfur, C.; Bucht, A.; Jonasson, S. 8-Isoprostane is an early biomarker for oxidative stress in chlorine-induced acute lung injury. Toxicol. Lett. 2018, 282, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of inci-dence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Havet, A.; Zerimech, F.; Sanchez, M.; Siroux, V.; Le Moual, N.; Brunekreef, B.; Stempfelet, M.; Künzli, N.; Jacquemin, B.; Matran, R.; et al. Outdoor air pollution, exhaled 8-isoprostane and current asthma in adults: The EGEA study. Eur. Respir. J. 2018, 51, 1702036. [Google Scholar] [CrossRef] [PubMed]
- Van’t Erve, T.J.; Lih, F.B.; Jelsema, C.; Deterding, L.J.; Eling, T.E.; Mason, R.P.; Kadiiska, M.B. Reinterpreting the best biomarker of oxidative stress: The 8-iso-prostaglandin F2alpha/prostaglandin F2alpha ratio shows complex origins of lipid peroxidation biomarkers in animal models. Free Radic. Biol. Med. 2016, 95, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes--25 years later. Biochim. Biophys Acta. 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Dybos, S.A.; Brustad, W.; Rolfseng, T.; Kvam, S.; Olsen, O.E.; Halgunset, J.; Skogseth, H. RNA-Integrity and 8-Isoprostane Levels Are Stable in Prostate Tissue Samples Upon Long-Term Storage at −80 °C. Biopreservation Biobanking 2021, 19, 2–10. [Google Scholar] [CrossRef]
- Baraldi, E.; Ghiro, L.; Piovan, V.; Carraro, S.; Ciabattoni, G.; Barnes, P.J.; Montuschi, P. Increased Exhaled 8-Isoprostane in Childhood Asthma. Chest 2003, 124, 25–31. [Google Scholar] [CrossRef]
- Wood, L.; Fitzgerald, D.A.; Gibson, P.; Cooper, D.M.; Garg, M.L. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids 2000, 35, 967–974. [Google Scholar] [CrossRef]
- Praticò, D.; Basili, S.; Vieri, M.; Cordova, C.; Violi, F.; Fitzgerald, G.A. Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane F2alpha-III, an index of oxidant stress. Am. J. Respir. Crit. Care Med. 1998, 158, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Mondino, C.; Ciabattoni, G.; Koch, P.; Pistelli, R.; Trové, A.; Barnes, P.J.; Montuschi, P. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J. Allergy Clin. Immunol. 2004, 114, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Collins, J.V.; Ciabattoni, G.; Lazzeri, N.; Corradi, M.; Kharitonov, S.A.; Barnes, P.J. Exhaled 8-Isoprostane as an In Vivo Biomarker of Lung Oxidative Stress in Patients with COPD and Healthy Smokers. Am. J. Respir. Crit. Care Med. 2000, 162, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, W.A.; Kharitonov, S.A.; Barnes, P.J. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax 2003, 58 Pt 1, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Quaggiotto, P.; Wood, L.; O’Loughlin, E.V.; Henty, R.L.; Garg, M.L. Elevated plasma levels of F2α isoprostane in cystic fibrosis. Lipids 1999, 34, 551–556. [Google Scholar] [CrossRef]
- Ciabattoni, G.; Davì, G.; Collura, M.; Iapichino, L.; Pardo, F.; Ganci, A.; Romagnoli, R.; Maclouf, J.; Patrono, C. In Vivo Lipid Peroxidation and Platelet Activation in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162 Pt 1, 1195–1201. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Carmella, S.G.; Wang, R.; Tan, Y.-T.; Adams-Haduch, J.; Gao, Y.-T.; Hecht, S.S. Relationship of the oxidative damage biomarker 8-epi-prostaglandin F2α to risk of lung cancer development in the Shanghai Cohort Study. Carcinogenesis 2018, 39, 948–954. [Google Scholar] [CrossRef]
- Gào, X.; Brenner, H.; Holleczek, B.; Cuk, K.; Zhang, Y.; Anusruti, A.; Xuan, Y.; Xu, Y.; Schöttker, B. Urinary 8-isoprostane levels and occurrence of lung, colorectal, prostate, breast and overall cancer: Results from a large, population-based cohort study with 14 years of follow-up. Free Radic. Biol. Med. 2018, 123, 20–26. [Google Scholar] [CrossRef]
- Tsikas, D.; Suchy, M.-T. Assessment of Urinary F2-Isoprostanes in Experimental and Clinical Studies: Mass Spectrometry Versus ELISA. Hypertension 2012, 60, e15. [Google Scholar] [CrossRef]
- Il’Yasova, D.; Morrow, J.D.; Ivanova, A.; Wagenknecht, L.E. Epidemiological marker for oxidant status: Comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann. Epidemiol. 2004, 14, 793–797. [Google Scholar] [CrossRef]
- Halliwell, B.; Lee, C.Y. Using isoprostanes as biomarkers of oxidative stress: Some rarely con-sidered issues. Antioxid Redox Signal. 2010, 13, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Byrd, G.D.; Ogden, M.W. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J. Lipid Res. 2007, 48, 1607–1617. [Google Scholar] [CrossRef]
- Baldwin, N.A.; Sarnowski, C.P.; Reddy, S.A.; Blair, I.A.; Clapper, M.; Lazarus, P.; Li, M.; Muscat, J.E.; Penning, T.M.; Vachani, A.; et al. Development of a Genotyping Microarray for Studying the Role of Gene-Environment Interactions in Risk for Lung Cancer. J. Biomol. Tech. JBT 2013, 24, 198–217. [Google Scholar] [CrossRef][Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Detection of inflammatory biomarkers in saliva and urine: Potential in diagnosis, prevention, and treatment for chronic diseases. Exp. Biol. Med. 2016, 241, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Fois, S.S.; Piras, B.; Carru, C.; Pirina, P. Reliability and Usefulness of Different Biomarkers of Oxidative Stress in Chronic Obstructive Pulmonary Disease. Oxidative Med. Cell. Longev. 2020, 2020, 4982324. [Google Scholar] [CrossRef]
- Barreiro, E.; Fermoselle, C.; Mateu-Jimenez, M.; Sánchez-Font, A.; Pijuan, L.; Gea, J.; Curull, V. Oxidative stress and inflammation in the normal airways and blood of pa-tients with lung cancer and COPD. Free Radic. Biol. Med. 2013, 65, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Sunnetcioglu, A.; Alp, H.H.; Ndan, B.S.; Balaharoglu, R.; Gunbatar, H. Evaluation of Oxidative Damage and Antioxidant Mechanisms in COPD, Lung Cancer, and Obstructive Sleep Apnea Syndrome. Respir. Care 2015, 61, 205–211. [Google Scholar] [CrossRef]
- van der Vaart, H.; Postma, D.S.; Timens, W.; ten Hacken, N.H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef]
- Penning, T.M. Human Aldo-Keto Reductases and the Metabolic Activation of Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2014, 27, 1901–1917. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Penninx, B.W.J.H. Sociodemographic and Lifestyle Determinants of Plasma Oxidative Stress Markers 8-OHdG and F2-Isoprostanes and Associations with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2016, 2016, 7530820. [Google Scholar] [CrossRef]
- Van’t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox Biol. 2017, 12, 582–599. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, A.M.; Boyce, P.D.; Cavallari, J.M.; Fang, S.C.; Eisen, E.A.; Christiani, D.C. Urinary 8-Isoprostane and 8-OHdG Concentrations in Boilermakers With Welding Exposure. J. Occup. Environ. Med. 2008, 50, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Peres, B.U.; Allen, A.J.H.; Shah, A.; Fox, N.; Laher, I.; Almeida, F.; Jen, R.; Ayas, N. Obstructive Sleep Apnea and Circulating Biomarkers of Oxidative Stress: A Cross-Sectional Study. Antioxidants 2020, 9, 476. [Google Scholar] [CrossRef]
- Janicka, M.; Kot-Wasik, Á.; Paradziej-Łukowicz, J.; Sularz-Peszyńska, G.; Bartoszek, A.; Namieśnik, J. LC-MS/MS Determination of Isoprostanes in Plasma Samples Collected from Mice Exposed to Doxorubicin or Tert-Butyl Hydroperoxide. Int. J. Mol. Sci. 2013, 14, 6157–6169. [Google Scholar] [CrossRef] [PubMed]
- Van’t Erve, T.J.; Lih, F.B.; Kadiiska, M.B.; Deterding, L.J.; Mason, R.P. Elevated plasma 8-iso-prostaglandin F2alpha levels in human smokers originate primarily from enzymatic instead of non-enzymatic lipid peroxidation. Free Radic. Biol. Med. 2018, 115, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Dalaveris, E.; Kerenidi, T.; Katsabeki-Katsafli, A.; Kiropoulos, T.; Tanou, K.; Gourgoulianis, K.I.; Kostikas, K. VEGF, TNF-α and 8-isoprostane levels in exhaled breath condensate and serum of patients with lung cancer. Lung Cancer 2009, 64, 219–225. [Google Scholar] [CrossRef]
- Syslová, K.; Kačer, P.; Kuzma, M.; Klusáčková, P.; Fenclová, Z.; Lebedová, J.; Pelclová, D. Determination of 8-iso-prostaglandin F2α in exhaled breath condensate using combination of immunoseparation and LC–ESI-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 867, 8–14. [Google Scholar] [CrossRef]
- Morrow, J.D.; Roberts, L. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999, 300, 3–12. [Google Scholar] [CrossRef]
- Morrow, J.D.; Zackert, W.E.; Yang, J.P.; Kurhts, E.H.; Callewaert, D.; Dworski, R.; Kanai, K.; Taber, D.; Moore, K.; Oates, J.A.; et al. Quantification of the Major Urinary Metabolite of 15-F2t-Isoprostane (8-iso-PGF2α) by a Stable Isotope Dilution Mass Spectrometric Assay. Anal. Biochem. 1999, 269, 326–331. [Google Scholar] [CrossRef]
- Soffler, C.; Campbell, V.L.; Hassel, D.M. Measurement of Urinary F2-Isoprostanes as Markers of in Vivo Lipid Peroxidation: A Comparison of Enzyme Immunoassays with Gas Chromatography–Mass Spectrometry in Domestic Animal Species. J. Veter-Diagn. Investig. 2010, 22, 200–209. [Google Scholar] [CrossRef]
- Barden, A.; Mas, E.; Croft, K.; Phillips, M.; Mori, T. Minimizing artifactual elevation of lipid peroxidation products (F2-isoprostanes) in plasma during collection and storage. Anal. Biochem. 2014, 449, 129–131. [Google Scholar] [CrossRef]
- Epplein, M.; Franke, A.A.; Cooney, R.V.; Morris, J.S.; Wilkens, L.R.; Goodman, M.T.; Murphy, S.P.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Association of Plasma Micronutrient Levels and Urinary Isoprostane with Risk of Lung Cancer: The Multiethnic Cohort Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.R.; Devaraj, A. Lung cancer risk in new pulmonary nodules: Implications for CT screening and nodule management. Lancet Oncol. 2016, 17, 849–850. [Google Scholar] [CrossRef]


| Cases | Controls (Benign Nodules) | Controls (COPD) | |
|---|---|---|---|
| Mean age | 59.3 ± 7.4 | 59.9 ± 6.4 | 48.4 ± 6.38 |
| Sex Males Females | 20 (41%) 29 (59%) | 24 (44%) 31 (56%) | 22 (54%) 19 (465) |
| Smoking status Current Former | 16 (33%) 33 (67%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Sun, D.; Xiu, G.; Lazarus, P.; Vachani, A.; Penning, T.M.; Whitehead, A.S.; Muscat, J.E. Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer. Int. J. Environ. Res. Public Health 2022, 19, 12488. https://doi.org/10.3390/ijerph191912488
Ma L, Sun D, Xiu G, Lazarus P, Vachani A, Penning TM, Whitehead AS, Muscat JE. Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer. International Journal of Environmental Research and Public Health. 2022; 19(19):12488. https://doi.org/10.3390/ijerph191912488
Chicago/Turabian StyleMa, Lin, Dongxiao Sun, Guangli Xiu, Philip Lazarus, Anil Vachani, Trevor M. Penning, Alexander S. Whitehead, and Joshua E. Muscat. 2022. "Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer" International Journal of Environmental Research and Public Health 19, no. 19: 12488. https://doi.org/10.3390/ijerph191912488
APA StyleMa, L., Sun, D., Xiu, G., Lazarus, P., Vachani, A., Penning, T. M., Whitehead, A. S., & Muscat, J. E. (2022). Quantification of Plasma 8-Isoprostane by High-Performance Liquid Chromatography with Tandem Mass Spectrometry in a Case-Control Study of Lung Cancer. International Journal of Environmental Research and Public Health, 19(19), 12488. https://doi.org/10.3390/ijerph191912488







